Published online Jun 27, 2012. doi: 10.4240/wjgs.v4.i6.146
Revised: June 20, 2012
Accepted: June 23, 2012
Published online: June 27, 2012
AIM: To investigate the mechanism of pentoxifylline (PTX) improvement in liver regeneration.
RESULTS: Rats were randomized into 4 groups: Control rats; Sham - sham-operation rats; Saline - 70% hepatectomy plus saline solution; PTX - 70% hepatectomy plus PTX. At 2 and 6 h after hepatectomy, aspartate aminotransferase, alanine aminotransferase, tumor necrosis factor (TNF)-α and interleukin-6 (IL-6) serum and hepatic tissue levels were determined. Tumor growth factor (TGF)-β1 gene expression in liver tissue was evaluated 24 h after hepatectomy by quantitative reverse transcriptase polymerase chain reaction analysis. Proliferation was analyzed by mitotic index and proliferating cell nuclear antigen (PCNA) staining 48 h after hepatectomy.
RESULTS: TNF-α and IL-6 serum levels increased at 2 and 6 h after hepatectomy. At 2 h after hepatectomy serum PTX was reduced but not hepatic levels of TNF-α and IL-6. A decrease in liver TGF-β1 gene expression and an increase in mitotic index and PCNA after hepatectomy were observed in the PTX treatment group in comparison to the saline group.
CONCLUSION: PTX improves liver regeneration by a mechanism related to down regulation of TNF-α production and TGF-β1 gene expression.
- Citation: Martino RB, Coelho AMM, Kubrusly MS, Leitão R, Sampietre SN, Machado MCC, Bacchella T, D’Albuquerque LAC. Pentoxifylline improves liver regeneration through down-regulation of TNF-α synthesis and TGF-β1 gene expression. World J Gastrointest Surg 2012; 4(6): 146-151
- URL: https://www.wjgnet.com/1948-9366/full/v4/i6/146.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v4.i6.146
Liver regeneration in small-for-size liver grafts and in subtotal hepatectomies may be suppressed, thereby increasing the morbidity and mortality in these situations[1,2].
The regenerative capacity of the liver has been known for a long time although the mechanisms of this complex physiological process are not completely understood. This process has been reported to be regulated by cytokines [interleukin-6 (IL-6) and tumor necrosis factor (TNF)] and growth factors[3,4]. TNF-α and IL-6 blood levels increase after partial hepatectomy and are strong promoters of hepatic regeneration after hepatectomy[2,5]. Indeed, inhibition of TNF-α production retards liver regeneration after partial hepatectomy[6].
TNF-α regulates the initial phase of liver regeneration after partial hepatectomy by enhancing the mitogenic effects of hepatic growth factor (HGF) in vivo and in vitro and through the release of IL-6 which activates Stat 3[7,8]. However, TNF-α and IL-6 are recognized as initial phase cytokines in inflammatory response to systemic infection or injury, and play a pivotal role in liver damage following hepatectomy or ischemia/reperfusion injury[9,10]. Its excessive production after 90% hepatectomy has been associated with adverse effects on the hepatic microcirculation and liver regeneration[11]. It has also been recently demonstrated that pentoxifylline (PTX), an inhibitor of TNF-α production[12], reduces liver injury and improves liver regeneration in a model of small-for-size liver transplantation through a mechanism thought to be related to interruption of TNF-α signaling[13]. Indeed a recent randomized controlled trial demonstrated the beneficial effects of PTX in major liver resections[14]. However the mechanisms of the effect of PTX on hepatic regeneration have not been completely evaluated.
Tumor growth factor (TGF)-β1 is an anti-inflammatory cytokine and also a potent growth inhibitor that suppresses the production of HGF and, when administered in high doses, reduces the peak of DNA synthesis at 24 h after partial hepatectomy[15-17]. TGF-β1 is produced during hepatic regeneration and released in the peripheral circulation after partial hepatectomy[18]. There are several pieces of evidence suggesting that TNF-α induces TGF-β1 expression in many cell types[19-22].
The aim of the present study was to test the hypothesis that PTX could improve liver regeneration through inhibition of TNF-α synthesis and reduction of liver TGF-β1 gene expression
One hundred and twelve adult male Wistar rats weighing 230-270 g were housed in individual cages and kept under standard conditions (12 h of light-dark cycle and temperature between 22 °C and 28 °C) with free access to a standard rat chow and water ad libitum. The experimental protocol was approved by the Ethics Commission of the Hospital das Clínicas: São Paulo University.
The partial hepatectomy was carried out under general anesthesia with intraperitoneal ketamine chloride (Ketalar, Parke Davis, São Paulo, Brazil) (100 mg/kg) through an upper abdominal midline incision. A 70% hepatectomy was performed using the technique described by Higgins and Andersen[23]. No mortality was observed in this model.
Animals were randomized into the four experimental groups: Group control - twenty four animals were not submitted to hepatectomy; Group sham - twenty four sham-operated rats underwent laparotomy and liver manipulation; Group saline - thirty two animals submitted to 70% hepatectomy and saline administration; Group PTX - thirty two animals with 70% hepatectomy and PTX administration.
Intraperitoneal administration of PTX, at 25 mg/kg of animal weight (1.25 mL/kg) (Trental™, Sanofi Aventis Pharma, São Paulo, Brazil), was performed immediately after the operation and the same dose was repeated after 12 h.
Intraperitoneal administration of saline solution, at 1.25 mL/kg of animal weight, was performed immediately after the operation and was repeated after 12 h.
At 2 and 6 h after hepatectomy animals were anesthetized for blood sampling through cardiac puncture and killed by exsanguination. Serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), as well as TNF-α and IL-6 serum and hepatic tissue levels were determined. TGF-β1 gene expression was evaluated 24 h after hepatectomy in remnant liver tissue. Proliferation was analyzed in remnant regenerating liver by mitotic index and proliferating cell nuclear antigen (PCNA) staining 48 h after hepatectomy.
Serum levels of AST and ALT were assayed by using optimized ultraviolet kit from Roche (Rotkrenz, Switzerland).
Serum and liver tissue levels of TNF-α and IL-6 were determined using an enzyme-linked immunosorbent assay kit from Biosource International Cytoscreen (Camarillo, California - USA).
Livers were rapidly removed and were immediately frozen in liquid nitrogen. All tissues were stored at -70º C until use. Total RNA was isolated from fresh-frozen liver tissues from the two groups using Trizol reagent (Invitrogen, Life Technologies, USA) according the manufacturer’s protocol. RNA quality was analyzed with an aliquot of 500 ng on a 1% agarose gel. Samples were kept at -80 °C until processing by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR). qRT-PCR analysis for TGF-β1 gene was performed in the Rotor-Gene RG-3000 (Corbett Research, Sidney, Australia) using a SuperScriptTM III Platinum SYBR Green One-Step qRT-PCR kit (Invitrogen, Life Technologies) with 100 ng of total RNA and 0.2 mmol/L sense/antisense primer per reaction, according to the manufacturer’s recommendations. The reaction was carried out under the following cycling conditions: 10 min at 50 °C, 5 min at 95 °C, followed by 35 cycles of 15 s at 95 °C, 30 s at 55 °C, and 30 s at 72 °C. To verify amplification reaction specificity, melting curves were determined using the following conditions: 72 °C ramping to 99 °C at 0.2 °C/s. A primer set was designed to amplify two separate intron-spanning regions, enabling us to assess the possible genomic DNA contamination: TGF-β1 sense 5'-CGGCAGCTGTACATTGACTT-3' and antisense 5'-AGCGCACGATCATGTTGGAC-3' β-actin sense 5'-TGTCACCAACTGGGACGATA-3' and β-actin antisense 5'-GGGGTGTTGAAGGTCTCAAA-3'. Primers were designed using primer 3_http://www.cgi v 0.2 program.13. RNA template concentrations (500, 100, 20, 4 and 0.8 ng/μL) were used to generate a standard curve to evaluate the amplification efficiency of the TGF-β1 gene in comparison to β-actin. The content of TGF-β1 RNA was determined as the number of transcripts relative to those of β-actin and additionally normalized to the mean value of control liver tissue. Quantification was obtained according to the ΔΔCT method.
Hepatocyte proliferation was assessed by PCNA and by mitotic index staining. Liver specimens were fixed in 2% formalin solution and stained with hematoxylin-eosin for histological analysis. The PCNA-labeling index was obtained by measuring the number of PCNA-positive hepatocytes in 20 consecutive high power fields (HPF: 400 ×). For mitotic index evaluation mitotic hepatocytes were counted in 20 consecutive high power fields (HPF: 400 ×).
Results were expressed as a mean ± SD. Statistical analysis was conducted with one-way ANOVA and post hoc testing with Tukey-Kramer multiple comparison test. Mann-Whitney’s test was performed for histological data. P values less than 0.05 were considered significant. Graph pad prism 4.0 Software was used for statistical data analysis.
Transaminase levels were not significantly reduced in rats treated by PTX (data not shown).
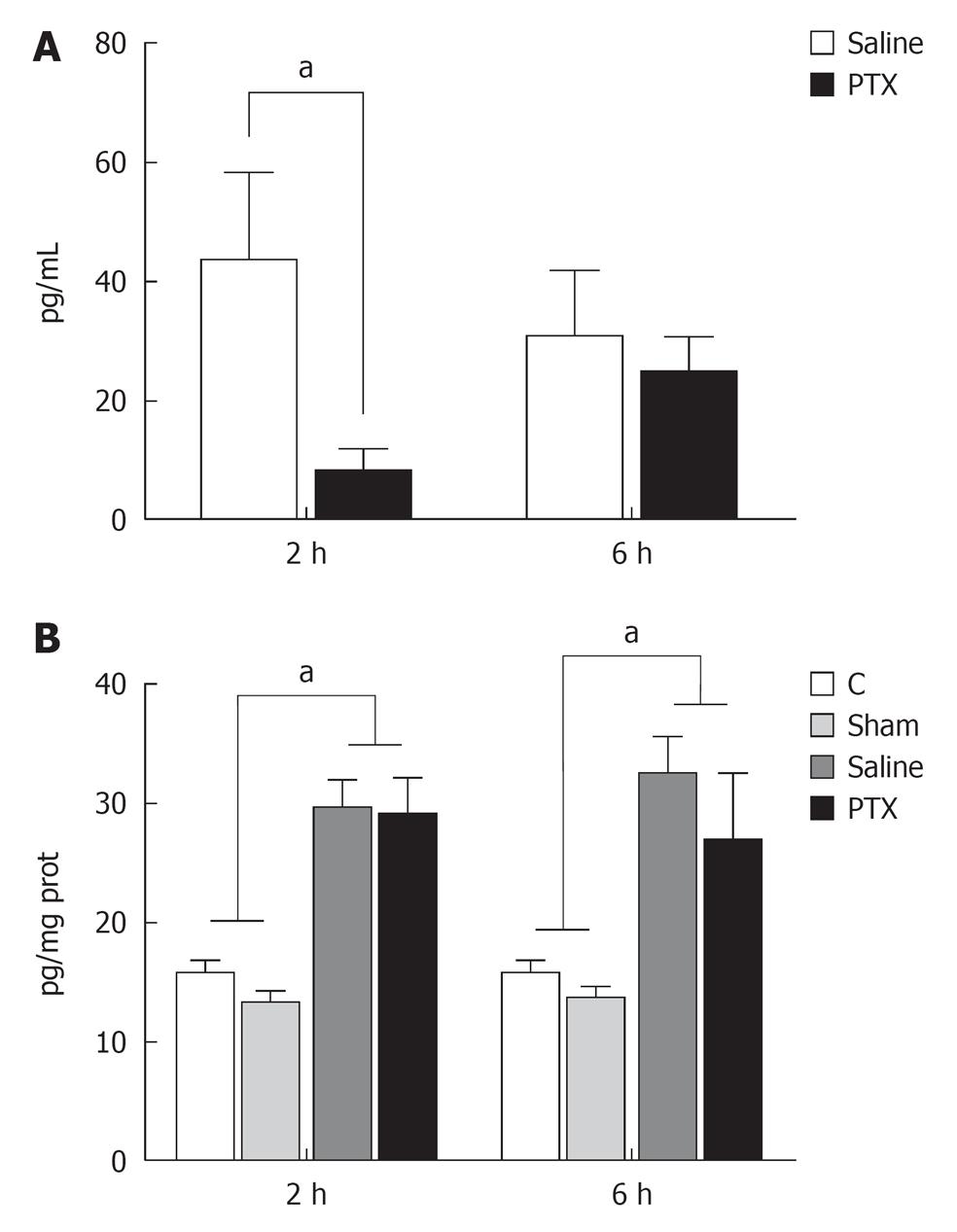
Serum levels of TNF-α were undetectable in control and sham operated groups but increased at 2 and 6 h after partial hepatectomy. Administration of PTX significantly decreased serum levels of TNF-α at 2 h but not at 6 h after hepatectomy, when compared to non-treated animals (Figure 1A).
Liver tissue levels of TNF-α increased after hepatectomy when compared to Control and Sham operation groups. However no difference was observed between Saline and PTX groups at 2 and 6 h after liver resection (Figure 1B).
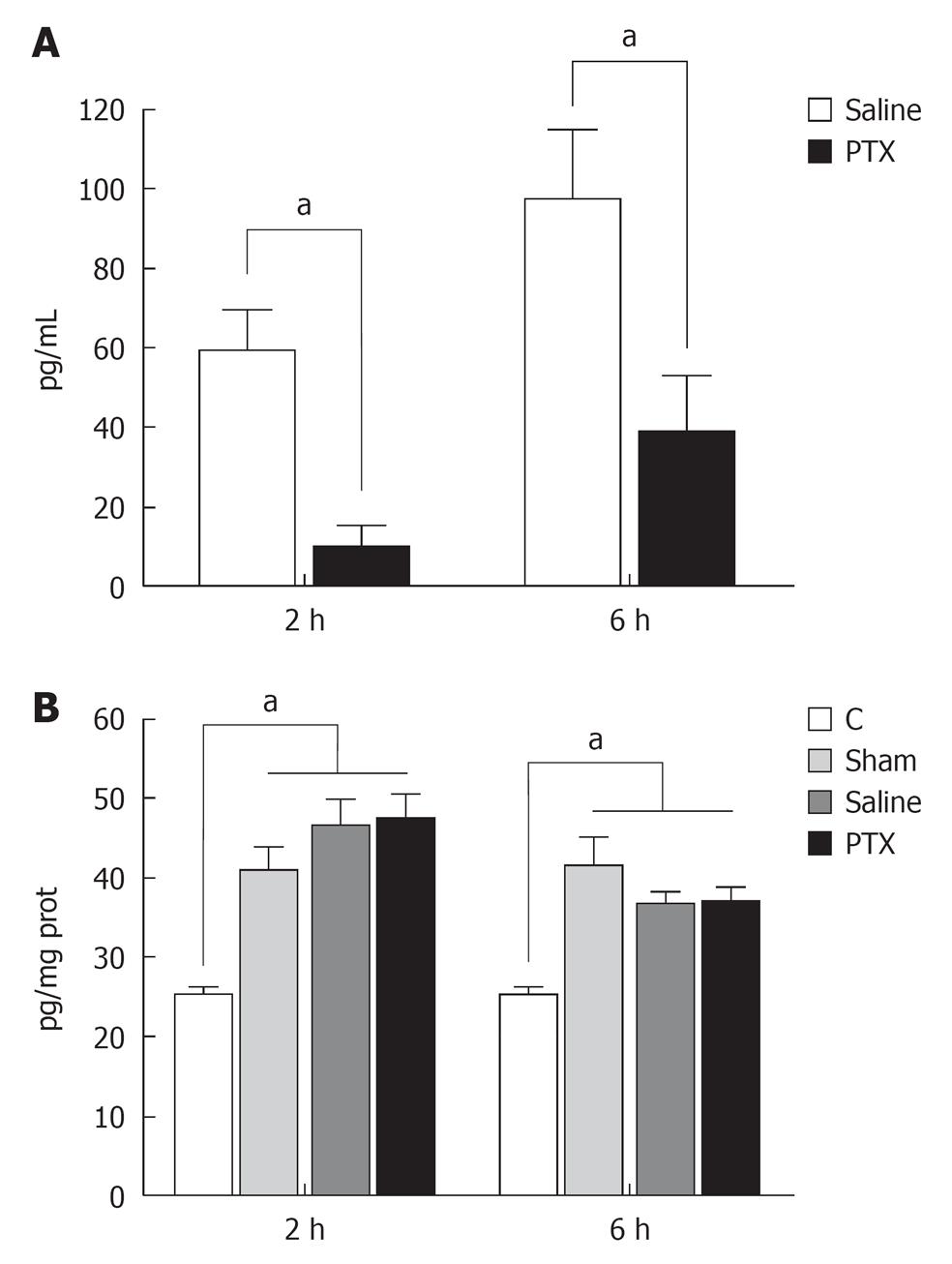
Serum levels of IL-6 were undetectable in Control and Sham operated groups and increased at 2 and 6 h after liver resection in group saline. Administration of PTX significantly decreases serum levels of IL-6 at 2 and 6 h after hepatectomy (Figure 2A).
Compared to control group, liver tissue levels of IL-6 increased in laparotomy and liver resection groups. However no differences were observed among these three groups (Figure 2B).
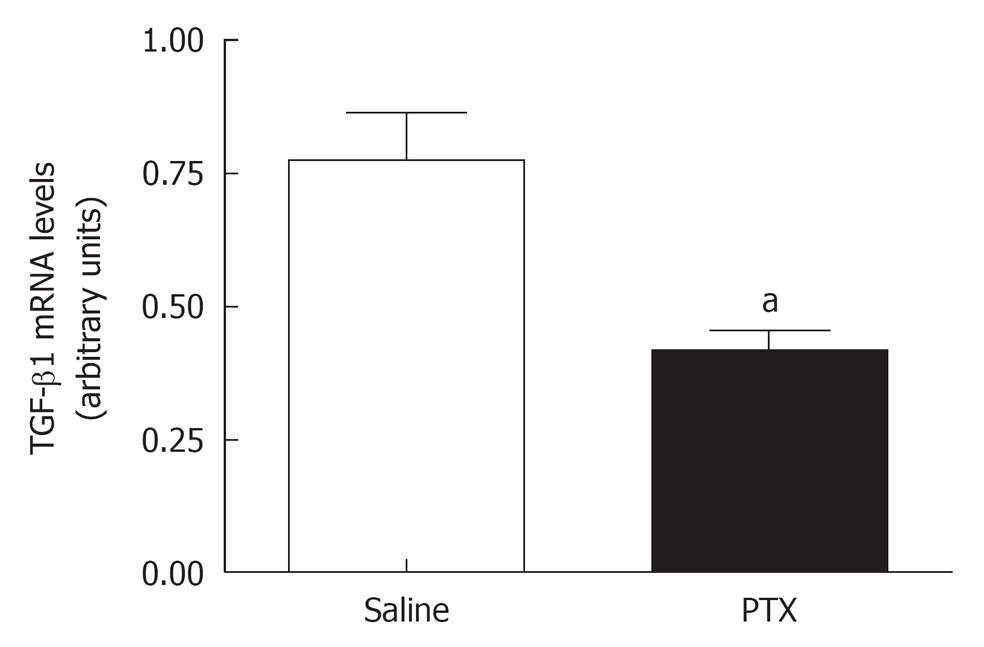
A significant reduction in TGF-β1 mRNA liver tissue levels was observed in group PTX when compared to group Saline (Figure 3).
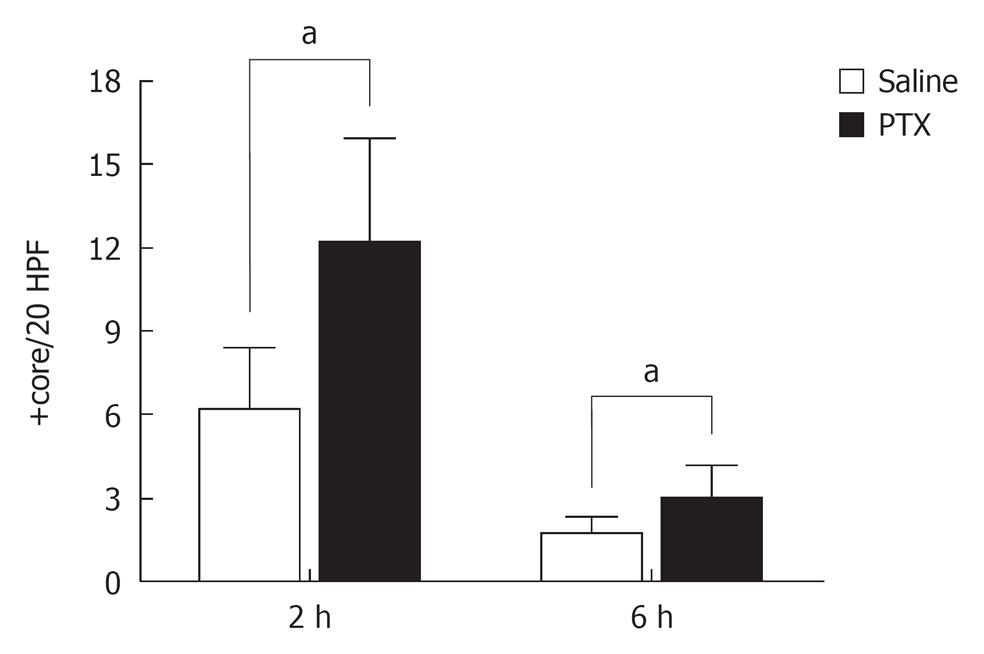
The pathological results obtained 48 h after the hepatectomy showed an increased PCNA labeling index (12.1 ± 3.8 core/20 HPF) and mitotic index (2.9 ± 1.2 core/20 HPF) in animals treated with PTX compared to saline treated rats (PCNA: 6.1 ± 2.2 core/20 HPF; mitotic index: 1.7 ± 0.5 core/20 HPF) (Figure 4).
In the present study, PCNA expression was used as an indicator of hepatic cell proliferation since there is a perfect correlation between this method, liver weight and 5-bromo-2-deoxyuridine incorporation[24].
After resection or injury the remaining hepatocytes enter into the G1 phase. Several previous studies demonstrated that cytokines, mainly TNF-α and IL-6, are involved in liver regeneration after partial hepatectomy[1,6,10,25].
TNF-α is recognized as a regulator of the initial phase of hepatic regeneration after liver resection and injury, given that it is able to increase the activity of DNA polymerase-α and the incorporation of [H3] thymidine into DNA in the liver cells of intact adult rats[26].
TNF-α also induces IL-6 production through NF-κB activation[27]. IL-6 is a strong promoter of liver regeneration in rats after 70% hepatectomy[28]. Antibody against TNF-α inhibits IL-6 production and liver regeneration, and the administration of suppressive agents against TNF-α also inhibits hepatic regeneration[6,26].
However, TNF-α and IL-6 are recognized as initial phase cytokines in the inflammatory response following systemic injury, stimulating the expression of chemoatractants which induce neutrophil accumulations and tissue injury[9]. Indeed TNF-α is involved in liver injury due to galactosamin, and endotoxin[11,29].
It has been demonstrated that excessive production of IL-6 in 90% hepatectomized rats is associated with adverse effects on liver regeneration. Indeed reduction of initial cytokine response in extensive hepatic resection in rats improves liver regeneration[11].
In the present study we demonstrated that the administration of PTX reduces TNF-α and IL-6 serum levels but not liver tissue levels (Figures 1 and 2). It has also been demonstrated that PTX administration increases the production of anti-inflammatory and growth promoting factors (IL-6 and IL-10) in a small-for-size liver transplantation in mice[13]. It was concluded that these effects were related to inhibition of the TNF-α signaling pathway[13]. However, in the present study we did not find a reduction in TNF-α levels in liver tissue following the administration of PTX, suggesting that the production of TNF-α in the liver tissue follows a different signaling pathway from that of macrophages. Indeed it has been demonstrated that the bile duct and the portal and central veins are major producers of TNF-α in regenerating livers[30]. Depletion of Kupffer cells by gadolinium chloride does not reduce liver TNF-α and actually enhances liver regeneration after partial hepatectomy[31]. This complex relationship between hepatocytes and non parenchimal cells was also underscored by findings that blocking nuclear factor (NF)-κB activation in hepatocytes increases NF-κB activation and TNF-α production in non parenchimal cells, augmenting proliferative response following partial hepatectomy[32]. It is, therefore, possible that PTX blocks TNF-α production specifically in Kupffer cells, thereby increasing the production of TNF-α by parenchimal cells.
TGF-β1 is a strong inhibitor of hepatocyte regeneration. It inhibits proliferation of hepatocytes in culture and suppresses the production of HGF[16,33]. TGF-β expression increases in liver tissue after hepatectomy in response to the regenerative process[34]. It has been also demonstrated that TNF-α influences TGF-β1 expression in many cell types[19-21]. There is also a correlation between TNF-α mRNA expression and TGF-β1 mRNA expression in vivo[22]. Therefore, suppression of TNF-α may also suppress TGF-β1 production, thereby enhancing liver regeneration. Indeed, in the present study we demonstrated that PTX administration reduces TGF-β1 expression in liver tissue and also improves liver regeneration. Recently it was demonstrated that liver regeneration is inhibited in small-for-size liver grafts and that this effect is prevented by over-expression of smad 7 which blocks TGF-β 1 induced activation of smad 2/3[24]. Suppression of TGF-β1 expression may be one of the mechanisms related to the beneficial effect of PTX in liver regeneration.
After extensive hepatectomy, gut-derived endotoxins cause increase production of TNF-α and IL-1, stimulating the production of other cytokines, and further amplifying the inflammatory response, thereby possibly contributing to hepatic injury[35]. Therefore reduction of cytokine serum levels could also be beneficial for liver regeneration, particularly in extended hepatectomy or small-for-size liver transplantation.
As demonstrated in the present study (Figure 4), the decrease of TNF-α and IL-6 serum levels without changing TNF-α and IL-6 liver tissue levels (Figures 1 and 2), and the reduction of liver TGF-β1 expression (Figure 3) may explain some of the beneficial effects of PTX in liver regeneration. Reduction of serum levels of cytokines seems to be crucial for the improvement of liver regeneration. Indeed, in rats that had undergone subtotal hepatectomy, serum cytokines levels were persistently elevated and associated with decrease in liver regeneration[11].
We can speculate that suppression of TNF-α liver tissue levels is deleterious for liver regeneration. However, reduction of cytokine serum levels without change in liver tissue levels, as observed both in the present study and by others, enhances liver regeneration[6,26,36].
In conclusion, our results demonstrate that PTX decreases the systemic inflammatory response and reduces liver TGF-β 1 mRNA expression, thereby enhancing liver regeneration. This may be useful to improve the function of small liver remnants in extended resection and in small-for-size liver grafts.
Liver regeneration in small-for-size liver grafts and following hepatectomy may be suppressed, thereby increasing mortality. The complex physiological processes of liver regeneration are not completely understood. Cytokines, such as tumor necrosis factor (TNF)-α, play a pivotal role in this process and in liver damage following hepatectomy or ischemia/ reperfusion injury.
Pentoxifylline (PTX), an inhibitor of TNF-α production, reduces liver injury and improves liver regeneration. However the mechanisms of this have not been completely evaluated. Tumor growth factor (TGF)-β1 is an anti-inflammatory cytokine and also a potent growth inhibitor. Given that TNF-α induces TGF-β1 expression, the authors demonstrated that PTX could improve liver regeneration through inhibition of TNF-α synthesis and reduction of liver TGF-β1 gene expression
Previous publications have stated that PTX improves liver regeneration by the inhibition of the liver TNF-α signaling pathway. However, in this study the authors did not find a reduction in TNF-α levels in liver tissue. The authors found a down-regulation of TGF-β1 gene expression in liver tissue and reduction in serum levels of TNF-α. The authors concluded that the effect of PTX on liver regeneration is related to down-regulation of systemic TNF-α production and liver tissue TGF-β1 gene expression.
PTX may be useful for improving the function of small liver remnant in extended resection and in small-for-size liver grafts.
This is a nicely written preclinical study on liver regeneration which investigate surrogate of inflammation and their anti-inflammatory cytochine as promoter and treatment of liver regeneration after large hepatectomy.
Peer reviewer: Salvatore Gruttadauria, MD, PhD, Abdominal Transplant Surgery, ISMETT-UPMC, Via E. Tricomi N. 1, Palermo 90127, Italy
S- Editor Wang JL L- Editor Hughes D E- Editor Zheng XM
| 1. | Zhong Z, Schwabe RF, Kai Y, He L, Yang L, Bunzendahl H, Brenner DA, Lemasters JJ. Liver regeneration is suppressed in small-for-size liver grafts after transplantation: involvement of c-Jun N-terminal kinase, cyclin D1, and defective energy supply. Transplantation. 2006;82:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1246] [Cited by in RCA: 1158] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 3. | Fausto N, Laird AD, Webber EM. Liver regeneration. 2. Role of growth factors and cytokines in hepatic regeneration. FASEB J. 1995;9:1527-1536. [PubMed] |
| 4. | Court FG, Wemyss-Holden SA, Dennison AR, Maddern GJ. The mystery of liver regeneration. Br J Surg. 2002;89:1089-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Fausto N, Riehle KJ. Mechanisms of liver regeneration and their clinical implications. J Hepatobiliary Pancreat Surg. 2005;12:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Kimura T, Sakaida I, Terai S, Matsumura Y, Uchida K, Okita K. Inhibition of tumor necrosis factor-alpha production retards liver regeneration after partial hepatectomy in rats. Biochem Biophys Res Commun. 1997;231:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Webber EM, Bruix J, Pierce RH, Fausto N. Tumor necrosis factor primes hepatocytes for DNA replication in the rat. Hepatology. 1998;28:1226-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 214] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1213] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 9. | Tracey KJ, Cerami A. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu Rev Med. 1994;45:491-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 784] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 10. | Miyazawa Y, Tsutsui H, Mizuhara H, Fujiwara H, Kaneda K. Involvement of intrasinusoidal hemostasis in the development of concanavalin A-induced hepatic injury in mice. Hepatology. 1998;27:497-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Tsutsumi R, Kamohara Y, Eguchi S, Azuma T, Fujioka H, Okudaira S, Yanaga K, Kanematsu T. Selective suppression of initial cytokine response facilitates liver regeneration after extensive hepatectomy in rats. Hepatogastroenterology. 2004;51:701-704. [PubMed] |
| 12. | Bernard C, Barnier P, Merval R, Esposito B, Tedgui A. Pentoxifylline selectivity inhibits tumor necrosis factor synthesis in the arterial wall. J Cardiovasc Pharmacol. 1995;25 Suppl 2:S30-S33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Tian Y, Jochum W, Georgiev P, Moritz W, Graf R, Clavien PA. Kupffer cell-dependent TNF-alpha signaling mediates injury in the arterialized small-for-size liver transplantation in the mouse. Proc Natl Acad Sci USA. 2006;103:4598-4603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Petrowsky H, Breitenstein S, Slankamenac K, Vetter D, Lehmann K, Heinrich S, DeOliveira ML, Jochum W, Weishaupt D, Frauenfelder T. Effects of pentoxifylline on liver regeneration: a double-blinded, randomized, controlled trial in 101 patients undergoing major liver resection. Ann Surg. 2010;252:813-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Barnard JA, Lyons RM, Moses HL. The cell biology of transforming growth factor beta. Biochim Biophys Acta. 1990;1032:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 99] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Gohda E, Matsunaga T, Kataoka H, Yamamoto I. TGF-beta is a potent inhibitor of hepatocyte growth factor secretion by human fibroblasts. Cell Biol Int Rep. 1992;16:917-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Russell WE, Coffey RJ, Ouellette AJ, Moses HL. Type beta transforming growth factor reversibly inhibits the early proliferative response to partial hepatectomy in the rat. Proc Natl Acad Sci USA. 1988;85:5126-5130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 283] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 18. | Michalopoulos GK, DeFrances M. Liver regeneration. Adv Biochem Eng Biotechnol. 2005;93:101-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Phan SH, Gharaee-Kermani M, McGarry B, Kunkel SL, Wolber FW. Regulation of rat pulmonary artery endothelial cell transforming growth factor-beta production by IL-1 beta and tumor necrosis factor-alpha. J Immunol. 1992;149:103-106. [PubMed] |
| 20. | Samad F, Uysal KT, Wiesbrock SM, Pandey M, Hotamisligil GS, Loskutoff DJ. Tumor necrosis factor alpha is a key component in the obesity-linked elevation of plasminogen activator inhibitor 1. Proc Natl Acad Sci USA. 1999;96:6902-6907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 150] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Warshamana GS, Corti M, Brody AR. TNF-alpha, PDGF, and TGF-beta(1) expression by primary mouse bronchiolar-alveolar epithelial and mesenchymal cells: tnf-alpha induces TGF-beta(1). Exp Mol Pathol. 2001;71:13-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Helmig S, Stephan P, Döhrel J, Schneider J. TNF-α mRNA expression correlates with TGF-β mRNA expression in vivo. Inflammation. 2011;34:255-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Higgins GM, Anderson RM. Experimental pathology of the liver: restoration of liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186-202. |
| 24. | Zhong Z, Tsukada S, Rehman H, Parsons CJ, Theruvath TP, Rippe RA, Brenner DA, Lemasters JJ. Inhibition of transforming growth factor-beta/Smad signaling improves regeneration of small-for-size rat liver grafts. Liver Transpl. 2010;16:181-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Higashitsuji H, Arii S, Furutani M, Mise M, Monden K, Fujita S, Ishiguro S, Kitao T, Nakamura T, Nakayama H. Expression of cytokine genes during liver regeneration after partial hepatectomy in rats. J Surg Res. 1995;58:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Akerman P, Cote P, Yang SQ, McClain C, Nelson S, Bagby GJ, Diehl AM. Antibodies to tumor necrosis factor-alpha inhibit liver regeneration after partial hepatectomy. Am J Physiol. 1992;263:G579-G585. [PubMed] |
| 27. | Yamada Y, Kirillova I, Peschon JJ, Fausto N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci USA. 1997;94:1441-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 740] [Cited by in RCA: 755] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 28. | Camargo CA, Madden JF, Gao W, Selvan RS, Clavien PA. Interleukin-6 protects liver against warm ischemia/reperfusion injury and promotes hepatocyte proliferation in the rodent. Hepatology. 1997;26:1513-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 322] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 29. | Wang Y, Singh R, Lefkowitch JH, Rigoli RM, Czaja MJ. Tumor necrosis factor-induced toxic liver injury results from JNK2-dependent activation of caspase-8 and the mitochondrial death pathway. J Biol Chem. 2006;281:15258-15267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 175] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 30. | Loffreda S, Rai R, Yang SQ, Lin HZ, Diehl AM. Bile ducts and portal and central veins are major producers of tumor necrosis factor alpha in regenerating rat liver. Gastroenterology. 1997;112:2089-2098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Rai RM, Yang SQ, McClain C, Karp CL, Klein AS, Diehl AM. Kupffer cell depletion by gadolinium chloride enhances liver regeneration after partial hepatectomy in rats. Am J Physiol. 1996;270:G909-G918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Malato Y, Sander LE, Liedtke C, Al-Masaoudi M, Tacke F, Trautwein C, Beraza N. Hepatocyte-specific inhibitor-of-kappaB-kinase deletion triggers the innate immune response and promotes earlier cell proliferation during liver regeneration. Hepatology. 2008;47:2036-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Houck KA, Michalopoulos GK. Altered responses of regenerating hepatocytes to norepinephrine and transforming growth factor type beta. J Cell Physiol. 1989;141:503-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Sowa JP, Best J, Benko T, Bockhorn M, Gu Y, Niehues EM, Bucchi A, Benedetto-Castro EM, Gerken G, Rauen U. Extent of liver resection modulates the activation of transcription factors and the production of cytokines involved in liver regeneration. World J Gastroenterol. 2008;14:7093-7100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Nolan JP, Camara DS. The importance of intestinal endotoxins in liver disease. Prog Clin Biol Res. 1985;189:347-367. [PubMed] |
| 36. | Hou Z, Yanaga K, Kamohara Y, Eguchi S, Tsutsumi R, Furui J, Kanematsu T. A new suppressive agent against interleukin-1beta and tumor necrosis factor-alpha enhances liver regeneration after partial hepatectomy in rats. Hepatol Res. 2003;26:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |