Published online Nov 27, 2013. doi: 10.4240/wjgs.v5.i11.287
Revised: October 1, 2013
Accepted: October 17, 2013
Published online: November 27, 2013
Processing time: 147 Days and 7.7 Hours
AIM: To investigate the clinical relevance and prognosis regarding survival according to the changes of the tumor-node-metastasis (TNM) in gastric cancer patients.
METHODS: We retrospectively studied 347 consecutive subjects who underwent surgery for gastric adenocarcinoma at the Division of General Surgery, Hospital of Busto Arsizio, Busto Arsizio, Italy between June 1998 and December 2009. Patients who underwent surgery without curative intent, patients with tumors of the gastric stump and patients with tumors involving the esophagus were excluded for survival analysis. Patients were staged according to the 6th and 7th edition TNM criteria; 5-year overall survival rates were investigated, and the event was defined as death from any cause.
RESULTS: After exclusion, our study population included 241 resected patients with curative intent for gastric adenocarcinoma. The 5-year overall survival (5-year OS) rate of all the patients was 52.8%. The diagnosed stage differed in 32% of 241 patients based on the TNM edition used for the diagnosis. The patients in stage II according to the 6th edition who were reclassified as stage III had significantly worse prognosis than patients classified as stage II (5-year OS, 39% vs 71%). According to the 6th edition, 135 patients were classifed as T2, and 75% of these patients migrated to T3 and exhibited a significantly worse prognosis than those who remained T2, regardless of lymph node involvement (37% vs 71%). The new N1 patients exhibited a better prognosis than the previous N1 patients (67% vs 43%).
CONCLUSION: 7th TNM allows new T2 and N1 patients to be selected with better prognosis, which leads to different staging. New stratification is important in multimodal therapy.
Core tip: The 7th edition of the tumor-node-metastasis (TNM) staging system appears to exhibit improved accuracy in staging and prognostic stratification with more precise indication for adjuvant and neoadjuvant therapy in the multimodal treatment era. Our data show the importance of standardization of treatment and the type of surgical lymphadenectomy for comparing different experiences. Further studies are necessary to improve the TNM system, particularly regarding the parameter N and the division into substages.
- Citation: Zurleni T, Gjoni E, Ballabio A, Casieri R, Ceriani P, Marzoli L, Zurleni F. Sixth and seventh tumor-node-metastasis staging system compared in gastric cancer patients. World Journal of Gastrointestinal Surgery 2013; 5(11): 287-293
- URL: https://www.wjgnet.com/1948-9366/full/v5/i11/287.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v5.i11.287
In addition to age, comorbidities, lesion site, macro- and microscopic type of tumor, quality of surgery and residual tumors, the main factors that influence the long-term survival of patients with gastric cancer are (1) the depth of tumor penetration into the gastric wall (T parameter); (2) the amount of the metastatic regional lymph nodes involved (N parameter); and (3) the presence of distant metastases (M parameter).
The tumor-node-metastasis (TNM) classification of cancer was developed between 1943 and 1952 by Prof. Pierre Denoix at the Institute Gustave-Roussy. In 1987, the Union for International Cancer Control (UICC) and the American Joint Committee on Cancer (AJCC) TNM classifications were unified. The following are the main objectives of the classifications: to aid the clinician in the planning of treatment, to provide an indication of prognosis, to assist in the evaluation of the results of treatment; to facilitate the exchange of information between treatment centers, to contribute to the continuing investigation of human cancer and to support cancer control activities[1,2]. Since January 1st 2010, the UICC/AJCC TNM 7th edition differs from the previous version regarding some aspects of the T parameter and is completely renewed regarding the N parameter (Table 1).
| TNM staging system 6th edition | TNM staging system 7th edition | ||||||
| Stage | T | N | M | Stage | T | N | M |
| 0 | Tis | N0 | M0 | 0 | Tis | N0 | M0 |
| IA | T1 | N0 | M0 | IA | T1 | N0 | M0 |
| IB | T1 | N1 | M0 | IB | T2 | N0 | M0 |
| T2a | N0 | M0 | T1 | N1 | M0 | ||
| T2b | N0 | M0 | IIA | T3 | N0 | M0 | |
| II | T1 | N2 | M0 | T2 | N1 | M0 | |
| T2a | N1 | M0 | T1 | N2 | M0 | ||
| T2b | N1 | M0 | IIB | T4a | N0 | M0 | |
| T3 | N0 | M0 | T3 | N1 | M0 | ||
| IIIA | T2a | N2 | M0 | T2 | N2 | M0 | |
| T2b | N2 | M0 | T1 | N3 | M0 | ||
| T3 | N1 | M0 | IIIA | T4a | N1 | M0 | |
| T4 | N0 | M0 | T3 | N2 | M0 | ||
| IIIB | T3 | N2 | M0 | T2 | N3 | M0 | |
| IV | T4 | N1 | M0 | IIIB | T4b | N0, N1 | M0 |
| T4 | N2 | M0 | T4a | N2 | M0 | ||
| T4 | N3 | M0 | T3 | N3 | M0 | ||
| T1 | N3 | M0 | IIIC | T4a | N3 | M0 | |
| T2 | N3 | M0 | T4b | N2, N3 | M0 | ||
| T3 | N3 | M0 | IV | AnyT | AnyN | M1 | |
| Any T | Any N | M1 | |||||
Particularly, the subserosa infiltration by the tumor, which was previously classified as T2b, is now classified as T3, and the perforation of serosa changed from T3 to T4a. Regarding the parameter N, the UICC/AJCC TNM 7th edition changes the lymph nodes “cut-off”. Tumors classified as N1 in the 6th edition with more than 2 positive nodes are classified as N2 in the 7th edition, while N2 is classified as N3a, and N3 is classified as N3b. In the new stratification by stage, the number of substages is increased. According to the 7th edition, only patients with distant metastases are classified as the fourth stage. Another important change to the criteria concerns distant metastases. In the new edition of the TNM staging system, a positive peritoneal cytology is considered as M1.
Several studies, which were mostly performed in eastern countries, have demonstrated the superiority of the 7th edition TNM criteria and highlighted issues still in dispute for improvement.
The aim of the present study is to compare the sixth and the seventh edition of the TNM classification in patients who underwent surgery for gastric cancer in a single center to confirm the superiority of the new edition regarding its prognostic stratification and reliability. We considered the parameters T, N and the lymph node ratio (LNR) individually regardless of stage as additional prognostic parameters. We observed and followed how these changes in the allocation of pT and pN parameters according to the two editions of the classification affect determining the prognosis and the type of treatment for these patients.
We retrospectively studied 347 consecutive patients who underwent surgery for gastric adenocarcinoma at the Division of General Surgery, Hospital of Busto Arsizio (Varese), Italy from June 1998 through December 2009. For the survival analysis, we excluded the following patients: (1) patients with distant metastases; (2) patients who underwent surgery without curative intent; (3) patients with tumors of the gastric stump after gastric resection for benign disease; (4) patients with other tumors at the time of diagnosis; and (5) patients with a large involvement of the esophagus requiring total esophagectomy.
None of the patients considered for inclusion in the study underwent neoadjuvant chemotherapy or radiochemotherapy. Because of the heterogeneous and unsystematic indication for adjuvant chemotherapy, treatment protocols and number of cycles, details of the postoperative chemotherapy were not considered in this study.
Regarding the surgical method, en bloc resection of the primary tumor and lymphatic drainage area was routinely performed. D2 lymphadenectomy was performed in 87% of patients, while the remaining 13% underwent a D1 lymphadenectomy according to the Japanese Guidelines[3,4]. The principles of tumor resection and lymphadenectomy by experienced surgeons were similar among all the resected patients. No local excision was performed.
For all patients, a regular 6th month follow-up within 5 years from surgery consisted of the following procedures: serum tumor biomarker and laboratory biochemical examinations, radiological and UltraSound imaging, endoscopic control (1/year) and physical examination. Annual follow-ups after 5 years were performed until the patients died. In this study, a period of 120 mo was considered as the end of the patient’s observation. The median follow-up was 48 mo (range: 0-120 mo).
The depth of primary tumor invasion (T) and lymph node involvement (N) were classified according to the 6th and 7th UICC/AJCC edition TNM classification. All patients were restaged using the 6th and 7th editions of the UICC/AJCC TNM staging system. Survival curves were estimated using the Kaplan-Meier method[5]. The overall survival (OS) rates were investigated, and the event was defined as death for any cause. The Log rank test was used to identify the differences between the survival estimates of the different patient groups. Hazard ratio (HR) and 95%CI were also generated. A P value of less than 0.05 was considered significant. All tests were two-tailed. Statistical analysis and graphics were performed with MedCalc Software bvba, Mariakerke, Belgium.
From June 1998 until December 2009, a total of 347 patients in our department underwent surgery for gastric adenocarcinoma. After exclusion, the study population consisted of 241 resected patients, and 112 patients are currently alive.
The clinical and pathological characteristics are shown in Table 2. The median age was 71 years (range: 37-94 years), and 51.9% of the patients (n = 125) were male.
| Variable | n(%) | 5-year overall survival rate (%) | P value |
| All | 241 | 52.80 | |
| Sex | 0.740 | ||
| Female | 116 (48.1) | 50.40 | |
| Male | 125 (51.9) | 54.30 | |
| Age (yr) | 0.000 | ||
| 1 ( ≤50) | 14 (5.8) | 78.60 | |
| 2 (51-60) | 18 (7.5) | 32.00 | |
| 3 (61-70) | 78 (32.3) | 57.50 | |
| 4 (71-80) | 87 (36.1) | 57.30 | |
| 5 (> 80) | 44 (18.3) | 35.20 | |
| Site | 0.006 | ||
| S | 40 (16.6) | 33.30 | |
| M | 50 (20.7) | 70.80 | |
| I | 150 (62.2) | 51.50 | |
| Surgery | 0.400 | ||
| Total gastrectomy | 191 (79.3) | 53.10 | |
| Subtotal gastrectomy | 50 (20.7) | 52.60 | |
| Lauren | 0.500 | ||
| Intestinal | 150 (62.2) | 56.50 | |
| Diffuse | 58 (24.0) | 48.50 | |
| Mixed | 15 (6.2) | 33.90 | |
| T stage (6th edition) | < 0.0001 | ||
| T1 | 64 (26.6) | 86.20 | |
| T2 | 135 (56.0) | 45.40 | |
| T3 | 37 (15.3) | 23.30 | |
| T4 | 5 (2.1) | 0.00 | |
| T stage (7th edition) | < 0.0001 | ||
| T1 | 64 (26.6) | 86.20 | |
| T2 | 33 (13.7) | 71.00 | |
| T3 | 102 (42.3) | 37.30 | |
| T4 | 42 (17.4) | 20.50 | |
| N stage (6th edition) | < 0.0001 | ||
| N0 | 81 (33.6) | 77.30 | |
| N1 | 73 (30.3) | 55.70 | |
| N2 | 50 (20.7) | 27.60 | |
| N3 | 37 (15.4) | 22.90 | |
| N stage (7th edition) | < 0.0001 | ||
| N0 | 81 (33.6) | 77.30 | |
| N1 | 39 (16.2) | 67.50 | |
| N2 | 35 (14.5) | 43.00 | |
| N3 | 86 (35.7) | 25.90 | |
| Stage (6th edition) | < 0.0001 | ||
| I | 87 (36.1) | 76.40 | |
| II | 59 (24.5) | 61.50 | |
| III | 55 (22.8) | 24.40 | |
| IV | 40 (16.6) | 21.20 | |
| Stage (7th edition) | < 0.0001 | ||
| I | 70 (29) | 85.60 | |
| II | 56 (23.3) | 61.50 | |
| III | 115 (47.7) | 27.00 | |
| Lymph node ratio | < 0.0001 | ||
| I (0) | 81 (33.6) | 77.30 | |
| II (0.01-0.09) | 41 (17.1) | 65.40 | |
| III (0.1-0.25) | 50 (20.7) | 44.50 | |
| IV (> 0.25) | 69 (28.6) | 21.00 |
Total gastrectomy was performed in 191 (79%) patients, and subtotal gastrectomy was performed in 50 (21%) patients.
A D2 lymphadenectomy was performed in 208 (87%) patients. The median number of lymph nodes retrieved was 37 (range: 5-100); the value reached 40 (range: 13-100) in D2 lymphadenectomy and 16 (range: 5-45) in D1. The incidence of positive node patients was 67%. The 5-year overall survival of the 241 patients was 52.8%, and the ten-year overall survival was 34.7%. In the univariate analysis, age, site, T parameter, N parameter and Stage were significantly associated with overall survival.
We also studied the LNR as a prognostic factor among parameters of the univariate analysis. We considered 4 cutoff based on Marchet et al[6] (Table 2).
Stage migration occured in 33% of the patients: 19.5% of the Ist stage were reclassified to IInd stage, and 33.9% of the IInd stage patients were reclassified as IIIrd stage. All the patients we considered as stage IV in the 6th ed. of the TNM staging system were reclassified as IIIrd stage using the 7th edition TNM staging system.
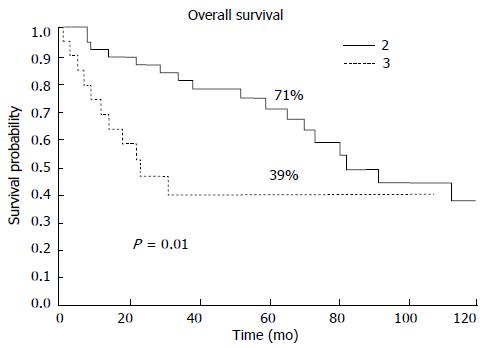
The patients classified as stage II according to the 6th edition and reclassified as stage III exhibited significantly worse prognosis than the patients who remained in stage II (5-year OS, 71% vs 39%; P = 0.01, HR = 2.3, 95%CI: 0.9-5.8) (Figure 1).
Important changes regarding the survival rates and stage reclassification were observed in our analysis. As shown in Table 3, the patients assigned stages using the sixth edition (orizzontally) exhibit a statistically significant difference in the prognosis when reclassified in a different stage according to the seventh edition criteria. However, a statistically significant difference in the prognosis was not observed when comparing the prognosis of patients assigned stages using the seventh edition criteria (vertically) with the stages assigned using the sixth edition (Table 3).
| 7th edition TNM | |||||
| 6th edition TNM | Stage (patients) | I | II | III | P |
| I | 70 | 17 | 0.004 | ||
| II | 39 | 20 | 0.040 | ||
| III | 55 | ||||
| IV | 40 | ||||
| P | 0.09 | P(II-III) = 0.3P(IV-III) = 0.1 | |||
Regarding the substages in the 7th edition, the 5-year survival rates are comparable between substage IB and IIA (5-year OS 59% vs 55%; P = 0.8). However, there is a significant difference regarding the survival probability at 5 years among substages IIIA, IIIB and IIIC (5-year OS IIIA: 47%, IIIB: 20%, IIIC: 24%; P = 0.07). The patients who belong to substage IIIC exhibit similar survival to M+ patients.
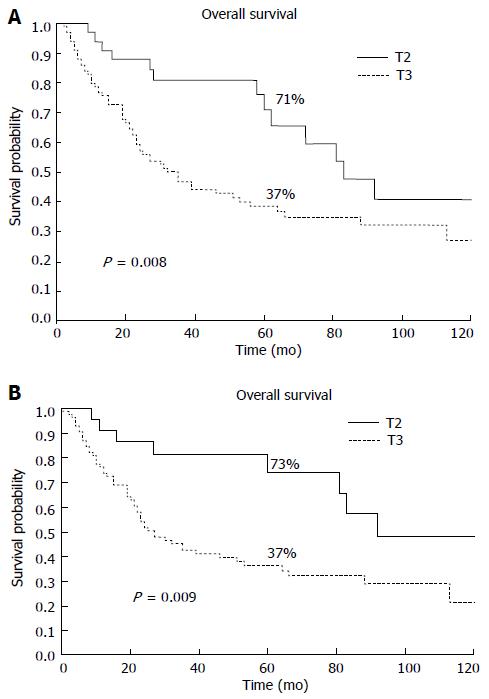
We also analyzed the T category on T2b patients reclassified as T3 in the new edition of the TNM. In our population, 135 T2 patients were classified according to the 6th edition (56%), and 75% of these patients were reclassified as T3 using the most recent revision of the TNM system. The 5-year survival rates of the migrated patients and the patients who remained as T2 were 71% and 37%, respectively (P = 0.008, HR = 2.1, 95%CI: 1.3-3.5) (Figure 2A). The T2aN+ patients exhibited significantly better survival compared with the T2b patients with lymph node involvement (N+) according to the 6th edition (5-year OS 73% vs 37%; P = 0.009, HR = 2.5, 95%CI: 1.4-4.4) (Figure 2B).
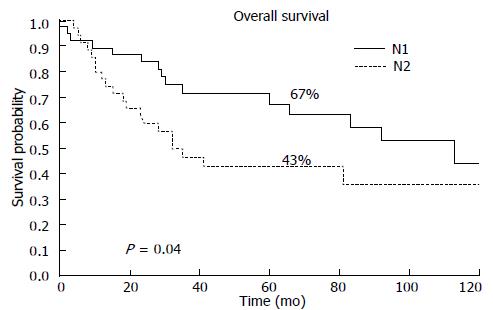
Patients stratified according to the N-stage using the 6th and 7th editions of the TNM are now classified as N1 with the 7th edition and exhibit a 5-year OS probability of 67%. The N2 patients classified according to the 7th edition. TNM exhibit a 5-year OS of 43% (P = 0.04) (Figure 3).
In this retrospective study, we focused on the major changes between the 6th and 7th edition of the TNM system regarding gastric cancer. The analysis of this migration reveals the most important prognostic factors and possible modifications introduced in multimodal treatment.
We observed an OS of 52.8%. That goes to 54% of survival in the D2 type of lymphadenectomy that represented 87% of the sample. In our study population, more than 50% of the patients were diagnosed with T2 lesions according to the 6th edition regarding the parameter of infiltration of the tumor in the gastric wall (T parameter). Other studies reported variable incidences of T2 (Sarela et al[7]: 30%; Marchet et al[8]: 32%; Nitti et al[9]: 51.4%; Park et al[10]: 30%; Lu et al[11]: 40%).
In our study, 102 out of 135 patients (75%) classified as T2 according to the 6th edition of the TNM system were reclassified as T3 based on the 7th edition of the TNM system. The shift exhibits a statistically significant prognostic difference in 5-year OS regardless of nodal involvement (Figure 2).
Our results concerning the prognostic differentiation between T2 and T3 are also confirmed by other studies[12,13]. Sarela et al[7] reported a statistically significant difference between patients classified as T2N1 and T3N1, (56% vs 44%; P = 0.3). Fotia et al[14] obtained different results (74% vs 67% for T2 to T1 to 5 years; P = 0.2).
Recently, Marchet et al[8] reported 5-year survival values of 67% for T2 and 52% for T3. When N+ patients were included in their analysis, 5-year survival rates of 66% and 47% were obtained for T2N+ and T3N+. In conclusion, the results of this study emphasize the prognostic value of T2/T3 categories and the importance of identifying subgroups of patients (T2b 6th edition) that may benefit from adjuvant chemotherapy. Based on our results, these patients would also be candidates for neoadjuvant treatment[15-17].
The renewal of the lymph node cut-off (N parameter) has allowed us to select patients with better prognosis (new N1). The involvement of 1-2 lymph nodes was associated with a better prognosis in our cases than patients with N2 (3-6 positive nodes). The 5-year OS rates were 67.5% for N1 and 43% for N2; (P = 0.04). Similar results were obtained from the study published by Ahn et al[13] (N1: 76.5% vs N2: 58%).
In our analysis, we did not investigate the difference between N3a and N3b because of the small sample size. However, according to other reports, a possible reclassification of the N3 category would be desirable because N3a and N3b exhibit significant differences in survival[13,18,19].
The analysis of the LNR (linf+/linftot) showed good prognostic stratification among the 4 curves (P < 0.0001). Some studies have described the usefulness of the LNR in Japan and South Korea[20,21].
As demonstrated by the work of Kong et al[22], the power of the differential staging of the LNR system was fortified with a higher number of examined lymph nodes and represents appropraite N-staging.
In a retrospective multicenter study of 1853 patients operated for gastric cancer, Marchet et al[6] showed that the LNR was an independent prognostic factor regardless of the type of lymphadenectomy.
Wang et al[23] showed that the “TNratioM System” may predict survival more accurately in patients who undergo limited lymph node analysis.
The changes in the parameters N and T have generated stage migration, which confirms the superiority of the 7th edition. of the TNM system. The new TNM edition groups patients with similar prognoses and separates subjects with different prognoses better that the previous version of the TNM system (Table 3). Similar rates of survival are shown in the analysis by Marrelli et al[24]. Evaluating the substages in our population, we observed that the 5-year survival values were similar between IB and IIA. Similar findings were reported in a large series of western patients with gastric cancer[18].
A significant difference regarding the 5-year survival was observed between the substages of stage III (IIIA, IIIB and IIIC). In a study by Wang et al[12] on 1503 patients, the tumor size (> 5 cm or < 5 cm) was a determining factor in the differentiation of the prognosis between IB and IIA. According to Wang et al[12], three subgroups of the fourth stage exhibit different biologic behaviors of relapse or metastasis models and need further analysis.
In conclusion, the 7th edition of the TNM system seems to have improved accuracy in staging and prognostic stratification, the 7th edition provides more precise indication for adjuvant and neoadjuvant therapy in the multimodal treatment era, our data show the importance of standardization of treatment and the type of surgical lymphadenectomy to compare different experiences and further studies are necessary to improve the TNM system particularly regarding the parameter N and the division into substages.
The Union for International Cancer Control and the American Joint Committee on Cancer tumor-node-metastasis (TNM) staging system is the most important classification of tumors. The main objectives of TNM cancer staging are to help the clinician plan the treatment, to give an indication of prognosis and to evaluate the results of treatment. In the new edition of the TNM (7th) staging system, there are important changes in the field of gastric cancer.
The 7th edition of the TNM system appears to exhibit improved accuracy in staging and prognostic stratification. Different experiences need to be compared to improve the reliability of the TNM classification system.
The TNM 7th edition differs from the previous version regarding gastric cancer on some aspects of the T and M parameters and is completely renewed regarding the N parameter. Several studies, which were predominantly performed in Eastern countries have demonstrated the superiority of the new edition criteria and the highlighted issues still require improvement.
The study results suggest that the 7th edition of the TNM system is superior to the previous version regarding prognostic stratification. However, further studies are necessary to improve the TNM system particularly regarding the N parameter and the division into substages.
The TNM classification uses three parameters to divide the patients into different stages: depth of tumor penetration into the gastric wall (T parameter), the number of metastatic regional lymph nodes involved (N parameter) and the presence of distant metastases (M parameter).
The retrospective study compares the 6th and 7th edition of the TNM classification in a single Italian institution to confirm the superiority of the new edition for prognostic accuracy. According to the experience, standardization of surgical therapy and a multidisciplinary approach are necessary to develop a multimodal tailored treatment.
P- Reviewers: Hiraki M, Jia L S- Editor: Gou SX L- Editor: A E- Editor: Wu HL
| 1. | Sobin LH, Gospodarowicz MK, Wittekind C. International Union Against Cancer (UICC) TNM classification of malignant tumours, 7th edition. New York: Wiley-Liss 2009; 1-336. |
| 2. | Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. editors. American Joint Committee on Cancer (AJCC). AJCC Cancer Staging Manual, 7th edition. New York: Springer-Verlag 2010; 1-649. |
| 3. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1897] [Article Influence: 135.5] [Reference Citation Analysis (0)] |
| 5. | Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481 Available from: http://www.jstor.org/stable/2281868. |
| 6. | Marchet A, Mocellin S, Ambrosi A, Morgagni P, Garcea D, Marrelli D, Roviello F, de Manzoni G, Minicozzi A, Natalini G. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg. 2007;245:543-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 314] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 7. | Sarela AI, Turnbull AD, Coit DG, Klimstra D, Brennan MF, Karpeh MS. Accurate lymph node staging is of greater prognostic importance than subclassification of the T2 category for gastric adenocarcinoma. Ann Surg Oncol. 2003;10:783-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Marchet A, Mocellin S, Ambrosi A, Morgagni P, Vittimberga G, Roviello F, Marrelli D, de Manzoni G, Minicozzi A, Coniglio A. Validation of the new AJCC TNM staging system for gastric cancer in a large cohort of patients (n = 2155): focus on the T category. Eur J Surg Oncol. 2011;37:779-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Nitti D, Marchet A, Mocellin S, Rossi GM, Ambrosi A, Mencarelli R. Prognostic value of subclassification of T2 tumours in patients with gastric cancer. Br J Surg. 2009;96:398-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Park do J, Kong SH, Lee HJ, Kim WH, Yang HK, Lee KU, Choe KJ. Subclassification of pT2 gastric adenocarcinoma according to depth of invasion (pT2a vs pT2b) and lymph node status (pN). Surgery. 2007;141:757-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Lu Y, Liu C, Zhang R, Li H, Lu P, Jin F, Xu H, Wang S, Chen J. Prognostic significance of subclassification of pT2 gastric cancer: a retrospective study of 847 patients. Surg Oncol. 2008;17:317-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Wang W, Sun XW, Li CF, Lv L, Li YF, Chen YB, Xu DZ, Kesari R, Huang CY, Li W. Comparison of the 6th and 7th editions of the UICC TNM staging system for gastric cancer: results of a Chinese single-institution study of 1,503 patients. Ann Surg Oncol. 2011;18:1060-1067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Ahn HS, Lee HJ, Hahn S, Kim WH, Lee KU, Sano T, Edge SB, Yang HK. Evaluation of the seventh American Joint Committee on Cancer/International Union Against Cancer Classification of gastric adenocarcinoma in comparison with the sixth classification. Cancer. 2010;116:5592-5598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 167] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 14. | Fotia G, Marrelli D, De Stefano A, Pinto E, Roviello F. Factors influencing outcome in gastric cancer involving muscularis and subserosal layer. Eur J Surg Oncol. 2004;30:930-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 1943] [Article Influence: 107.9] [Reference Citation Analysis (0)] |
| 16. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4609] [Article Influence: 242.6] [Reference Citation Analysis (0)] |
| 17. | Boige V, Pignon J, Saint-Aubert B, Lasser P, Conroy T, Bouché O, Segol P, Bedenne L, Rougier P, Ychou M. Final results of a randomized trial comparing preoperative 5-fluorouracil (F)/cisplatin (P) to surgery alone in adenocarcinoma of stomach and lower esophagus (ASLE): FNLCC ACCORD07-FFCD 9703 trial. J Clin Oncol. 2007;25:4510. |
| 18. | Marrelli D, Morgagni P, de Manzoni G, Coniglio A, Marchet A, Saragoni L, Tiberio G, Roviello F. Prognostic value of the 7th AJCC/UICC TNM classification of noncardia gastric cancer: analysis of a large series from specialized Western centers. Ann Surg. 2012;255:486-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 19. | Fang WL, Huang KH, Chen JH, Lo SS, Hsieh MC, Shen KH, Li AF, Niu DM, Chiou SH, Wu CW. Comparison of the survival difference between AJCC 6th and 7th editions for gastric cancer patients. World J Surg. 2011;35:2723-2729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Cheong JH, Hyung WJ, Shen JG, Song C, Kim J, Choi SH, Noh SH. The N ratio predicts recurrence and poor prognosis in patients with node-positive early gastric cancer. Ann Surg Oncol. 2006;13:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Inoue K, Nakane Y, Iiyama H, Sato M, Kanbara T, Nakai K, Okumura S, Yamamichi K, Hioki K. The superiority of ratio-based lymph node staging in gastric carcinoma. Ann Surg Oncol. 2002;9:27-34. [PubMed] |
| 22. | Kong SH, Lee HJ, Ahn HS, Kim JW, Kim WH, Lee KU, Yang HK. Stage migration effect on survival in gastric cancer surgery with extended lymphadenectomy: the reappraisal of positive lymph node ratio as a proper N-staging. Ann Surg. 2012;255:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 23. | Wang J, Dang P, Raut CP, Pandalai PK, Maduekwe UN, Rattner DW, Lauwers GY, Yoon SS. Comparison of a lymph node ratio-based staging system with the 7th AJCC system for gastric cancer: analysis of 18,043 patients from the SEER database. Ann Surg. 2012;255:478-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 173] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 24. | Marrelli D, Pedrazzani C, Morgagni P, de Manzoni G, Pacelli F, Coniglio A, Marchet A, Saragoni L, Giacopuzzi S, Roviello F. Changing clinical and pathological features of gastric cancer over time. Br J Surg. 2011;98:1273-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |