Published online Jul 27, 2013. doi: 10.4240/wjgs.v5.i7.229
Revised: May 17, 2013
Accepted: June 20, 2013
Published online: July 27, 2013
Processing time: 82 Days and 15.6 Hours
The Carney triad (CT) describes the coexistence of multiple neoplasms including gastrointestinal stromal tumors (GISTs), extra-adrenal paraganglioma and pulmonary chondroma. At least two neoplastic tumors are required for diagnosis. In most cases, however, CT is incomplete. We report a case of an incomplete CT in a 34-year-old woman with a multifocal GIST and non-functional paraganglioma of the liver. Preoperative evaluation with a gastrofiberscope and abdominal computed tomography revealed multiple gastric tumors resembling GISTs and a single liver lesion which was assumed to have metastasized from the gastric tumors. The patient underwent total gastrectomy and partial hepatectomy. Histologic findings confirmed multiple gastric GISTs and paraganglioma of the liver. We report a case of a patient with incomplete expression of CT.
Core tip: The Carney triad (CT) describes the coexistence of multiple neoplasms including gastrointestinal stromal tumors (GISTs), extra-adrenal paraganglioma and pulmonary chondroma. We report a case of an incomplete CT. CT is a very rare syndrome but Carney et al thoroughly documented its clinical manifestations. The presence of pulmonary chondroma and paraganglioma should be verified, especially in young women with multifocal GISTs to rule out CT.
- Citation: Hong SW, Lee WY, Lee HK. Hepatic paraganglioma and multifocal gastrointestinal stromal tumor in a female: Incomplete Carney triad. World J Gastrointest Surg 2013; 5(7): 229-232
- URL: https://www.wjgnet.com/1948-9366/full/v5/i7/229.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v5.i7.229
The Carney triad (CT) initially described the triad of gastric leiomyosarcoma, functioning extra-adrenal paraganglioma, and pulmonary chondroma[1]. Later, gastric leiomyosarcoma was replaced with gastric gastrointestinal stromal tumors (GISTs) and several cases of paragangliomas were known to be non-functioning[2]. GISTs in childhood or adolescence can occur as sporadic diseases unrelated to a syndrome, and can present as either a familial disorder [Carney-Stratakis syndrome (CSS)] or part of non-hereditary CT[3]. The differential diagnosis of CT from sporadic gastric GISTs is crucial because CT differ considerably from sporadic gastric GISTs in clinical both course and prognosis[2]. Here, we report a CT case with multiple gastric GISTs and paraganglioma of the liver.
An otherwise healthy 34-year-old woman visited our hospital after being diagnosed with gastric tumors via gastrofiberscopic screening at a community hospital. She had no specific symptoms or signs related to gastric tumors. She had no specific past medical history involing hypertesion. There was no abnormality of her past or familial history related to endocrine disease or gastrointestinal malignancies. An initial physical examination showed no significant abnormalities. Laboratory findings were also unremarkable. Tumor markers, such as carcinoembryonic antigen and carbohydrate antigen 19-9, were within normal limits.
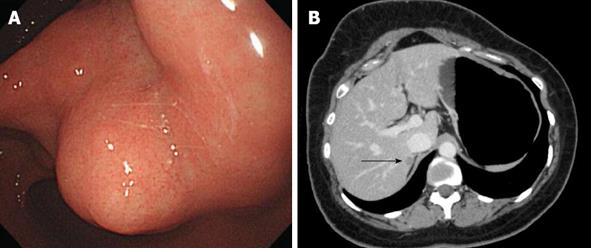
Esophagogastroduodenoscopy revealed a multifocal gastric tumor, suspicious for a GIST, in the whole stomach. The tumors were multiple small exophytic polypoid lesions in the whole stomach from fundus to antrum. The largest tumor was approximately 4 cm, was located in the antrum and was not ulcerated (Figure 1A). Abdominal computed tomography revealed pathologic lesions in the proximal antrum, enlarged perigastric lymph nodes, and a lesion in segment VII of the liver. This hepatic lesion was assumed to have metastasized from the gastric tumors (Figure 1B). Chest radiography revealed no evidence of lung metastasis. Positron emission tomography revealed hyper-dense areas in the antrum and perigastric lymph nodes in areas other than the liver.
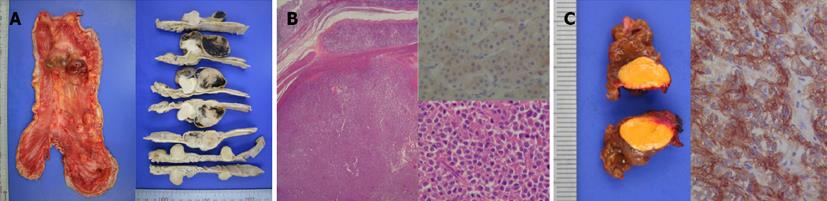
The patient underwent total gastrectomy, regional lymphadenectomy, and partial hepatectomy of segment VII. Based on macroscopic examination, the sporadic gastric stromal tumorlets were multifocal subserosal exophytic polypoid nodules (n = 8). The tumors ranged from 7 mm × 5 mm to 40 mm × 35 mm (Figure 2A). The largest tumor was located at the lesser curvature of the antrum. The gastric mucosa was grossly unremarkable, without ulceration or tumefaction. All eight tumors were diffusely immunoreactive with CD 117 (KIT) and CD34 antibodies, confirming the diagnosis of GISTs (Figure 2B). The average mitotic count was 6/50 high power fields suggesting an intermediate risk of malignancy (prognostic group 2C). The resection margins and lymph nodes were free of neoplasia. The hepatic tumor was 12 mm × 7 mm in diameter, with a bright orange yellowish color and a relatively well-defined timorous nodule. Microscopically, the tumor cells were arranged in small nests (so called “zellballen”, which are distinctive cell balls), set in a vascularly rich stroma. Immunohistochemical stains disclosed synaptophysin-positive tumor cell nests surrounded by S100 protein-positive sustentacular cells (Figure 2C). These findings were consistent with benign primary paraganglioma. A 24 h urine study assaying for metanephrine, epinephrine, and norepinephrine was performed 10 d after the operation and revealed no abnormalities.
The postoperative course was uneventful, and the patient was discharged from the hospital in good condition. No adjuvant chemotherapy was administered, and no evidence of recurrence was detected at the 1-year follow-up.
Multifocal hyperplasia of the interstitial cells of Cajal is a precursor of hereditary GIST in patients with germline mutations of c-KIT or alpha platelet-derived growth factor receptor (PDGFRA), but precursor lesions of sporadic GISTs have not yet been defined[4]. Carney et al[1] first described the association of gastric epithelioid leiomyosarcoma with pulmonary chondroma and functioning extra-adrenal paraganglioma of unknown origin, which today is known as CT. CT is defined by the coexistence of the following three tumors: extra-adrenal paraganglioma: only functioning extra-adrenal paragangliomas were initially included and non-functioning extra-adrenal paragangliomas were added later; gastric GISTs, previously known as gastric epithelioid leiomyosarcoma; and pulmonary chondroma (hamartoma)[2]. For the diagnosis of CT, at least two of the these major components are necessary. Recently, CSS was reported. This syndrome is a dyad of paraganglioma and gastric stromal sarcoma. CSS is inheritable in an autosomal dominant pattern, affects both males and females, and does not present pulmonary chondroma[5]. We considered our case as CT because the patient had no family history of related tumors.
CT predominately affects females (over 80% of cases) in their 2nd and 3rd decades and often presents with unpredictable outcomes[6]. The first tumor identified is usually a gastric GIST. The most common initial clinical manifestation is a GIST with bleeding. Associated symptoms and signs are anemia, hematemesis, and melena. Gastric GISTs in CT are usually multifocal and, antrumal based. These tumors are wild-type for common mutations in the receptor tyrosine kinase gene KIT and for the homologous oncogene PDGFRA in contrast to most sporadic GISTs in adults[7,8]. Gastric GISTs in CT frequently metastasize to regional lymph nodes, thus contrasting with common GISTs. The reason for this high rate of lymph node metastasis is not known[9]. Surgical resection is the only curative therapy for gastric GISTs with CT. Although partial resection is initially performed, further resection or total gastrectomy is required when multiple tumors reside in the entire stomach or recur after tumor resection.
In our case, the preoperative detection of perigastric lymph nodes suggested metastasis. However, a pathologic assessment did not reveal metastasis. According to clinical practice guidelines for GISTs in Japan, for the treatment of a GIST that has already metastasized to other organs but is considered to be resectable, surgery is the preferred treatment modality[10]. In this case, percutaneous biopsy of the liver lesion had a substantial risk of tumor cell spillage through the needle track. Moreover the tumor was located immediately adjacent to the inferior vene cava, so percutaneous biopsy was considered to be difficult and risky for this patient. Therefore, we performed a total gastrectomy and resection of the liver tumor simultaneously, without preoperative pathologic confirmation of the liver lesion.
Other CT neoplasms are usually found when the lesion is evaluated for gastric GISTs. These neoplasms are often misinterpreted as metastatic GISTs and are treated as such. Our patient was preoperatively diagnosed with a multifocal gastric GIST with hepatic metastasis. The incidence of hepatic metastasis from gastric GISTs in CT was reported to be 17.7%[2]. Postoperative histologic findings were consistent with primary hepatic paraganglioma and we confirmed the diagnosis of CT.
The most common combination is the association of GISTs with pulmonary chondroma (75%)[6]. Combinations of GIST and paraganglioma as observed in our case, account for 44% of CT cases[6].
Frequent sites of paraganglioma in CT are the aortopulmonary body, sympathetic chain, retroperitoneum, and carotid body. Only two cases of hepatic paraganglioma similar to our case were reported among 79 patients with CT in Carney’s series[2]. Paragangliomas are rare neuroendocrine tumors arising from neural-crest-derived chromaffin cells. Although paragangliomas may present anywhere along the sympathetic paraganglia chains from the neck to the pelvis, most reside intra-abdominally, in the superior para-aortic area[11].
Mortality from the triad depends on gastrointestinal hemorrhage, metastatic disease and hypertensive phenomena. Symptoms and signs of catecholamine excess were observed in 13 (35%) of 37 paraganglioma patients with CT[2]. In our case, we could not confirm whether the hepatic paraganglioma was functioning because the preoperative diagnosis of the liver lesion was metastasis and because initial blood and urine chemistry evaluation for catecholamine could not be performed. Considering the lack of symptoms or signs, the paraganglioma was assumed to be non-functioning. When CT is suspected in patients with multiple gastric GISTs, a radiologic and chemical work-up should be undertaken to rule out paraganglioma.
Although most CD117-expressing GISTs are aggressive, these tumors respond to imatinib. Tumors in younger patients with CT are less aggressive and less responsive to imatinib but still metastasize[12]. Most deaths of CT patients are due to malignant GISTs but several cases are due to paraganglioma (3/77, 3.8%)[13]. At the onset of the syndrome, all three types of tumors are detected in very few cases (1%). The mean interval between the detection of the first and second tumors has been reported to be 8.4 years[2]. Eventually, early surgery can reduce both short and long-term mortality from bleeding and metastasis, respectively.
In conclusion, CT is a very rare syndrome, but Carney and colleagues thoroughly documented its clinical manifestations. The presence of pulmonary chondroma and paraganglioma should be verified, especially in young women with multifocal GISTs, to rule out CT. A careful long-term follow-up is required to detect metachronous tumors.
P- Reviewers Ding XW, Hornick JL, Mussi C S- Editor Gou SX L- Editor A E- Editor Lu YJ
| 1. | Carney JA, Sheps SG, Go VL, Gordon H. The triad of gastric leiomyosarcoma, functioning extra-adrenal paraganglioma and pulmonary chondroma. N Engl J Med. 1977;296:1517-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 259] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | Carney JA. Gastric stromal sarcoma, pulmonary chondroma, and extra-adrenal paraganglioma (Carney Triad): natural history, adrenocortical component, and possible familial occurrence. Mayo Clin Proc. 1999;74:543-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Stratakis CA, Carney JA. The triad of paragangliomas, gastric stromal tumours and pulmonary chondromas (Carney triad), and the dyad of paragangliomas and gastric stromal sarcomas (Carney-Stratakis syndrome): molecular genetics and clinical implications. J Intern Med. 2009;266:43-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 198] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 4. | Chen H, Hirota S, Isozaki K, Sun H, Ohashi A, Kinoshita K, O’Brien P, Kapusta L, Dardick I, Obayashi T. Polyclonal nature of diffuse proliferation of interstitial cells of Cajal in patients with familial and multiple gastrointestinal stromal tumours. Gut. 2002;51:793-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Carney JA, Stratakis CA. Familial paraganglioma and gastric stromal sarcoma: a new syndrome distinct from the Carney triad. Am J Med Genet. 2002;108:132-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 210] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 6. | Zhang L, Smyrk TC, Young WF, Stratakis CA, Carney JA. Gastric stromal tumors in Carney triad are different clinically, pathologically, and behaviorally from sporadic gastric gastrointestinal stromal tumors: findings in 104 cases. Am J Surg Pathol. 2010;34:53-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 7. | Agaimy A, Pelz AF, Corless CL, Wünsch PH, Heinrich MC, Hofstaedter F, Dietmaier W, Blanke CD, Wieacker P, Roessner A. Epithelioid gastric stromal tumours of the antrum in young females with the Carney triad: a report of three new cases with mutational analysis and comparative genomic hybridization. Oncol Rep. 2007;18:9-15. [PubMed] |
| 8. | Matyakhina L, Bei TA, McWhinney SR, Pasini B, Cameron S, Gunawan B, Stergiopoulos SG, Boikos S, Muchow M, Dutra A. Genetics of carney triad: recurrent losses at chromosome 1 but lack of germline mutations in genes associated with paragangliomas and gastrointestinal stromal tumors. J Clin Endocrinol Metab. 2007;92:2938-2943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Agaimy A, Carney JA. Lymphatics and D2-40/podoplanin expression in gastrointestinal stromal tumours of the stomach with and without lymph node metastasis: an immunohistochemical study with special reference to the Carney triad. J Clin Pathol. 2010;63:229-234. [PubMed] |
| 10. | Nishida T, Hirota S, Yanagisawa A, Sugino Y, Minami M, Yamamura Y, Otani Y, Shimada Y, Takahashi F, Kubota T. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol. 2008;13:416-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 321] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 11. | Lack EE. Extra-adrenal paragangliomas of the sympathoadrenal neuroendocrine system. Washington, DC: Armed Forces Institute of Pathology 1997; . |
| 12. | Savage DG, Antman KH. Imatinib mesylate--a new oral targeted therapy. N Engl J Med. 2002;346:683-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 573] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 13. | Carney JA. Carney triad: a syndrome featuring paraganglionic, adrenocortical, and possibly other endocrine tumors. J Clin Endocrinol Metab. 2009;94:3656-3662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |