CASE REPORT
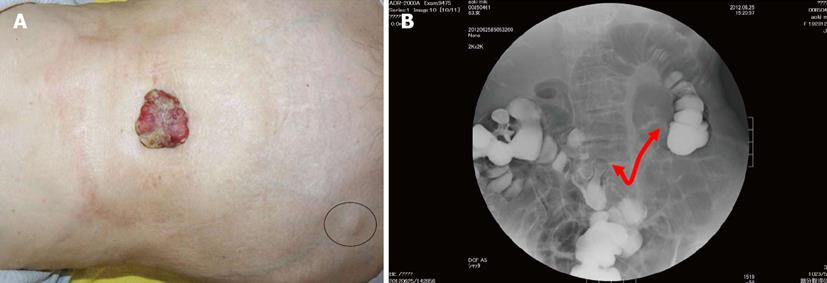
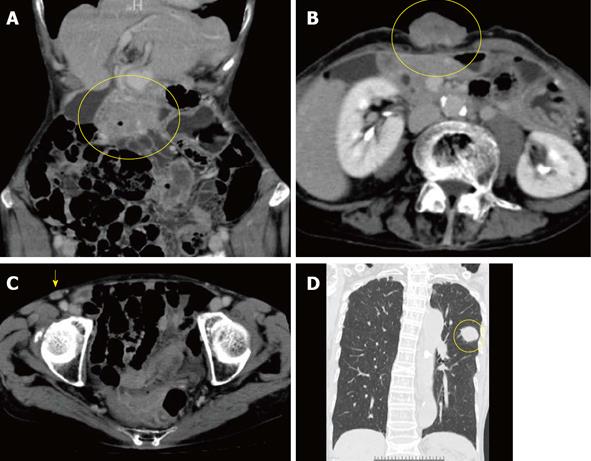
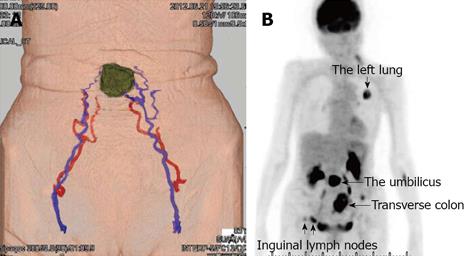
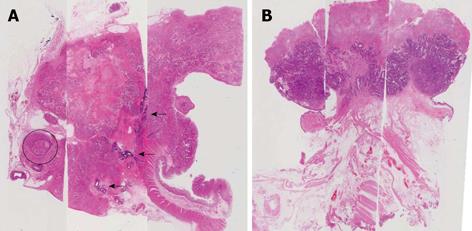
An 83-year-old woman presented to our hospital with a 1-month history of a rapidly enlarging umbilical mass with a necrotic odor. Physical examination revealed a 5.0 cm × 4.5-cm hard, ulcerated umbilical nodule (Figure 1A). She also had two swollen right inguinal lymph nodes. Needle biopsy of the umbilical tumor revealed tubular adenocarcinoma. Endoscopy and contrast radiography of the gastroduodenal and colorectum revealed advanced transverse colon cancer with a very narrowed lumen and oozing of blood (Figure 1B). Dynamic enhanced computer tomography showed tumors of the transverse colon, umbilicus, right inguinal lymph nodes, and left lung (Figures 2). Feeding arteries and drainage veins for the umbilical tumor were the inferior epigastric vessels (Figure 3A), and no vessels had developed along the round ligament of the liver. Fluorodeoxyglucose-positron emission tomography showed uptake at these same points (Figure 3B). Blood examination showed anemia and increased levels of carcinoembryonic antigen, carbohydrate antigen 19-9, and cytokeratin 19 fragment, although other tumor markers were within their normal ranges. Imaging of the left tumor indicated primary lung cancer, and a diagnosis of advanced transverse colon cancer with SMJN and primary lung cancer was made.
Figure 1 Findings of diagnostic nodule and colonorectal enema.
A: A 5.0- × 4.5-cm hard, ulcerated, caulescent tumor was observed in the umbilicus. Two swollen right inguinal lymph nodes were palpable (circle); B: Colonorectal enema showed an apple core sign at the transverse colon near the hepatic flexure, red arrow: the length of narrowed lumen.
Figure 2 Computer tomography showed tumors of the transverse colon (A, circle), umbilicus (B, circle), right inguinal lymph nodes (C, arrow) and left lung (D, circle).
The lung tumor was accompanied by a typical spicula. Vessels along the round ligament of the liver did not develop to and from the umbilical tumor.
Figure 3 Findings of dynamic computer tomography and fluorodeoxyglucose-positron emission tomography.
A: Dynamic computer tomography revealed feeding arteries and drainage veins for the umbilical tumor were the inferior epigastric vessels; B: Fluorodeoxyglucose-positron emission tomography showed uptake in the transverse colon, umbilicus, right inguinal lymph nodes, and left lung (arrows).
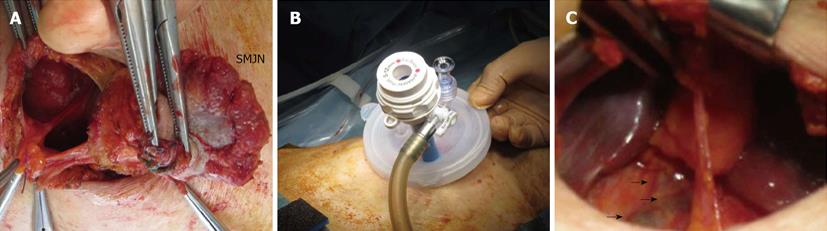
Ten days after the first medical examination, the patient underwent local resection of the umbilical tumor (Figure 4A) and subsequent single-site laparoscopic surgery involving right hemicolectomy with paracolic lymph node dissection (Figure 4B), by using a single access port (EZ Access: Hakko Medical Co. Ltd., Chikuma, Nagano, Japan) and trocar (EZ trocar, Hakko Medical Co. Ltd.). Indocyanine green (ICG) was injected into the paraumbilical portion beforehand. The umbilicus and caulescent tumor were resected en bloc with establishment of surgical margins and ligation of vessels and urachal tract. The round ligament of the liver and the urachus were also ligated. ICG injection revealed the drainage lymph nodes in the abdomen (Figure 4C), and these drainage lymph nodes were dissected. Intra-abdominal dissemination to the mesocolon was confirmed during surgery. After making skin flap, peritoneum was sutured with a running pattern. The fascia was tightly closed with interrupted sutures by using absorbable material. Buried absorbable sutures were used for skin closure. The operative time was 1 h 57 min, and the blood loss volume was 30 mL.
Figure 4 Intraoperative findings and surgical procedures.
A: The umbilicus and caulescent tumor were resected en bloc with establishment of a surgical margin and ligation of vessels; B: Single-site laparoscopic surgery involving right hemicolectomy with paracolic lymph node dissection was subsequently performed. C: Indocyanine green was injected into the paraumbilical portion beforehand, allowing for detection of the drainage lymph nodes in the abdomen (arrow). SMNJ: Sister Mary Joseph’s nodule.
Histopathologically, the transverse colon cancer was confirmed to be moderately differentiated tubular adenocarcinoma. Marked invasion into vessels was observed in the primary colon cancer, and multiple obvious arteriovenous shunts were present (Figure 5A). Immunohistological staining for CK7, CK20, TTF1, and CDX2 in the umbilical tumor were performed to identify the primary tumor. The positive CK 20/CDX2 and negative CK7/TTF1 status indicated that the umbilical tumor was consistent with a moderately differentiated adenocarcinoma of colonic origin, not lung origin. The peritoneum of the umbilicus showed normal findings (Figure 5B). Although the lymph nodes of the mesocolon near the primary colon cancer were positive for metastasis, the drainage lymph nodes from the SMJN, which were detected by ICG, were all negative.
Figure 5 Histopathological findings of the primary colon cancer and Sister Mary Joseph’s nodule are shown (hematoxylin and eosin staining).
A: In the primary colon cancer, severe invasion and tumor thrombosis were observed in the vessels (circle), and nontortuous arteries and multiple A-V shunts were also present (arrows); B: The umbilical tumor was consistent with a moderately differentiated adenocarcinoma of colonic origin. The peritoneum of the umbilicus revealed normal findings.
The postoperative course was uneventful. Although chemotherapy for the colon cancer and thoracoscopic surgery for the primary lung cancer were scheduled, the patient and her family desired home hospice. Seven months after surgery, she died of rapidly growing lung cancer.
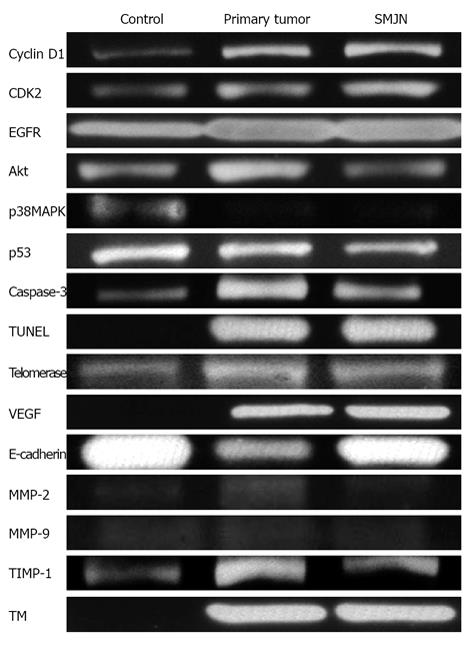
Normal colon, primary colon tumor, and SMJN specimens underwent analysis by western blotting, gelatin zymography, and real-time PCR. The following results were obtained: (1) Cell cycle: cyclin D1, cyclin-dependent kinase 2, epidermal growth factor receptor, Akt, mitogen-activated protein kinase, and p53; (2) Apoptotic induction: caspase-3 and terminal deoxynucleotidyl transferase dUTP nick end labeling; (3) Immortalization: telomerase; (4) Neoangiogenesis: vascular endothelial growth factor (VEGF); and (5) Invasion and metastasis: E-cadherin and matrix metalloproteinase-2 and -9. In addition, to evaluate vascular activity, tissue inhibitor of metalloproteinase-1 and thrombomodulin (TM) were evaluated. The actual intensities of each are shown in Figure 6.
Figure 6 Actual intensities of the normal colon, primary colon tumor, and Sister Mary Joseph’s nodule in western blotting gelatin zymography, and real-time polymerase chain reaction (telomerase) are shown.
CDK2: Cyclin-dependent kinase 2; EGFR: Epidermal growth factor receptor; MAPK: Mitogen-activated protein kinase; TUNEL: Terminal deoxynucleotidyl transferase dUTP nick end labeling; VEGF: Vascular endothelial growth factor; MMP: Matrix metalloproteinase; TIMP: Tissue inhibitor of metalloproteinase; TM: Thrombomodulin; SMNJ: Sister Mary Joseph’s nodule.
DISCUSSION
Although SMJN is a rare first manifestation of colon cancer, current documents report that this nodule usually arises from gastrointestinal and gynecological neoplasms, most commonly from the stomach, colon, pancreas, and ovary and less frequently from the uterus, cervix, gallbladder, and small intestine[7-11,13]. In addition, diagnostic characteristics and imaging findings have been described[10,14-18]. SMJN is associated with a grave prognosis[9,12]. Our case also had a poor prognosis.
SILS was rapidly accepted as a minimally invasive surgical technique. This approach has been applied to a variety of surgical procedures[19,20]. Although SILS has advantages in terms of superior cosmetics, fewer wound complications, and less pain[20,21], many surgeons currently consider that SILS is associated with a higher procedure failure rate with more blood loss and longer operative times than conventional laparoscopic surgery[19,22-25]. The confined surgical field and limited approach direction in SILS may compromise its safety and certainty[22,24,25]. In our case, the incision for resection of the umbilicus with a safe margin was suitable for SILS, caused less pain, was attractive for palliative surgery. Although we agreed that SILS is associated with some problems in terms of its safety and certainty[22,24,25], we consider that SILS still has an advantage in terms of being a palliative surgery that minimizes wound complications and postoperative pain. In cases of malignancy, we suggested that SILS is still available for palliative surgery, even though SILS accompanies some problems.
Metastasis of SMJN occurs mainly by a direct disseminative pathway[26], and may involve lymphogenous and hematogenous pathways[27-29]. Metastatic lesions can reach the umbilicus via spread through lymphatic ducts, the venous network, the arterial network, contiguous extension, or even via iatrogenic seeding with laparoscopy[29]. These different pathways may help to explain why such a wide variety of malignant tumors can produce SMJN[30]. The tumor may spread to the umbilicus through the lymph ducts or blood vessels, by contiguous extension, or through embryologic remnants[26-28]. Regarding the metastatic pathway in our case, the histopathological findings denied direct dissemination and revealed less invasion to the lymphogenous tract. However, to our knowledge, SMJN via the hematogenous pathway is very rare.
Histopathological findings revealed umbilical tumor without direct disseminations and developed lymphoid ducts. The ICG injection detected drainage lymph nodes from umbilical tumor to intra-abdominal lymph nodes, and these “secondary” lymph nodes were not metastatic. Histopathological and ICG injection showed metastatic lymphatic networks did not develop around umbilical tumor. Histopathological assessments revealed that primary and umbilical tumors showed advanced invasions into vessels with arteriovenous shunts. The imaging findings of the inferior epigastric vessels with inguinal lymphoid metastasis as well as the results of VEGF and TM may support the development of the hematogenous pathway in our case. Metastatic route to umbilicus is so complicate[26-30], and it seems difficult to establish the haematogenic route. We just speculated that our SMJN maybe mainly occur via haematogenic pathway.
The presence of SMJN is often a poor prognostic factor because affected patients have advanced metastatic disease at the time of initial diagnosis[9,12]. The average survival time after the appearance of an SMJN is reportedly 10-11 mo[12]. Our patient actually died approximately 7 mo after surgery.
Sister Mary is gone, and 74 years have passed since she died. Is umbilical metastasis, namely SMJN, necessarily associated with a poor prognosis? Future studies are still needed to elucidate the carcinogenesis of her signature nodule.
ACKNOWLEDGMENTS
We are grateful to Satoshi Seo, Yuhei Hamaguchi, Tatsuki Arimitsu, Gozo Kiguchi and Naoki Yamashita (Mitsubishi Kyoto Hospital) to establish laparoscopic surgery in our institution, and to Kagemasa Kuribayashi, Takuma Kato, Kanako Saito, Linan Wang and Mie Torii (Department of Cellular and Molecular Immunology, Mie University Graduate School of Medicine, Tsu, Japan) for their help to perform protein assays.