Published online Sep 27, 2012. doi: 10.4240/wjgs.v4.i9.214
Revised: August 17, 2012
Accepted: September 17, 2012
Published online: September 27, 2012
AIM: To investigate the value of staging laparoscopy with laparoscopic ultrasound (LUS) and peritoneal lavage cytology in patients with newly-diagnosed gastric tumours in our department.
METHODS: Retrospective review of prospectively-collected data was conducted in all patients with newly-diagnosed gastric tumours on oesophagogastroduodenoscopy between December 2003 and July 2008. All the patients had a pre-treatment histological diagnosis and were discussed at the hospital multidisciplinary tumour board meeting for their definitive management. Computed tomography scan was performed in all patients as a part of standard preoperative staging work up. Staging laparoscopy was subsequently performed in selected patients and staging by both modalities was compared.
RESULTS: Twenty seven patients were included. Majority of patients had cardio-oesophageal junction adenocarcinoma. Thirteen patients (48%) were upstaged following staging laparoscopy and one patient was downstaged (3.7%). None of the patients had procedure-related complications. None of the patients with metastasis detected at laparoscopy underwent laparotomy. Gastrectomy after staging laparoscopy was performed in 13 patients (9 R0 resections, 3 R1 resections and 1 R2 resection). Only one patient did not have gastrectomy at laparotomy because of extensive local invasion. Three patients were subjected to neoadjuvant therapy following laparoscopy but only one patient subsequently underwent gastrectomy.
CONCLUSION: In this small series reflecting our institutional experience, staging laparoscopy appears to be safe and more accurate in detecting peritoneal and omental metastases as compared to conventional imaging. Peritoneal cytology provided additional prognostic information although there appeared to be a high false negative rate.
- Citation: Shelat VG, Thong JF, Seah M, Lim KH. Role of staging laparoscopy in gastric malignancies - our institutional experience. World J Gastrointest Surg 2012; 4(9): 214-219
- URL: https://www.wjgnet.com/1948-9366/full/v4/i9/214.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v4.i9.214
Peritoneal metastasis is one of the common sites of metastasis in patients with gastric adenocarcinoma. In patients with metastatic disease, life expectancy is limited and as such, if possible, laparotomy should be avoided. However, in many occasions, preoperative staging is not accurate. Staging by conventional imaging techniques is fraud with limitations and often results in understaging and unnecessary laparotomy. Computed tomography (CT) scanning is the mainstay of staging and is useful in assessment of hepatic metastasis, ascites and lymphadenopathy. However, CT scans miss 30% to 45% of peritoneal and liver nodules especially if these are smaller than 5mm[1-3]. It is estimated that CT scan staging has an accuracy of 50% to 65%[4].
Other imaging modalities used for preoperative staging include endoscopic ultrasound (EUS) and positron emission tomography (PET) scan. In EUS, the transducer is placed directly next to the gastric wall, and high-frequency sound waves are used to determine the depth of tumor invasion and detect local lymph node involvement. It is more accurate than CT scan in assessing depth of tumour infiltration and also a useful means for guiding fine needle aspiration for cytology. However, EUS cannot permit assessment of distant lymph node involvement or liver metastases. It is also highly operator-dependent. PET scan is an expensive imaging tool that is useful in confirming malignancy in CT-detected lymphadenopathy or distant metastases. However, accuracy in detection of lymphadenopathy has little impact on the decision for surgery. Furthermore, the sensitivity of PET scan for detection of peritoneal metastasis is only 50% and signet-ring cell gastric carcinoma is undetectable with this mode of imaging. The role of PET scan in gastric malignancies is therefore limited.
Staging laparoscopy is a safe and fast, albeit invasive, procedure that can improve detection of peritoneal deposits and liver metastasis by direct visualization. It can be performed in combination with laparoscopic ultrasound (LUS) and allow further evaluation of the tumour for local invasion or liver metastasis. Staging laparoscopy has been advocated for use in patients with more than gastric mucosal involvement, no histologically confirmed metastatic disease, patients not being considered for palliative open gastrectomy and patients who may be candidates for neoadjuvant chemotherapy trials. Although there is no level one evidence at present, staging laparoscopy has been shown to avoid unnecessary laparotomy[5,6].
Data obtained by cytological examination of peritoneal washes at the time of surgery has been included as one of the prognostic factors in the staging of gastric cancer by the Japanese Research Society for Gastric cancer, along with tumour depth, degree of nodal metastasis and liver metastasis[7]. However, the role of cytology during staging laparoscopy in advanced gastric cancer is controversial and has been reported to provide little additional information compared to laparoscopy findings alone[8,9].
In this study, our experience with staging laparoscopy in combination with LUS and peritoneal lavage cytology in patients with newly-diagnosed gastric tumours is evaluated.
All patients with newly diagnosed gastric tumours on oesophagogastroduodenoscopy between December 2003 and July 2008 had a staging CT scan performed. Staging laparoscopy was subsequently performed in selected patients. The patient selection criteria: (1) not early gastric cancer; (2) no histological evidence of metastatic disease; (3) not a candidate for palliative procedure; and (4) possible candidate for neo-adjuvant chemotherapy.
Staging by CT scan (Somatom Sensation 64, Siemens, Germany) was obtained after 90 mL of intravenous Iohexol (Omnipaque 350, Nycomed Imaging A.S) injection and CT scan images were completed without multiplanar reconstruction. The CT scan was reviewed and reported by a qualified radiologist. The CT scan images were reviewed by operating surgeon and confirmed the radiologist report.
Staging laparoscopy is done as a day case procedure under general anaesthesia. Three ports (one 10 mm paraumbilical, one 10 mm port at the right upper quadrant and one 5mm port at the left upper quadrant) are used and pneumoperitoneum is created with carbon dioxide and maintained at a pressure of 12 mmHg. The 30 degree scope is used to assess the primary tumour for serosal involvement and local infiltration, the liver, peritoneum and omentum for metastasis, and lymph nodes for enlargement. Inspection of omental bursa by extended laparoscopy was not performed. LUS is performed using a 7.5 MHz probe. Peritoneal washing for cytology is obtained by introducing 100 mL of 0.9% saline into the peritoneal cavity and reaspirating 20 mL to 50 mL of the fluid from several areas of the peritoneal cavity.
Staging CT scan was compared with staging diagnostic laparoscopy. Subsequent patient management and survival outcomes were analysed. For patients who underwent resection with a curative intent, gastrectomy was classified as R0 (histologically-proven complete tumour excision), R1 (residual microscopic tumour) or R2 (residual macroscopic tumour).
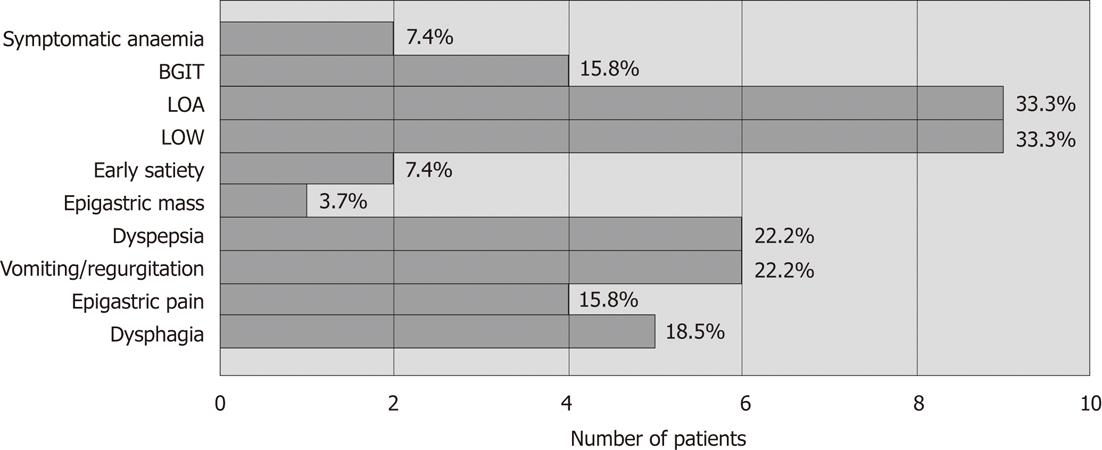
Twenty seven patients were included in the study. Mean age of presentation was 63.3 years (range 41 years to 87 years). Eighteen (66.7%) patients were male. Twenty four (88.9%) patients were Chinese. Eleven (33.3%) patients had a history of smoking. Presenting symptoms of patients are as shown in Figure 1.
In our study, the commonest site of gastric adenocarcinoma was the cardio-oesophageal junction (58%). Other sites of involvement were the antrum (15%), greater curve (8%), lesser curve (8%) and body (8%). One patient had linitis plastica.
Nineteen patients had staging laparoscopy as a day procedure. The rest of the patients had a variable length of stay in hospital because of a variety of reasons (metastatic workup, initiation of treatment, rapid deterioration). Mean duration of procedure was 45 min (range 10-140 min, median 50 min). There were no procedure-related complications in any of the patients. Twenty patients had laparoscopic ultrasonography along with staging laparoscopy. None of the patients underwent extended laparoscopy.
Thirteen (48%) patients were upstaged following laparoscopy. Of these, 10 patients had obvious metastasis (six patients had peritoneal metastasis, two patients had omental metastasis and two patients had both peritoneal and omental metastasis). Three patients were found to have locally-advanced disease - one patient each had bulky tumour, local invasion into the lesser omentum and local invasion into the left lobe of liver. None of the patients had liver metastasis. The CT scan of all the three patients showed diffuse gastric wall thickening along with perigastric lymphadenopathy. One patient was downstaged following laparoscopy. CT scan had shown possible liver metastasis but this was not confirmed on LUS. The comparison of CT scan staging and laparoscopic staging has been summarized in Table 1.
| Computed tomography scan | Laparoscopy | Peritoneal cytology | |
| Upstaging | |||
| 1 | CEJ thickening | Peritoneal nodules | Positive |
| 2 | Irregular thickening of anterior stomach wall? Left lobe liver invasion | Omental nodules; no liver invasion | Not done |
| 3 | Diffuse thickening with perigastric nodes | Peritoneal and omental nodules | Atypical cells |
| 4 | Diffuse thickening with perigastric nodes | Peritoneal nodules | Positive |
| 5 | Thickening of gastric wall | Peritoneal nodules; invasion to left lobe of liver | Positive |
| 6 | CEJ tumour with diffuse abdominal lymphadenopathy | Omental nodules | Atypical cells |
| 7 | Irregular thickening of gastric wall with enlarged splenic hilar, porta hepatic, peripancreatic and para-aortic nodes | Moderate ascites; secondary deposits in pylorus | Atypical cells? Suspicious for malignancy |
| 8 | Grossly distended stomach with circumferential enhancing tumour with multiple abdominal nodes and? liver lesion | Omental nodules | Atypical cells |
| 9 | Diffuse thickening of gastric mucosa and abdominal lymphadenopathy with? liver hypodensity | Ascites; peritoneal nodules | Positive |
| 10 | CEJ malignancy with lesser curvature lymphadenopathy with? Invasion into the pancreas | Peritoneal nodules | Positive |
| 11 | CEJ tumour with perigastric lymph nodes | Serosal involvement | Negative |
| 12 | Diffuse gastric wall thickening with abdominal lymphadenopathy | Bulky tumour invading serosa and left lobe of liver | Atypical cells, favour reactive |
| 13 | Diffuse gastric wall thickening with abdominal lymphadenopathy | Omental nodules | Positive |
| Down staging | |||
| 1 | Regional lymphadenopathy with liver metastases | Mobile tumour at antrum with no liver metastases on laparoscopic ultrasound | Negative |
None of the 10 patients with metastatic disease underwent laparotomy. Six patients received palliative chemotherapy and/or radiotherapy, two of which also had palliative stenting and one patient had palliative laparoscopic gastrojejunostomy. Survival ranged from 2 to 18 mo.
Of the four patients who refused palliative chemo-radiotherapy, survival was dismal and ranged from 11 d to 8 mo.
Three patients with locally-advanced disease at laparoscopy underwent neoadjuvant chemoradiotherapy. Only one of these patients eventually underwent radical total gastrectomy and is still alive at 40 mo. The other two patients had rapid disease progression despite chemoradiotherapy and both died within six months.
One patient was downstaged and underwent curative R0 subtotal gastrectomy with adjuvant chemoradiotherapy.
One patient had surgery postponed because of fever and repeat CT scan a month later showed rapid progression of disease and widespread metastasis. The patient died at 10 mo (peritoneal cytology was negative at laparoscopy).
The rest of the patients (n = 12) underwent laparotomy with curative intent. Eight patients with gastric adenocarcinoma underwent R0 resections, three patients underwent R1 resections and one patient underwent a R2 resection.
Peritoneal lavage cytology was done in twenty six patients. Six (27.3%) patients had positive cytology and these were also patients with obvious metastasis on laparoscopy. Two other patients had overt intra-abdominal metastasis and negative cytology.
Five patients had atypical cells on cytology. One patient had bulky tumour and subsequently died at four months during neoadjuvant chemoradiotherapy. One patient subsequently underwent R1 total gastrectomy, retrocolic Roux-en-Y oesophagojejunostomy and jejunojejunostomy but declined adjuvant chemoradiotherapy and died at two months. One patient had obvious omental metastasis and died at four months. One patient had inoperable tumour at laparotomy and died at four months. One patient was lost to follow-up.
Thirteen patients had negative peritoneal cytology. Two patients had obvious metastasis at laparoscopy. One patient had locally invasive tumour involving the left lobe of liver.
Management of gastrointestinal malignancies depends on preoperative assessment and staging. Despite advances in science and technology, accurate preoperative evaluation is not a rule and many times a metastatic disease is only unraveled at a laparotomy. Multimodal management of advanced gastric cancer demands accurate preoperative staging and staging laparoscopy has made a significant impact on the patient care.
From our study, staging laparoscopy had a significant impact on decisions regarding the treatment plan in patients with advanced gastric malignancies. Staging laparoscopy avoided unnecessary laparotomy in 48% of patients. In 3.7% of patients, the tumour was downstaged and a curative resection was possible. Nakagawa et al[6] published similar findings in which 44% of patients were upstaged and 3% downstaged after laparoscopy. One patient deemed resectable by laparoscopy in our study, was found to be unresectable at laparotomy. Sensitivity of laparoscopy in detecting peritoneal, hepatic and nodal metastases has been shown to be superior to CT scan or EUS[10]. In fact; laparoscopy has been shown to be more sensitive than both CT scan and EUS combined. O’Brien et al[11] found that laparoscopy had a sensitivity of 77% compared to 38% with CT scan and EUS combined. The role of staging laparoscopy will continue to evolve due to lack of a non invasive staging modality that provides adequate and accurate information. The potential advantages and disadvantages of staging laparoscopy are summarized in Table 2. Staging laparoscopy is contraindicated when there are complications that mandate upfront surgical intervention.
| Advantages | Disadvantages |
| Less invasive procedure compared to open laparotomy | It is an invasive procedure with risk of general anesthesia |
| Can avoid unnecessary laparotomy and indirect cost savings | Possible delay in definitive treatment |
| Additional palliative procedures can be performed | Added resource utilization and cost |
In our study, none of the patients had liver metastasis at laparoscopy. One patient with possible liver metastasis on CT scan was subsequently found not to have metastasis with the aid of LUS. Samee et al[12] had evaluated the role of LUS during staging laparoscopy in oesophagogastric cancers and found that the addition of LUS increased the detection rate of lymph node and liver metastasis and local extension by 8%. Inspection of omental bursa with extended laparoscopy has a role in posterior gastric and pancreatic malignancies. It has longer operating time and it can complicate gastrectomy due to adhesions or inflammation developing due to tissue dissection. We are not sure if extended staging laparoscopy could have identified the sole patient that was unresectable after staging laparoscopy.
Peritoneal lavage cytology during laparotomy is included as a staging process in the 13th edition of Japanese Classification of Gastric cancer[7]. However, the usefulness of peritoneal lavage cytology during staging laparoscopy is controversial. Some authors have concluded from their studies that peritoneal lavage cytology during laparoscopic staging of gastrointestinal malignancies offered little benefit[8,9]. The sensitivity of peritoneal lavage cytology has been reported to be relatively low ranging from 22% to 30%[13,14]. In our group of patients, 19% of patients with obvious peritoneal metastasis did not have positive cytology or atypical cells on cytology. However, Nakagawa et al[6] reported that peritoneal deposits not found initially at laparoscopy were found in patients with positive cytology. Cytology of peritoneal lavage fluid at laparoscopy could therefore be beneficial and make up for false-negative results at laparoscopy.
In addition, patients with positive cytology or atypical cells on cytology in our study seemed to have a worse prognosis even in the absence of obvious metastasis at laparoscopy. Survival was dismal and ranged from two to four months in these patients without overt metastasis. In fact, the patient deemed resectable at laparoscopy but later found to be unresectable at laparotomy, had atypical cells on peritoneal lavage cytology. Bentrem et al[15] found that patients with positive peritoneal cytology obtained during staging laparoscopy was the preoperative factor most predictive of early recurrence and death from gastric cancer following R0 resection. Badgwell et al[16] showed that patients with positive peritoneal cytology at laparoscopy had similar survival outcomes compared to those with gross metastatic disease. Nakagawa et al[6] also found that 29% of patients had positive cytology in the absence of obvious malignant deposits and that these patients had a poorer survival rate compared to those with negative cytology. The treatment of patients with positive cytology alone is not well-established. Although there are studies that have shown a survival benefit for intraperitoneal chemotherapy in conjunction with radical resection in patients with positive peritoneal cytology vs resection alone, this remains experimental[17].
Neoadjuvant therapy has been shown to result in down staging of the primary tumour such that initially unresectable tumours subsequently have higher curative resection rate[18,19]. The use of neoadjuvant chemotherapy also appears to improve survival in patients with positive peritoneal cytology without obvious metastatic disease. Use of neoadjuvant therapy resulted in a 3-year overall survival rate of 12% vs 0% for patients who did not receive neoadjuvant therapy[16]. In a recent publication, Nath et al[20] found that the median survival for patients with positive cytology who did not undergo chemotherapy was six months in patients with overt metastases vs nine months in patients without. When chemotherapy was given, the comparable survival was nine months vs 15 mo respectively. In our study, three patients with locally advanced disease underwent neoadjuvant chemoradiotherapy but only one subsequently had curative resection. The other two patients had rapid disease progression and died within four months. The role of a second staging laparoscopy after neoadjuvant therapy to assess clinical response is yet of questionable benefit and hence not routinely performed. Conventional imaging techniques are still being used routinely for this purpose. However, this again brings back the question of accuracy especially in detection of peritoneal metastasis. Yano et al[21] reported the use and accuracy of second staging laparoscopy to assess for chemotherapeutic response prior to decision for salvage surgery. In addition to avoiding unnecessary laparotomy, accurate staging by laparoscopy is able to identify patients with advanced gastric malignancies for decisions on neoadjuvant therapy to be considered and subsequent response to therapy assessed.
In conclusion, our study demonstrates that laparoscopy is a safe and effective staging tool in gastric malignancies. It is able to detect small peritoneal and liver metastasis missed by conventional imaging techniques. LUS and cytological examination of peritoneal washing improves the accuracy of disease staging, and is helpful in predicting prognosis and assisting in patient selection for neoadjuvant treatment.
Peritoneal carcinomatosis is a frequent means of metastasis in patients with gastric cancer. Intra-abdominal tumour deposits are difficult to detect by conventional imaging techniques and often results in under-staging and unnecessary laparotomy. There has been few studies showing the effectiveness of staging laparoscopy in reducing unnecessary laparotomy. This study adds to the evidence.
Multimodal management of advanced gastric cancer demands accurate preoperative staging and staging laparoscopy with peritoneal cytology has made a significant impact on the patient care. Peritoneal lavage cytology is included as a staging process in the 13th edition of Japanese Classification of Gastric cancer.
Staging by conventional imaging techniques is fraud with limitations and often results in understaging and unnecessary laparotomy. Staging laparoscopy is a safe and fast, albeit invasive, procedure that can improve detection of peritoneal deposits and liver metastasis by direct visualization. It can be performed in combination with laparoscopic ultrasound and allow further evaluation of the tumour for local invasion or liver metastasis. Data obtained by cytological examination of peritoneal washes at the time of surgery has been included as one of the prognostic factors in the staging of gastric cancer by the Japanese Research Society for Gastric cancer.
If a patient without radiological evidence of metastatic disease does not require a palliative gastrectomy, then staging laparoscopy should be considered to exclude metastatic disease. Staging laparoscopy should also be considered within neo-adjuvant chemotherapy protocols.
Inspection of omental bursa during staging laparoscopy is defined as extended staging laparoscopy and is useful to determine posterior invasion. There has also been an attempt to stage gastric cancer based on staging laparoscopy: Stage I - no serosal invasion; Stage II - serosal invasion; Stage III - adjacent organ invasion; Stage IV - metastatic disease.
This is a well written retrospective study from a small volume centre regarding their experience on staging laparoscopy and authors have demonstrated a substantial upstaging and reduction in unnecessary laparotomy.
Peer reviewers: Theodoros E Pavlidis, Professor, 2nd Surgical Prop. Department, Hippocration Hospital, Aristotle University of Thessaloniki Medical School, 54248 Thessaloniki, Greece; Shingo Noura, MD, PhD, Department of Surgery, Osaka Medical Center for Cancer and Cardiovascular Diseases,1-3-3 Nakamichi, Higashinari-ku, Osaka 537-8511, Japan; Dr. Stefano Scabini, Department of Emato-Oncology, AOU San Martino Hospital, Largo R. Benzi 10, 16100 Genoa, Italy
S- Editor Wen LL L- Editor A E- Editor Xiong L
| 1. | Ziegler K, Sanft C, Zimmer T, Zeitz M, Felsenberg D, Stein H, Germer C, Deutschmann C, Riecken EO. Comparison of computed tomography, endosonography, and intraoperative assessment in TN staging of gastric carcinoma. Gut. 1993;34:604-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 107] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Oñate-Ocaña LF, Gallardo-Rincón D, Aiello-Crocifoglio V, Mondragón-Sánchez R, de-la-Garza-Salazar J. The role of pretherapeutic laparoscopy in the selection of treatment for patients with gastric carcinoma: a proposal for a laparoscopic staging system. Ann Surg Oncol. 2001;8:624-631. [PubMed] |
| 3. | Nieveen van Dijkum EJ, de Wit LT, van Delden OM, Rauws EA, van Lanschot JJ, Obertop H, Gouma DJ. The efficacy of laparoscopic staging in patients with upper gastrointestinal tumors. Cancer. 1997;79:1315-1319. [PubMed] [DOI] [Full Text] |
| 4. | Angelelli G, Ianora AA, Scardapane A, Pedote P, Memeo M, Rotondo A. Role of computerized tomography in the staging of gastrointestinal neoplasms. Semin Surg Oncol. 2001;20:109-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 5. | Lowy AM, Mansfield PF, Leach SD, Ajani J. Laparoscopic staging for gastric cancer. Surgery. 1996;119:611-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 99] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Nakagawa S, Nashimoto A, Yabusaki H. Role of staging laparoscopy with peritoneal lavage cytology in the treatment of locally advanced gastric cancer. Gastric Cancer. 2007;10:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition -. Gastric Cancer. 1998;1:10-24. [PubMed] |
| 8. | van Dijkum EJM, Sturm PD, de Wit LT, Offerhaus J, Obertop H, Gouma DJ. Cytology of peritoneal lavage performed during staging laparoscopy for gastrointestinal malignancies: is it useful? Ann Surg. 1998;228:728-733. [RCA] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Sotiropoulos GC, Kaiser GM, Lang H, Treckmann J, Brokalaki EI, Pottgen C, Gerken G, Paul A, Broelsch CE. Staging laparoscopy in gastric cancer. Eur J Med Res. 2005;10:88-91. [PubMed] |
| 10. | Stell DA, Carter CR, Stewart I, Anderson JR. Prospective comparison of laparoscopy, ultrasonography and computed tomography in the staging of gastric cancer. Br J Surg. 1996;83:1260-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | O'Brien MG, Fitzgerald EF, Lee G, Crowley M, Shanahan F, O'Sullivan GC. A prospective comparison of laparoscopy and imaging in the staging of esophagogastric cancer before surgery. Am J Gastroenterol. 1995;90:2191-2194. [PubMed] |
| 12. | Samee A, Moorthy K, Jaipersad T, Crisp W, Cheruvu C, Elder J, Deakin M. Evaluation of the role of laparoscopic ultrasonography in the staging of oesophagogastric cancers. Surg Endosc. 2009;23:2061-2065. [PubMed] |
| 13. | Bentrem D, Wilton A, Mazumdar M, Brennan M, Coit D. The value of peritoneal cytology as a preoperative predictor in patients with gastric carcinoma undergoing a curative resection. Ann Surg Oncol. 2005;12:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 164] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 14. | Badgwell B, Cormier JN, Krishnan S, Yao J, Staerkel GA, Lupo PJ, Pisters PW, Feig B, Mansfield P. Does neoadjuvant treatment for gastric cancer patients with positive peritoneal cytology at staging laparoscopy improve survival? Ann Surg Oncol. 2008;15:2684-2691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Suzuki T, Ochiai T, Hayashi H, Hori S, Shimada H, Isono K. Peritoneal lavage cytology findings as prognostic factor for gastric cancer. Semin Surg oncol. 1999;4:27-33. |
| 16. | Boku T, Nakane Y, Minoura T, Takada H, Yamamura M, Hioki K, Yamamoto M. Prognostic significance of serosal invasion and free intraperitoneal cancer cells in gastric cancer. Br J Surg. 1990;77:436-439. [PubMed] |
| 17. | Wilke H, Preusser P, Fink U, Gunzer U, Meyer HJ, Meyer J, Siewert JR, Achterrath W, Lenaz L, Knipp H. Preoperative chemotherapy in locally advanced and nonresectable gastric cancer: a phase II study with etoposide, doxorubicin, and cisplatin. J Clin Oncol. 1989;7:1318-1326. [PubMed] |
| 18. | Nashimoto A, Yabusaki H, Tanaka O, Sasaki J, Akiyama N. Neoadjuvant chemotherapy in advanced gastric cancer with non-curative factors: a Phase II study with 5-fluorouracil, leucovorin, and cisplatin. Gastric Cancer. 1999;2:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Shimada S, Tanaka E, Marutsuka T, Honmyo U, Tokunaga H, Yagi Y, Aoki N, Ogawa M. Extensive intraoperative peritoneal lavage and chemotherapy for gastric cancer patients with peritoneal free cancer cells. Gastric Cancer. 2002;5:168-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Nath J, Moorthy K, Taniere P, Hallissey M, Alderson D. Peritoneal lavage cytology in patients with oesophagogastric adenocarcinoma. Br J Surg. 2008;95:721-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Yano M, Tsujinaka T, Shiozaki H, Inoue M, Sekimoto M, Doki Y, Takiguchi S, Imamura H, Taniguchi M, Monden M. Appraisal of treatment strategy by staging laparoscopy for locally advanced gastric cancer. World J Surg. 2000;24:1130-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |