Published online Mar 27, 2013. doi: 10.4240/wjgs.v5.i3.30
Revised: October 3, 2012
Accepted: December 20, 2012
Published online: March 27, 2013
AIM: To assess the effects of preoperative treatment on the hepatic histology of non-tumoral liver and the postoperative outcome.
METHODS: One hundred and six patients underwent hepatic resection for colorectal metastases between 1999 and 2009. The surgical specimens were reviewed with established criteria for diagnosis and grading of pathological hepatic injury. The impact of preoperative therapy on liver injury and postoperative outcome was analyzed.
RESULTS: Fifty-three patients (50%) received surgery alone, whereas 42 patients (39.6%) received neoadjuvant chemotherapy and 11 (10.4%) patients received preoperative hepatic artery infusion (HAI). Chemotherapy included oxaliplatin-based regimens (31.1%) and irinotecan-based regimens (8.5%). On histopathological analysis, 16 patients (15.1%) had steatosis, 31 (29.2%) had sinusoidal dilation and 20 patients (18.9%) had steatohepatitis. Preoperative oxaliplatin was associated with sinusoidal dilation compared with surgery alone (42.4% vs 20.8%, P = 0.03); however, the perioperative complication rate was not significantly different between the oxaliplatin group and surgery group (27.3% vs 13.2%, P = 0.1). HAI was associated with more steatosis, sinusoidal dilation and steatohepatitis than the surgery group, with higher perioperative morbidity (36.4% vs 13.2%, P = 0.06) and mortality (9.1% vs 0% P = 0.02).
CONCLUSION: Preoperative oxaliplatin was associated with sinusoidal dilation compared with surgery alone. However, the preoperative oxaliplatin had no significant impact on perioperative outcomes. HAI can cause pathological changes and tends to increase perioperative morbidity and mortality.
- Citation: Lu QY, Zhao AL, Deng W, Li ZW, Shen L. Hepatic histopathology and postoperative outcome after preoperative chemotherapy for Chinese patients with colorectal liver metastases. World J Gastrointest Surg 2013; 5(3): 30-36
- URL: https://www.wjgnet.com/1948-9366/full/v5/i3/30.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v5.i3.30
Colorectal cancer (CRC) is one of the most common causes of cancer death in the Western world, ranking second in Europe and third in the United States[1]. The incidence of CRC in China is lower than that in the West, but has increased in recent years[2,3] and has become a substantial burden in China. Some studies have reported changes in the characteristics of colorectal cancers in China[4,5]. Approximately 50% of patients with colorectal cancer develop liver metastases at some point during the course of their disease[6,7]. Surgical resection remains the first choice of treatment, with a 25%-40% long-term survival rate[8,9]. However, only 15%-20% of patients with colorectal liver metastases are suitable for surgical resection[10]. Chemotherapy is the first choice of treatment for unresectable patients but it is very rare for patients treated with chemotherapy alone to survive longer than 5 years.
Neoadjuvant chemotherapy has been evaluated in patients with initially resectable liver metastases. The rationale for using preoperative chemotherapy in patients with initially resectable disease includes an opportunity to demonstrate regimen-specific efficacy, as well as allowing time to identify those patients who will progress and who therefore may not benefit from liver resection. In addition, preoperative chemotherapy may decrease the magnitude of resection needed[11].
Although the use of new chemotherapeutic agents has a number of theoretical benefits, concern about liver injury after surgery led investigators to examine the impact of chemotherapy[12-16]. In the current study, we analyze the histopathological changes associated with preoperative chemotherapy and report the postoperative outcome.
In addition, hepatic artery infusion (HAI) has been increasingly used in China as a palliative treatment of unresectable colorectal metastases (CRM) or the edge of the liver function in an effort to reduce lesion size and thus make surgery feasible when the remnant liver is insufficient in size, based on cross-sectional imaging volumetrics. Therefore, we also collected data to evaluate whether HAI before surgery can have an impact on hepatic histopathology.
A retrospective review was undertaken on patients who underwent hepatic surgery for CRM with a curative intent at Peking University Cancer Hospital between January 1999 and April 2009. Hepatic resections were defined according to the Brisbane terminology[17,18]. Patients were divided into the following four groups based on their preoperative therapy: (1) no preoperative therapy; (2) Oxaliplatin-based chemotherapy with fluorouracil (FU) or Xeloda; (3) Irinotecan-based chemotherapy plus FU; and (4) preoperative HAI. Only patients who received regional therapy with HAI were included in the HAI group.
Standard demographic data were collected on all patients, including type and duration of preoperative treatment, details of the resection, estimated blood loss (EBL), characteristics of the resected tumor, postoperative morbidity and 90 d mortality.
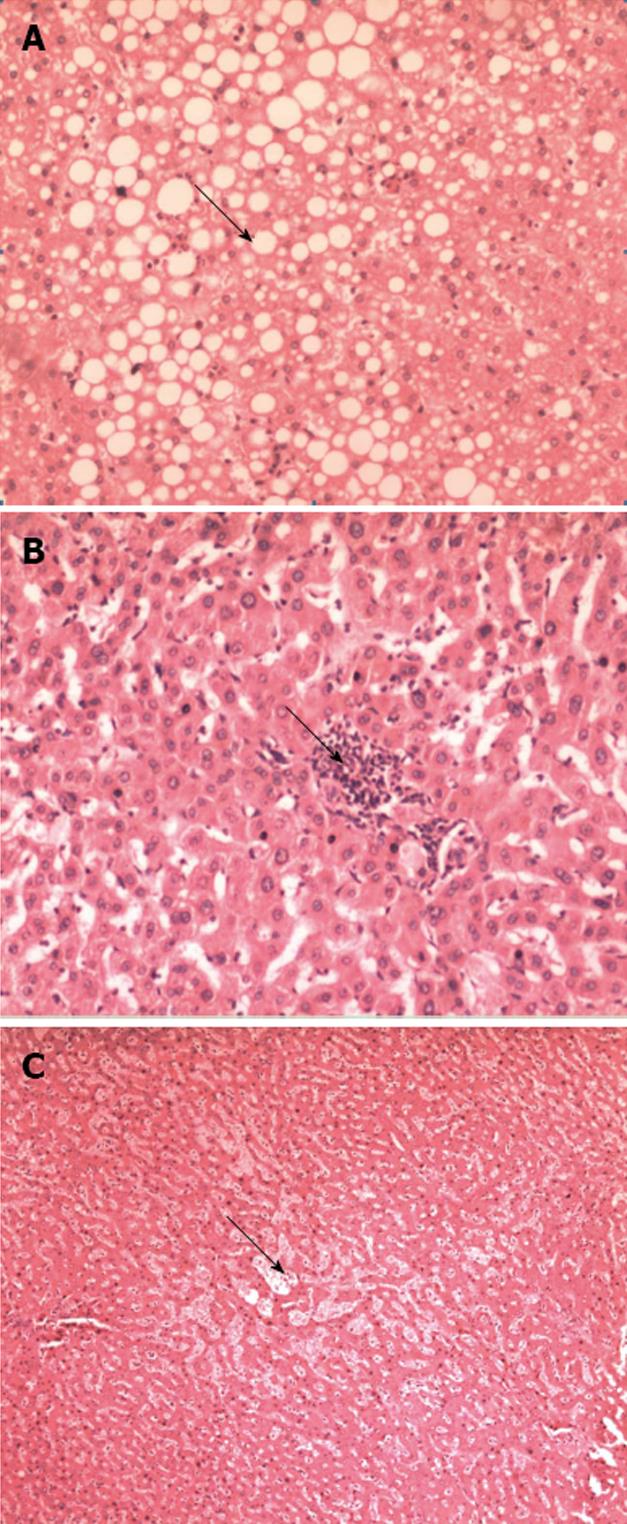
The archival slides (original formalin-fixed, paraffin-embedded and HE staining) from those resected hepatic specimens were blindly reviewed by a pathologist (Zhao AL). The histopathological findings in the non-tumoral liver tissue were evaluated semi-quantitatively as follows: (1) degree of steatosis was graded as none, mild (< 30%), moderate (≥ 30% to 50%) or severe (≥ 50%; Figure 1A); (2) steatohepatitis was graded as defined by Kleiner et al[19] based on steatosis (score 0: < 5%; 1: 5% to 33%; 2: > 33% to 66%; and 3: > 66%), lobular inflammation (score 0: no foci; 1: one foci; 2: two to four foci; and 3: > four foci per 200 × field) and ballooning (score 0: none; 1: few balloon cells; and 2: many cells/prominent ballooning, Figure 1B); and (3) sinusoidal injury was graded according to an established grading system of sinusoidal dilation (grade 0: absent; grade 1: centrilobular involvement limited to one-third of the lobular surface; grade 2: centrilobular involvement extending in two-thirds of the lobular surface; grade 3: complete lobular involvement)[12] (Figure 1C). Hepatic injury was defined as steatosis more than 30%, steatohepatitis Kleiner score ≥ 4 and/or grade 2-3 sinusoidal dilation.
Summary statistics were performed using the χ2 test and Fisher’s exact test for comparing categorical variables; the Kruskal-Wallis test was used to compare continuous variables among the treatment groups. The odds ratios (OR) and the 95%CI were estimated and P value < 0.05 was considered to be statistically significant. All statistical analyses were performed using SAS software, version 9.0.
Table 1 presents the clinicopathological features of the 106 patients in the study. A total of 106 patients were included in the analysis. There were 50 (47.2%) men and 56 (52.8%) women and the median patient age was 60 years (range 32-79 years). The presentation of hepatic metastases was metachronous in most patients (n = 62; 58.5%), while synchronous metastases accounted for 41.5%. The median number of hepatic metastases was 3 (range 1-5) and the median size of the largest lesion was 4 cm (range 0.7-22 cm).
| Variable | Patients |
| Sex | |
| Female | 56 (52.8) |
| Male | 50 (47.2) |
| Site of primary tumor | |
| Colon | 55 (51.9) |
| Rectum | 51 (48.1) |
| Hepatic metastases | |
| Median | 3 |
| Solitary | 61 (57.5) |
| Multiple | 45 (42.5) |
| Metastases type | |
| Synchronous | 44 (41.5) |
| Metachronous | 62 (58.5) |
| Extent of hepatic resection | |
| Minor (1-2 segment) | 81 (76.4) |
| Major (≥ 3 segment or hemihepatectomy) | 25 (23.6) |
At the time of the operation, the extent of hepatic resection was less than 3 segments or hemihepatectomy in 81 patients (76.4%) and a hemihepatectomy or more than 3 segments removal in 25 patients (23.6%). The median EBL was 200 mL (range 50-3000 mL). The median EBL in the preoperative chemotherapy arm was 575 mL, which was obviously higher than those without preoperative treatment (200 mL).
A total of 42 patients received neoadjuvant chemotherapy therapy, consisting of oxaliplatin plus FU regimen (33, 31.1%) and irinotecan plus FU regimen (9, 8.5%). While 11 (10.4%) patients received preoperative HAI before surgical treatment of the hepatic metastases, in which 8 patients received Cisplatin plus Epirubicin, three patients received oxaliplatin plus FU/CF. Of the 42 patients who received preoperative chemotherapy, the median duration was 5 cycles with 2-3 wk per cycle (range 2-10 cycles). The median duration of the HAI group was 3 cycles with 1 mo per cycle (range 1-3 mo). In general, the tumor characteristics and surgery details were similar among all preoperative treatment groups (Table 2). There was also no significant difference between groups with regard to age, gender, site of primary tumor, number of hepatic CRM, EBL or hepatic CRM tumor size (all P > 0.05). Patients who received HAI before surgery tended to have less EBL than other groups.
| Variable | Patients (n = 106) | Chemotherapy (n = 53) | Oxaliplatin (n = 33) | Irinotecan (n = 9) | HAI (n = 11) | P value |
| Mean age, yr | 60 | 59.8 | 56.9 | 56.9 | 54.2 | 0.26 |
| Gender | ||||||
| Female | 56 (52.8) | 29 (54.7) | 16 (48.5) | 5 (55.6) | 6 (54.5) | 0.95 |
| Male | 50 (47.2) | 24 (45.3) | 17 (51.5) | 4 (44.4) | 5 (45.5) | |
| Site of primary tumor | ||||||
| Colon | 55 (51.9) | 28 (52.8) | 16 (48.5) | 5 (55.6) | 6 (54.5) | 0.97 |
| Rectum | 51 (48.1) | 25 (47.2) | 17 (51.5) | 4 (44.1) | 5 (45.5) | |
| Timing of hepatic metastases | ||||||
| Synchronous | 44( 41.5) | 19 (35.8) | 20 (60.6) | 2 (22.2) | 3 (27.3) | 0.05 |
| Metachronous | 62 (58.5) | 34 (64.2) | 13 (39.4) | 7 (77.8) | 8 (72.7) | |
| Surgery type | ||||||
| Minor (1-2 segment) | 81 (76.4) | 42 (79.2) | 24(72.7) | 9 (100) | 6 (54.5) | 0.10 |
| Major (≥ 3 segment or hemihepatectomy) | 25 (23.6) | 11 (20.8) | 9 (27.3) | 0 (0) | 5 (45.5) | |
| No. of hepatic CRM | ||||||
| Single | 61 (57.5) | 34 (64.2) | 17 (51.5) | 6 (66.7) | 4 (36.4) | 0.29 |
| Multiple | 45 (42.5) | 19 (35.8) | 16 (48.5) | 3 (33.3) | 7 (63.6) | |
| Largest hepatic CRM tumor size, cm | 10.4 | 4.87 | 4.55 | 4.73 | 4.05 | 0.87 |
| Median estimated blood loss, mL | 200 | 400 | 350 | 600 | 300 | 0.90 |
| Duration of chemotherapy, wk (median) | 4 | 0 | 4.8 | 4.1 | 0 | < 0.0001 |
| Postoperative complication | ||||||
| Yes | 20 (18.9) | 7 (13.2) | 9 (27.3) | 0 (0) | 4 (36.4) | 0.07 |
| No | 86 (81.1) | 46 (86.8) | 24 (72.7) | 9 (100) | 7 (63.6) |
The overall perioperative complication rate was 18.9%. Thirteen patients (12.3%) suffered from hepatic complications, including liver failure (n = 3), hepatic insufficiency (n = 2), bile leaks (n = 9) and hepatic abscess (n = 1). Non-hepatic complications occurred in 11 patients (10.3%); there were 6 pulmonary complications (5.7%; pleural effusion, n = 6), 1 cardiovascular complications (0.94%; rapid atrial fibrillation, n = 1), 1 stress ulcer (0.94%) and 1 pancreatic fistula (0.94%), 2 peritoneal effusion (1.8%) and 1 with abdominal infectious complications (0.94%). Overall, the perioperative complication rate was similar between the no-chemotherapy group (13.2%) and the chemotherapy group (21.4%) (P = 0.29). In addition, patients who received HAI tended to have more postoperative morbidity (36.4% vs 13.2%, P = 0.06) and mortality (9.1% vs 0% P = 0.02) than those who received no preoperative chemotherapy. The complication rate did not differ with a different type of preoperative therapy (HAI 36.4%; irinotecan 0%; oxaliplatin 27.3%) (P = 0.07).
During the final pathological analysis of the resected specimen, hepatic injury was shown in 51 patients (48.1%). Steatosis more than 30% was identified in 16 patients (15.1%), grade 2 to 3 sinusoidal dilation in 31 patients (29.2%) and steatohepatitis Kleiner score ≥ 4 in 20 patients (18.9%). Preoperative chemotherapy is associated with pathological liver injury compared with non treatment before surgery (57.1% vs 35.8%, P = 0.038; OR: 2.39; 95%CI: 1.0-5.4). When patients were stratified according to the duration of chemotherapy (1 to 5, 6 to 10 cycles), the rate of hepatic injury increased over time in patients who received preoperative chemotherapy (76.2% vs 38.1%, P = 0.01). In Table 3, specifics on hepatic injury stratified by preoperative therapy are listed. Neither oxaliplatin nor irinotecan as neo-adjuvant chemotherapy before liver resection was associated with an increased rate of steatosis. The type of chemotherapy regimen used was associated with distinct patterns of liver injury: oxaliplatin was associated with grade 2 to 3 sinusoidal dilation compared with no chemotherapy (42.4% vs 20.8%, respectively, P = 0.03; OR = 2.8; 95%CI: 0.97-8.2). Patients receiving irinotecan also tended to have a higher likelihood of steatohepatitis compared with non treatment before surgery (33.3% vs 11.3%, P = 0.08), although the P value was not statistically significant. Specifically, HAI was also associated with more steatosis, sinusoidal dilation and steatohepatitis than no preoperative treatment. HAI was associated with steatosis and steatohepatitis compared with non treatment before surgery (36.4% vs 9.4%, P = 0.02; 36.4% vs 11.3%, P = 0.03, respectively) and patients receiving HAI tended to have a higher likelihood of sinusoidal dilation compared with no chemotherapy (45.5% vs 20.8%, P = 0.08), although the P value was not statistically significant.
| Regimen | Liver toxicity (n = 51) | Steatosis > 30% (n = 16) | Sinusoidal dilation (n =31) | Steatohepatitis (n = 20) | ||||||||
| Yes | No | 1P value | Yes | No | 1P value | Yes | No | 1P value | Yes | No | 1P value | |
| No CTx | 19 (35.8) | 34 (64.2) | 5 (9.4) | 48 (90.6) | 11 (20.8) | 42 (79.2) | 6 (11.3) | 47 (88.7) | ||||
| Oxaliplatin | 19 (57.6) | 14 (42.4) | 0.04 | 5 (15.2) | 28 (84.8) | NS | 14 (42.4) | 19 (57.6) | 0.03 | 7 (21.2) | 26 (78.8) | NS |
| Irinotecan | 5 (55.6) | 4 (44.4) | NS | 2 (22.2) | 7 (77.8) | NS | 1 (11.1) | 8 (88.9) | NS | 3 (33.3) | 6 (66.7) | 0.08 |
| HAI | 8 (72.7) | 3 (27.3) | 0.02 | 4 (36.4) | 7 (63.6) | 0.02 | 5 (45.5) | 6 (54.5) | 0.08 | 4 (36.4) | 7 (63.6) | 0.03 |
There were three patients who died within 90 d of surgery, with a perioperative mortality rate of 2.8%. Of those three deaths, one was due to renal failure, one was associated with an abdominal infection and a bile leak and another from acute respiratory distress syndrome (ARDS). There were two deaths among the preoperative chemotherapy (1.8%), all from oxaliplatin preoperative treatment, while another death occurred in the HAI arm (0.9%). There is no association between preoperative chemotherapy and the risk of perioperative mortality (P = 0.1). Patients with oxaliplatin (n = 33) tended to have a higher risk of death (6.1%) vs no preoperative treatment (0%), although the P value was not statistically significant (P = 0.07). There were 2 deaths (3.9%) in 51 patients with hepatic injury (one death was associated with an abdominal infection and a bile leak, another from ARDS) compared with one death (1.8%) in 55 patients without hepatic injury (one from renal failure).
In our study, there were seven patients with concomitant hepatitis before surgery, six with hepatitis B virus infection and one with hepatitis C virus infection. Two of these received neoadjuvant chemotherapy. However no further liver injury or complication was observed in those two patients.
Currently, chemotherapy has been commonly used as a part of an integrated multimodality approach to CRM and sometimes as the first treatment choice. Recently, an increasing number of reports have shown that the administration of preoperative chemotherapy can be associated with pathological changes in liver parenchyma[12-16]. However, the question remains whether these hepatic injuries have any clinical significance.
In the current study, we performed a retrospective analysis on the result of the use of preoperative treatment, including chemotherapy and HAI, for any impact on pathological liver injury and on clinical outcome, including postoperative complication and mortality.
Our study results show that preoperative treatment with oxaliplatin was significantly associated with a greater likelihood of sinusoidal dilation compared with no chemotherapy (42.4% vs 20.8%, P = 0.03), which is consistent with other recently published studies[15,20-22].
Interestingly, we observed that the incidence of sinusoidal dilation with oxaliplatin was 42.4%, relatively higher than Vauthey et al[16] (18.9%) and Pawlik et al[22] reported (9.6%). The reason for the different prevalence of sinusoidal dilation is probably multifactorial. Although progress has been made in this area, cohesive guidelines have yet to be proposed and consensus is lacking on a uniform set of pathological terminology to define chemotherapy-associated liver injury. The subjective variability between expert pathologists can lead to a different incidence rate of pathological changes in liver parenchyma. That is why we decided to have only one pathologist with hepatobiliary expertise assess the degree of liver injury and follow Vauthey’s[16] strict definition.
Until now, only a few studies have been able to connect a given chemotherapeutic agent with a specific histopathological injury and a meaningful adverse outcome[16]. In our study, preoperative oxaliplatin was not significantly associated with an increase risk of postoperative complication (27.3% vs 13.2%, P = 0.1). Similar results were observed in other studies[22-24], indicating that preoperative oxaliplatin had no impact on postoperative morbidity or mortality.
Among previous reports, only Vauthey et al linked irinotecan-based chemotherapy with steatohepatitis and increased 90 d postoperative mortality[12-16]; 34 (8.4%) patients had steatohepatitis as defined by the nonalcoholic steatohepatitis score. Irinotecan was associated with steatohepatitis (20.2% incidence in the irinotecan group vs 4.4% in the non-chemotherapy group, P = 0.0001) and patients with steatohepatitis had an increased 90 d mortality rate compared with patients who did have steatohepatitis. In our study, steatohepatitis (Kleiner score ≥ 4) was observed in 20 patients (18.8%), a higher rate than that Vauthey reported (20.2%). However, no postoperative complication or mortality was observed in patients with irinotecan treatment. We need to closely monitor the patient’s status when we use irinotecan before surgery due to a relatively high steatohepatitis incidence rate, although data is not sufficient at present.
HAI has been used extensively in the palliative treatment of unresectable hepatocellular carcinoma. It was observed in several studies that it could improve quality of life, symptomatic control and survival time as a local therapy for CRM[25-28]. HAI is increasingly used in China as a palliative treatment of unresectable CRM as it may increase the possibility of surgery and can be used when surgery is not possible or not successful. However, less attention has been paid to the hepatic histological injures and perioperative complications after HAI, since it is commonly excluded from preoperative studies which observe the impact on hepatic histology and its outcomes for CRM. Until now, limited studies have explored whether HAI can affect the remaining liver for CRM and determine whether it can be used before surgery to improve postoperative recovery. Pulitanò et al[29] reported that postoperative morbidity rate were comparable between the HAI group and surgery alone group (14% vs 14%). He concluded that HAI of fluorodeoxyuridine does not negatively affect the outcome of subsequent liver resection. However, his article did not evaluate the hepatic pathological changes. In our study, we observed that HAI was associated with a higher risk of steatosis, sinusoidal dilation and steatohepatitis compared with non treatment before surgery. In addition, patients who received HAI tended to have more postoperative morbidity and mortality; those data alerted us to be more careful about its adverse impact on hepatic histology, despite a limited small sample size.
Discussion about the optimum interval between chemotherapy and hepatectomy has been based on the assumption that hepatic side effects of chemotherapy are time-related and reversible. Kopetz et al[30] reviewed the data and stated that a limited course of chemotherapy, with an interval of at least 5 wk, might minimize the incidence of surgical complications. Although the optimal timing of hepatic resection after completion of chemotherapy varies among institutions, a consensus is evolving for a minimum interval of 4 wk to allow the liver to recover, in the hope of reducing morbidity and mortality. In our study, almost all recruited patients received hepatic resection after completion of chemotherapy with an interval of 4-6 wk. Based on the clinical practice in our cancer center, the preoperative complication rate is observed at 13.2%, comparable with other reported papers[29].
Given this, the use of preoperative chemotherapy and HAI may need to be more carefully monitored and the choice of regimen and duration of treatment tailored to the particular individual’s situation. Future investigations will be needed to clarify the pathogenesis and molecular pathways underlying the cause of chemotherapy-associated liver injury and its relationship to other known pathways. In addition, only through a thorough understanding of the patient’s status and the patient’s liver condition prior to administration of systemic chemotherapy can potentially confounding variables be accounted for and the true impact of systemic chemotherapy on the liver be determined[22].
Preoperative oxaliplatin was associated with sinusoidal dilation compared with surgery alone. However, the preoperative oxaliplatin had no significant impact on perioperative outcomes. HAI can cause pathological changes and tends to increase perioperative morbidity and mortality.
Colorectal cancer (CRC) is one of the most common causes of cancer death in the Western world, ranking second in Europe and third in the United States. The incidence of CRC in China is lower than that in the West, but has increased in recent years and become a substantial burden in China. Some studies have reported changes in the characteristics of colorectal cancers in China.
Preoperative chemotherapy before resection of hepatic colorectal metastases may cause hepatic injury and affect the postoperative outcome. The objective of this study was to assess the effects of preoperative treatment on the hepatic histology of non-tumoral liver and the postoperative outcome.
Preoperative oxaliplatin was associated with sinusoidal dilation compared with surgery alone. However, preoperative oxaliplatin had no significant impact on perioperative outcomes. Hepatic artery infusion can cause pathological changes and tends to increase perioperative morbidity and mortality.
The data presented in this paper is very interesting, especially the references about the impact of the duration of chemotherapy and the effect of hepatic artery infusion on the liver parenchyma. It is worthy of being published.
P- Reviewers Morise Z, Tiberio GA S- Editor Wen LL L- Editor Roemmele A E- Editor Xiong L
| 1. | Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4489] [Cited by in RCA: 4286] [Article Influence: 214.3] [Reference Citation Analysis (0)] |
| 2. | Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4370] [Cited by in RCA: 4261] [Article Influence: 224.3] [Reference Citation Analysis (0)] |
| 3. | Cao KJ, Ma GS, Liu YL, Wan DS. [Incidence of colorectal cancer in Guangzhou City from 2000 to 2002]. Ai Zheng. 2009;28:441-444. [PubMed] |
| 4. | Zhang S, Cui Y, Weng Z, Gong X, Chen M, Zhong B. Changes on the disease pattern of primary colorectal cancers in Southern China: a retrospective study of 20 years. Int J Colorectal Dis. 2009;24:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Jiang SX, Wang XS, Geng CH, Wang GY. Altering trend of clinical characteristics of colorectal cancer: a report of 3,607 cases. Ai Zheng. 2009;28:54-56. [PubMed] |
| 6. | Stangl R, Altendorf-Hofmann A, Charnley RM, Scheele J. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343:1405-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 482] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 7. | Leonard GD, Brenner B, Kemeny NE. Neoadjuvant chemotherapy before liver resection for patients with unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2005;23:2038-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 236] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 8. | Nordlinger B, Jaeck D, Guiguet M, Vaillant JC, Balladur P, Schaal JC. Surgical reaction of hepatic metastases. Multicentre retrospective study by the French Association of Surgery. Treatment of hepatic metastases of colorectal cancer. Paris: Springer-Verlag 1992; 129-156. [DOI] [Full Text] |
| 9. | Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309-318; discussion 318-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2799] [Article Influence: 107.7] [Reference Citation Analysis (1)] |
| 10. | Scheele J. Hepatectomy for liver metastases. Br J Surg. 1993;80:274-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Adam R, Avisar E, Ariche A, Giachetti S, Azoulay D, Castaing D, Kunstlinger F, Levi F, Bismuth F. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal. Ann Surg Oncol. 2001;8:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 475] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 12. | Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, Dousset B, Morel P, Soubrane O, Chaussade S. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460-466. [PubMed] |
| 13. | Kooby DA, Fong Y, Suriawinata A, Gonen M, Allen PJ, Klimstra DS, DeMatteo RP, D’Angelica M, Blumgart LH, Jarnagin WR. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg. 2003;7:1034-1044. [PubMed] |
| 14. | Fernandez FG, Ritter J, Goodwin JW, Linehan DC, Hawkins WG, Strasberg SM. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg. 2005;200:845-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 355] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 15. | Karoui M, Penna C, Amin-Hashem M, Mitry E, Benoist S, Franc B, Rougier P, Nordlinger B. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 519] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 16. | Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, Xiong HQ, Eng C, Lauwers GY, Mino-Kenudson M. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 953] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 17. | Zorzi D, Mullen JT, Abdalla EK, Pawlik TM, Andres A, Muratore A, Curley SA, Mentha G, Capussotti L, Vauthey JN. Comparison between hepatic wedge resection and anatomic resection for colorectal liver metastases. J Gastrointest Surg. 2006;10:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Pang YY. The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000; 2: 333-39. HPB (Oxford). 2002;4:99; author reply 99-100. [PubMed] |
| 19. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [PubMed] |
| 20. | Nakano H, Oussoultzoglou E, Rosso E, Casnedi S, Chenard-Neu MP, Dufour P, Bachellier P, Jaeck D. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg. 2008;247:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 351] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 21. | Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007-1016. [PubMed] |
| 22. | Pawlik TM, Olino K, Gleisner AL, Torbenson M, Schulick R, Choti MA. Preoperative chemotherapy for colorectal liver metastases: impact on hepatic histology and postoperative outcome. J Gastrointest Surg. 2007;11:860-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 179] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 23. | Sahajpal A, Vollmer CM, Dixon E, Chan EK, Wei A, Cattral MS, Taylor BR, Grant DR, Greig PD, Gallinger S. Chemotherapy for colorectal cancer prior to liver resection for colorectal cancer hepatic metastases does not adversely affect peri-operative outcomes. J Surg Oncol. 2007;95:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Ryan P, Nanji S, Pollett A, Moore M, Moulton CA, Gallinger S, Guindi M. Chemotherapy-induced liver injury in metastatic colorectal cancer: semiquantitative histologic analysis of 334 resected liver specimens shows that vascular injury but not steatohepatitis is associated with preoperative chemotherapy. Am J Surg Pathol. 2010;34:784-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Wasser K, Giebel F, Fischbach R, Tesch H, Landwehr P. [Transarterial chemoembolization of liver metastases of colorectal carcinoma using degradable starch microspheres (Spherex): personal investigations and review of the literature]. Radiologe. 2005;45:633-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Popov I, Lavrnić S, Jelić S, Jezdić S, Jasović A. Chemoembolization for liver metastases from colorectal carcinoma: risk or a benefit. Neoplasma. 2002;49:43-48. [PubMed] |
| 27. | Müller H, Nakchbandi V, Chatzisavvidis I, von Voigt C. Repetitive chemoembolization with melphalan plus intra-arterial immuno-chemotherapy within 5-fluorouracil and granulocyte-macrophage colony-stimulating factor (GM-CSF) as effective first- and second-line treatment of disseminated colorectal liver metastases. Hepatogastroenterology. 2003;50:1919-1926. [PubMed] |
| 28. | Barber FD, Mavligit G, Kurzrock R. Hepatic arterial infusion chemotherapy for metastatic colorectal cancer: a concise overview. Cancer Treat Rev. 2004;30:425-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Pulitanò C, Arru M, Catena M, Guzzetti E, Vitali G, Ronzoni M, Venturini M, Villa E, Ferla G, Aldrighetti L. Results of preoperative hepatic arterial infusion chemotherapy in patients undergoing liver resection for colorectal liver metastases. Ann Surg Oncol. 2008;15:1661-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Kopetz S, Vauthey JN. Perioperative chemotherapy for resectable hepatic metastases. Lancet. 2008;371:963-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |