Published online Dec 27, 2014. doi: 10.4240/wjgs.v6.i12.248
Revised: May 18, 2014
Accepted: October 28, 2014
Published online: December 27, 2014
Processing time: 270 Days and 23.4 Hours
Inflammatory pseudotumor (IPT) of the spleen is an uncommon entity with an uncertain aetiology. Inflammatory pseudotumors present diagnostic difficulties because the clinical and radiological findings tend to suggest a malignancy. The symptoms include weight loss, fever, and abdominal pain. Most cases of splenic IPT present solitary relatively large well circumscribed masses on imaging. The diagnosis in the majority of the cases is made after histopathologic study of splenectomy specimens. The IPTs that occur in the spleen and liver are typically associated with Epstein-Barr virus. Thirty-seven percent of all new cases of active tuberculosis infection are extrapulmonary tuberculosis and tuberculous lymphadenitis the most commonly occurring form of extrapulmonary tuberculosis. We report the case of an inflammatory pseudotumor of the spleen associated with splenic tuberculous lymphadenitis in a 50-year-old female patient who was preoperatively diagnosed with a malignant spleen tumour based on her history of breast of carcinoma.
Core tip: A rare benign; lesion inflammatory pseudotumours (IPT) are infrequently found in the spleen with only sporadic case reports and short case series reported in the literature. Here we report a case of a spleen IPT associated with splenic tuberculous lymphadenitis in a 50-year-old female patient who was preoperatively diagnosed with a malignant spleen neoplasm.
- Citation: Prieto-Nieto MI, Pérez-Robledo JP, Díaz-San Andrés B, Nistal M, Rodríguez-Montes JA. Inflammatory pseudotumour of the spleen associated with splenic tuberculosis. World J Gastrointest Surg 2014; 6(12): 248-252
- URL: https://www.wjgnet.com/1948-9366/full/v6/i12/248.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v6.i12.248
Inflammatory pseudotumours (IPT) characterized microscopically by a proliferation of inflammatory cells, are infrequently found in the spleen and there are only sporadic case reports and short case series in the literature[1]. We report a case of a spleen IPT associated with splenic tuberculous lymphadenitis in a 50-year-old female patient who was preoperatively diagnosed with a malignant spleen neoplasm[1,2].
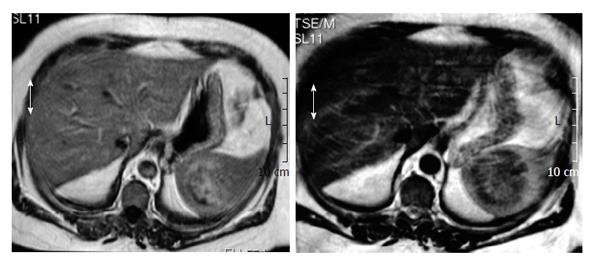
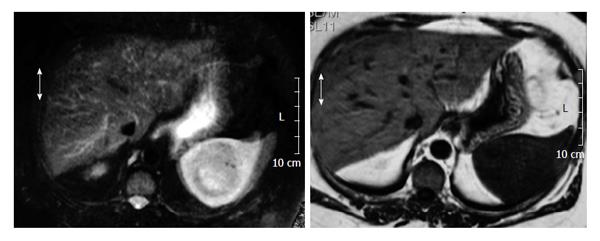
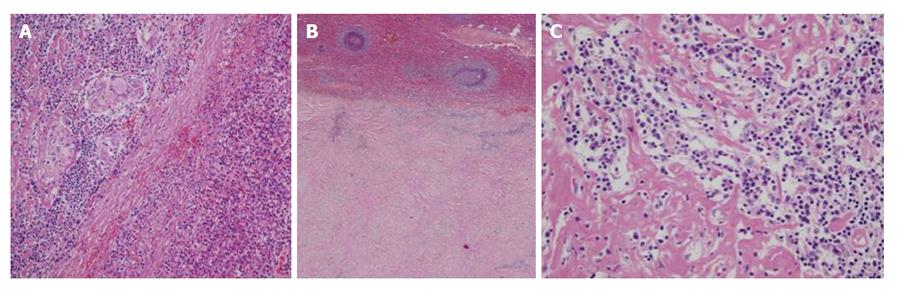
A 50-year-old woman had been undergone a quadrantectomy for an intraductal breast carcinoma intraductal. The sentinel ganglion was negative so the patient received radiotherapy followed by tamoxifen. Two years later her routine follow-up ultrasonography incidentally discovered a solid mass at the inferomedial pole of the spleen. There was no history of spleen trauma. Over the previous two months, she had experienced minor weight loss and tiredness, fatigue and left flank pain. Biochemical and blood count parameters were within normal ranges. Serum α-fetoprotein, carcinoembryonic antigen, and antinuclear antibody and CA 19.9, CA 125, CA 15 levels were normal and serum echinococcus granulosis IgE and hemagglutination tests were negative. Biochemical and haematological results were all within normal ranges. Physical examination did not reveal organomegalia or lymphadenopthy. Blood antibodies for Epstein-Barr Virus (EBV), cytomegalovirus, toxoplasmosis and Human Immunodeficiency Virus (HIV) were all negative. The chest x-ray was normal. Magnetic resonance imaging (MRI) revealed a 6 cm diameter mass. T1-weighted axial imaging, showed a hypoisointense signal, while the T2-weighted axial imaging showed an isointense signal with small areas of hyperintensity (Figures 1 and 2). Post-gadolinium-DTPA T1-weighted imaging showed a heterogeneous increase in the mass[3,4]. Splenectomy was performed. The spleen measured 17 cm × 10 cm × 8 cm and weighed 415 g. The histopathological study described an inflamed spleen with an inflammatory pseudotumor (Figure 3A) showing fibroblastic and myofibroblastic proliferation (Figure 3B) that was associated with con tuberculous lymphadenitis of the spleen (Figure 3C). A six month regimen consist in two months of isoniazide 300/d (INH), rifacin 600 mg/d (RIFADIN), pyrazinamide 1.5 g/d and ethambutol 1 g/d (MYAMBUTOL), followed by four months of isoniazid and rifampin. Eighteen months after surgery the patient remains asymptomatic and there is no evidence of tumoral recurrence.
IPT are benign in nature. Slow-growing, these tumors may be located in the lung, respiratory tract, gastrointestinal tract, liver, spleen or lymph nodes,but the liver is the most common extra pulmonary site[1,2]. About one half of the lesion is discovered incidentally during revision for other malignancies after splenectomy[1].
The aetiology and pathogenesis of IPT are not yet understood. It may represent an non specific response to a bacterial or viral infection. In up to 40% of cases, granulomata, giant cells and EBV are detected in the involved tissue. EBV can be found in 66.7% of splenic and hepatic pseudotumors but it is detectable in only 20% of pseudotumors of the lymph node[3]. The frequency of EBV may vary depending on the site of the IPT[3-5]. It has been hypothesised that IPT may have an autoimmune nature. This hypothesis is supported by the fact that, in some cases, IPT are associated with thrombocytopenia purpura[6,7]. In addition, autoimmunity is suggested by the high plasmatic cell content in histologic specimens. Infection, vascular causes and autoinmunes disorders have been hypothesised in their pathogenesis. Infection is one of the hypothesesis because of the presence of granulomas and giant cells in the masses, as occurred in our patient who had tuberculosis. Mycobacteria have been found in spindle cell pseudotumors (MSP). In most of the reported cases MSP occurs in the lymph node of inmunocompromised patients[8] and it should be noted that our patient had undergone radiotherapy so perhaps her autoinmune system had become compromised, allowing her to acquire the tuberculosis and then inflammatory pseudotumor appeared. Some 37% of all new cases of active tuberculosis infection are extrapulmonary tuberculosis[9]. Tuberculous lymphadenitis is the most commonly occurring form of extrapulmonary tuberculosis. Excisional biopsy of the lymph nodes with histology, acid fast-bacillus stain and mycobacterial culture are the diagnostic procedure of choice. A sample can be cultured in a specific medium (Lowenstein Jenssen) to diagnose the bacteria, but if there is not sufficient simple, a polymerase reaction (PCR) analysis which is highly sensitive and can distinguish tuberculosis from other mycobacteris, must be done[10,11]. Tuberculosis in the spleen is uncommon, usually associated with miliary dissemination and is most often observed in patients with immunodeficiency. Splenic tuberculoma can be micronodular or macronodular. The latter form is extremely rare and seen more often in HIV+ individuals. However there are sporadic case reports of splenic TB in immunocompetent patients. Our patient had neither a history of TB nor showed evidence of TB in any other organ. The bacteriological findings were confirmed by histopathology and acid-fast bacillus staining as well as culture and PCR. Results with interferon gamma release assays have shown that the yield of this test is poor in patients with altered immune systems and this was done in our patient[12].
In most cases, IPT of the spleen affects patients in their fifth or sixth decade of life, with men and women being affected in the same proportion[1], although some authors report a higher incidence in women[1,2]. Patients with IPT of the spleen present with unspecific symptoms, and the diagnosis is most often the result of an incidental finding. The most usual complaint is pain located on the left upper quadrant due to either increased spleen size or compression of adjacent anatomical structures. Less frequently, fever of unknown origin, anaemia and weight loss[2], thrombocytosis, polyclonal hypergammaglobulinemia, hypercalcemia and lekocytosis all of which suggest a lymphoproliferative disorder[13,14] may be reported. Physical examination can reveal splenomegalia, but findings are most often unspecific
Preoperative diagnosis is troublesome, and can not be made by relying on laboratory findings[1] although leukocytosis and an elevated erythrocyte sedimentation rate are common. Radiological studies reveal focal necrosis, cystic calcification and myxoid changes. An abdominal radiograph can show splenomegalia and curvilinear calcifications along the inside edge of the spleen[14]. Computed tomography (CT) is not specific in differentiating between splenic IPT and other malignancies[8]. CT reveals a mass with a central hypodense area that may correspond to a necrotic zone surrounded by a hyperdense area with outlying hypodense zones[1,2,15]. MRI reveals a lesion with a minimum increase in the signal on T1, a hypersignal on T2, and gadolinium injection, a moderate enhancement in signal intensity on T1 as occurred in our patient[14,16]. The only MRI difference between a splenic tuberculoma and an inflammatory tumor of the spleen is that the image of the former changes with the evolution of the disease and may even disappear at 5-10 mo while the inflammatory pseudotumor does not change[17,18].
Radiological findings may also be indistinguishable from those of a lymphoproliferative disorder or a malignancy of the spleen. Lymphoma is the most usual misdiagnosis. The differential diagnosis should also consider hamartoma and benign tumours, including as well as vascular malformations, granulomatous infections (which most often result in systemic affectation), splenic infarct, and spleen metastases, the latter being the initial presumptive diagnosis in our patient[13,14,19]. Surgery remains the gold stantard for definitive diagnosis.
In most cases the pseudotumor consists of a single well-circumscribed mass of 2-15 cm in diameter, composed of inflammatory cells, particularly plasmatic cells, and a fibroblastic proliferation of spindle cells, such as smooth muscle antibody (+), myofibroblasts and follicular dendritic cells (+). The spindle cells in IPT are most often myofibroblasts[5,20]. (1) IPT of the spleen is classified into three groups according to the cellular characteristics of the mass, clinical presentation and course, potential aetiology and prognosis: IPT-like follicular dendritic cell tumour. Is the kind that most frequently affects women. It is associated with a spindle cells and EBV infection; (2) The presence of myofibroblasts in a splenic IPT has led to the designation of inflammatory myofibroblastic (IMT) tumor. A considerable proportion of these tumours are EBV (+) and they have a potential for malignancy; and (3) IPT of the spleen was described by Cottelingam and Jaffe as having a predominance of spindle cells. Some 33%-48% of cases are found incidentally. This is the group which best deserves the term IPT of the spleen[5,20]. The World Health Organization classification places inflammatory myofibroblastic tumors in an intermediate category (rarely metastasizing, < 5%) between benign and malignant[14].
IPT of the spleen is a rare entity. It should be included in the differential diagnosis for splenic masses, especially when MRI discloses a single mass showing increased signal on T1 following gadolinium injection.
A 50-year-old woman with a inflammatory pseudotumour of the spleen that present diagnostic difficulties because the clinical and radiological findings tend to suggest a malignancy.
Unspecific symptoms and the diagnosis is most often the result of an incidental finding.
Lymphoproliferative disorder or a malignancy of the spleen. Lymphoma, hamartoma, vascular malformations, splenic infarct and spleen metastases.
Biochemical and blood count parameters were within normal ranges. Serum α-fetoprotein, carcinoembryonic antigen, and antinuclear antibody and CA 19.9, CA 125, CA 15 levels were normal and serum echinococcus granulosis IgE and hemagglutination tests were negative.
The histopathological study described an inflamed spleen with an inflammatory pseudotumor showing fibroblastic and myofibroblastic proliferation that was associated with con tuberculous lymphadenitis of the spleen.
A six month regimen consist in two months of isoniazide 300/d (INH), rifacin 600 mg/d (RIFADIN), pyrazinamide 1.5 g/d and ethambutol 1 g/d (MYAMBUTOL), followed by four months of isoniazid and rifampin. Splenectomy was performed.
The aetiology and pathogenesis of inflammatory pseudotumours (IPT) are not yet understood. It may represent an non specific response to a bacterial or viral infection. In up to 40% of cases, granulomata, giant cells and Epstein-Barr virus (EBV) are detected in the involved tissue. EBV can be found in 66.7% of splenic and hepatic pseudotumors but it is detectable in only 20% of pseudotumors of the lymph node.
Excisional biopsy of the lymph nodes with histology, acid fast-bacillus stain and mycobacterial culture are the diagnostic procedure of choice. A sample can be cultured in a specific medium (Lowenstein Jenssen) to diagnose the bacteria, but if there is not sufficient simple, a polymerase reaction analysis which is highly sensitive and can distinguish tuberculosis from other mycobacteris, must be done. Tuberculosis in the spleen is uncommon, usually associated with miliary dissemination and is most often observed in patients with immunodeficiency. Splenic tuberculoma can be micronodular or macronodular. The latter form is extremely rare and seen more often in Human Immunodeficiency Virus+ individuals.
The frequency of EBV may vary depending on the site of the IPT. It has been hypothesised that IPT may have an autoimmune nature. This hypothesis is supported by the fact that, in some cases, IPT are associated with thrombocytopenia purpura. In addition, autoimmunity is suggested by the high plasmatic cell content in histologic specimens. Infection, vascular causes and autoinmunes disorders have been hypothesised in their pathogenesis. Infection is one of the hypothesis because of the presence of granulomas and giant cells in the masses, as occurred in their patient who had tuberculosis. Mycobacteria have been found in spindle cell pseudotumors (MSP). In most of the reported cases MSP occurs in the lymph node of inmunocompromised patients and it should be noted that their patient had undergone radiotherapy so perhaps her autoinmune system had become compromised, allowing her to acquire the tuberculosis and then inflammatory pseudotumor appeared. Tuberculous lymphadenitis is the most commonly occurring form of extrapulmonary tuberculosis.
It is an interesting manuscript worthy of publication.
P- Reviewer: Demonacos C, Garcia-Elorriaga G, Iso Y, Li ZF, Mastroianni CM S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Ma ZH, Tian XF, Ma J, Zhao YF. Inflammatory pseudotumor of the spleen: A case report and review of published cases. Oncol Lett. 2013;5:1955-1957. [PubMed] |
| 2. | Ozkara SK, Gürbüz Y, Erçín C, Müezzínoğlu B, Türkmen M. Inflammatory pseudotumor of the spleen. Virchows Arch. 2001;438:629-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Oz Puyan F, Bilgi S, Unlu E, Yalcin O, Altaner S, Demir M, Cakir B. Inflammatory pseudotumor of the spleen with EBV positivity: report of a case. Eur J Haematol. 2004;72:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Lewis JT, Gaffney RL, Casey MB, Farrell MA, Morice WG, Macon WR. Inflammatory pseudotumor of the spleen associated with a clonal Epstein-Barr virus genome. Case report and review of the literature. Am J Clin Pathol. 2003;120:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Rosenbaum L, Fekrazad MH, Rabinowitz I, Vasef MA. Epstein-Barr virus-associated inflammatory pseudotumor of the spleen: report of two cases and review of the literature. J Hematop. 2009;2:127-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Alimoglu O, Cevikbas U. Inflammatory pseudotumor of the spleen: report of a case. Surg Today. 2003;33:960-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Noguchi H, Kondo H, Kondo M, Shiraiwa M, Monobe Y. Inflammatory pseudotumor of the spleen: a case report. Jpn J Clin Oncol. 2000;30:196-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Philip J, Beasley MB, Dua S. Mycobacterial spindle cell pseudotumor of the lung. Chest. 2012;142:783-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Golden MP, Vikram HR. Extrapulmonary tuberculosis: an overview. Am Fam Physician. 2005;72:1761-1768. [PubMed] |
| 10. | Velásquez MJ, Szigethi QM, Panace VR, Morales IR, Márquez CS, Pefaur PJ, Mocarquer MA, Salinas CP, Beltrán BC. [Hepatic-splenic micobacteriosis, unusual form of probable extrapulmonary tuberculosis. Case report and review]. Rev Chilena Infectol. 2007;24:59-62. [PubMed] |
| 11. | Rodarte-Shade M, Diaz-Elizondo JA. Splenic tuberculosis. Surg Infect (Larchmt). 2012;13:420-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Oikonomou A, Mantatzis M, Ritis K, Kartalis G, Prassopoulos P. Multiple splenic macronodular tuberculomas: MRI characteristics under treatment. Int J Tuberc Lung Dis. 2006;10:233-234. [PubMed] |
| 13. | Hsu CW, Lin CH, Yang TL, Chang HT. Splenic inflammatory pseudotumor mimicking angiosarcoma. World J Gastroenterol. 2008;14:6421-6424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Rajabi P, Noorollahi H, Hani M, Bagheri M. Inflammatory pseudotumor of spleen. Adv Biomed Res. 2014;3:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Kapoor R, Jain AK, Chaturvedi U, Saha MM. Ultrasound detection of tuberculomas of the spleen. Clin Radiol. 1991;43:128-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | McHenry CR, Perzy-Gall HB, Mardini G, Chung-Park M. Inflammatory pseudotumor of the spleen: a rare entity that may mimic hematopoietic malignancy. Am Surg. 1995;61:1067-1071. [PubMed] |
| 17. | Bastounis E, Pikoulis E, Varelas P, Cirochristos D, Aessopos A. Tuberculoma of the spleen: a rare but important clinical entity. Am Surg. 1999;65:131-132. [PubMed] |
| 18. | Dede F, Doğan E, Demir M, Sener D, Kös M, Tad M, Eskioğlu E. Unusual presentation of tuberculosis as a splenic mass. Tohoku J Exp Med. 2006;210:79-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Krishnan J, Frizzera G. Two splenic lesions in need of clarification: hamartoma and inflammatory pseudotumor. Semin Diagn Pathol. 2003;20:94-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Oshiro H, Nomura M, Yamanaka S, Watanabe S, Inayama Y. Splenic inflammatory pseudotumor (inflammatory myofibroblastic tumor). J Clin Exp Hematop. 2007;47:83-88. [PubMed] |