Published online Jun 27, 2012. doi: 10.4240/wjgs.v4.i6.141
Revised: June 22, 2012
Accepted: June 24, 2012
Published online: June 27, 2012
AIM: To determine the effectiveness of using multidetector computed tomography (MDCT) data in preoperative planning of robot-assisted surgery.
METHODS: Fourteen patients indicated for surgery underwent MDCT using 64 and 256-slice MDCT. Before the examination, a specially constructed navigation net was placed on the patient’s anterior abdominal wall. Processing of MDCT data was performed on a Brilliance Workspace 4 (Philips). Virtual vectors that imitate robotic and assistant ports were placed on the anterior abdominal wall of the 3D model of the patient, considering the individual anatomy of the patient and the technical capabilities of robotic arms. Sites for location of the ports were directed by projection on the roentgen-positive tags of the navigation net.
RESULTS: There were no complications observed during surgery or in the post-operative period. We were able to reduce robotic arm interference during surgery. The surgical area was optimal for robotic and assistant manipulators without any need for reinstallation of the trocars.
CONCLUSION: This method allows modeling of the main steps in robot-assisted intervention, optimizing operation of the manipulator and lowering the risk of injuries to internal organs.
- Citation: Berelavichus SV, Karmazanovsky GG, Shirokov VS, Kubyshkin VA, Kriger AG, Kondratyev EV, Zakharova OP. Virtual modeling of robot-assisted manipulations in abdominal surgery. World J Gastrointest Surg 2012; 4(6): 141-145
- URL: https://www.wjgnet.com/1948-9366/full/v4/i6/141.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v4.i6.141
Robotic techniques in modern surgery have undergone a number of stages from their initial introduction to their application in daily practice. Today, there are several surgery clinics where robotic complexes are used and applied in a range of fields. Urological applications of robotic technology have ranged from robot-assisted prostatectomy for prostate cancer to laparoscopic donor nephrectomy and cystectomy, renal transplant, and recently, robotic vasovasostomy[1-5]. Gynecology is one of the fastest growing fields of robotic surgery. Applications include the use of the da Vinci surgical system (Intuitive Surgical, USA) in benign gynecology and gynecologic oncology. Robotic surgery can be used to treat fibroids, abnormal periods, endometriosis, ovarian tumors, pelvic prolapse, and female cancers[6-13]. The role of this technology is now clearly established as it allows minimally invasive surgery with the benefits of traditional open surgical techniques.
Nonetheless, the published literature on robotic surgery reveals that the application of robotic technology in abdominal surgery is, as yet, quite limited. In our opinion the infrequent use of robot-assisted interventions on the peritoneum and retroperitoneal space is due to the limited instrument dexterity and the necessity to constantly manipulate in different regions of the abdomen during surgery. For example, transposition of the intestinal tract from one floor of the peritoneal cavity to another is difficult to perform using robotic manipulators.
However, in abdominal surgery there are many operations that require highly precise movements of the kind which can be achieved with the help of robotics, providing three-dimensional vision, tremor filtration, and motion scaling.
Therefore, it is important to find a compromise solution that includes the advantages of both methods and provides maximum utility, thereby achieving widespread acceptance of robot-assisted technologies in the treatment of various lesions in abdominal surgery.
One of the current advantages in robotic assisted abdominal surgery, which is driving its continued development and expansion, is precise computer-assisted precise of the laparoscopic and manipulation ports on the anterior abdominal wall in relation with the operation field. Reviewing the literature, we were unable to find any publications related to this problem.
Our goal was to determine possible intervention strategies and the capabilities of robot-assisted intervention in patients with surgical diseases of the peritoneum and retroperitoneal space.
From March 2009 to June 2010, forty three robot-assisted operations were performed in the department of abdominal surgery at the Vishnevsky Institute of Surgery (Russia, Moscow). Table 1 summarizes the range of diseases and the operations performed.
| Robot-assisted operations | Quantity | Surgical illnesses |
| Atypical liver resection | 16 | Non-parasitic liver cysts, FNH, cystadenoma |
| Pancreatic resection | 5 | Solid pseudopapillary tumor, cystadenocarcinoma, cystic lymphangioma, cystic adenocarcinoma and neuroendocrine tumor |
| Cholecystectomy | 2 | Polyps, adenomyosis of the gall bladder |
| Spleenectomy | 1 | Cystic lymphangioma of the spleen |
| Spleen resection | 2 | Spleen cysts, lymphangioma |
| Wedge gastric resection | 2 | Gastrointestinal stromal tumor |
| Adrenalectomy | 2 | Endothelial adenoma of the adrenal cortex |
| Phrenum plastic | 1 | Relaxation of the left cupola of the diaphragm |
| Adnexectomy | 1 | Luteal cyst of the left oophoron |
| Resection of gastric cysts | 1 | Duplication gastric cyst |
| Left colon resection | 1 | Cancer of the colon |
| Duodenojejunal passage resection | 2 | Gastrointestinal stromal tumor, teratoma |
| Anterior resection or the rectum | 1 | Rectal cancer |
| Erasion of the extra-organ retroperitoneal tumor | 1 | Lipoma |
| Cholecystectomy, resection of the gastric tumor | 1 | Submucous gastric tumor; BSD: chronic acalculous cholecystitis |
| Cholecystectomy, liver resection | 1 | Liver cysts, gall bladder polyps |
| Cholecystectomy, spleen resection | 1 | Non-parasitic spleen cyst. BSD |
| Left-sided lobectomy of the liver | 1 | FNH |
| PDR | 1 | Adenocarcinoma |
| Total | 43 |
Given the wide spectrum of diseases, we faced the problem of the adequate installation of ports on the anterior abdominal wall. In contrast with gynecological and urological surgery, we had to operate in various anatomic regions of the abdominal cavity. There were, therefore, some technical problems with the correct installation of the trocars that lead to the narrowing of the surgical field, interference of the robotic arms and complications for the surgical team’s work.
The aim of our study was to create a method that would allow preoperative planning of the optimal location of the laparoscopic and manipulation ports on the anterior abdominal wall in relation to the required surgical area in a range of operations.
We added computer modeling of the upcoming surgical procedure to the treatment algorithm of every patient in our study. Preoperative 3-D modeling was performed for 14 patients (age range 26 tо 72 years). Three patients underwent splenectomy (non-parasitic splenic cysts), 3 patients had pancreas resections and one patient underwent a combined cholecystectomy and gastric tumor resection. Seven patients were operated for non-parasitic cysts of the liver (segments VII, VIII). This location is considered one of the most hard-to-reach for conventional laparoscopic surgery, but with preoperative 3-D modeling and the advantages of robot-assisted surgery (increased dexterity, 3-D visualization, seven degrees of freedom, physiological tremor elimination and the ability to scale motions) these operations became much more precise and safe.
All patients underwent a preoperative contrast enhanced triphasic 64-slice and 256-slice multi-detector computed tomography (Phillips Brilliance). About 500 mL of water was routinely administrated 5-10 min before the examination to demarcate the duodenum and delineate the pancreatic head region. Each patient received 100 mL of non-ionic contrast material containing 370 mg iodine/mL (omnipaque 350, ultravist 370, optiray 350) via intravenous injection at the rate of 3-5 mL/s using automatic power injectors [OptiVantage DH (Mallinckrodt; Inc.)] through a 18-gauge or 20-gauge intravenous catheter in a antecubital vein. Unenhanced and triphasic (arterial phase, portal venous phase) enhanced scans were performed. Unenhanced and enhanced scan images were obtained from the top of the diaphragm through the pelvis. Monitoring of contrast media bolus was performed on the level of the aortic arch in all cases. The trigger threshold of density was set at 150 HU for the aortic ROI placed at the center of the vessel lumen. Delay after the start of injection was 10 s for arterial phase, and 35 s for portal venous phase. The levels of the tracker and starting position were the same in both cases.
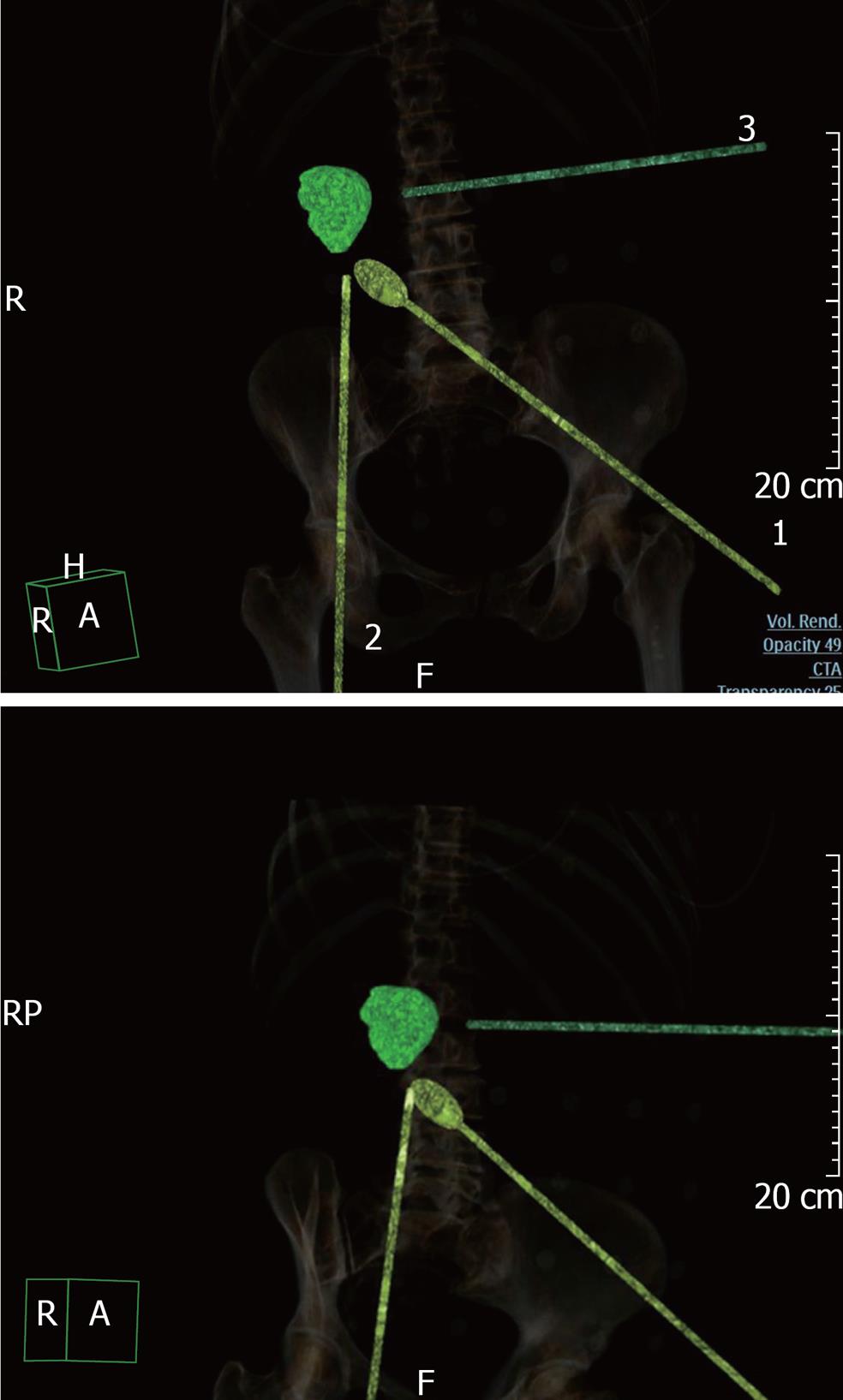
Virtual modeling of robot-assisted operations was performed with the Brilliance iCT workstation (Workstation Brilliance iCT 4.0). We used the Liver segmentation program with RFA planning section (virtual radiofrequency ablation). The examination of the portal phase was carried out semi-automatically with manual correction in different reconstructions, including 3-D visualization. Modeling started with “vector 1” that imitated the robotic laparoscopic camera. The first laparoscopic port was then installed virtually, taking into consideration features of the abdominal cavity, the anterior abdominal wall and bone structures. The internal part of the “vector 1” was directed to the surgical area. Usually, the installation point was the umbilical zone. The next two or three robotic ports were located in the optimal locations, considering all individual anatomical features (the distance between all the ports was not less than 10-12 cm). The internal parts of the ports were focused on the lesion (Figure 1). The assistant trocar location was selected after the robotic ports were placed (vectors 1, 2 and 3). The assistant port was situated on the opposite side of the surgical area in the largest interval between the robotic ports. Finally, the image of the virtually installed instruments (regarding the body surface) was saved and sent to the surgery department.
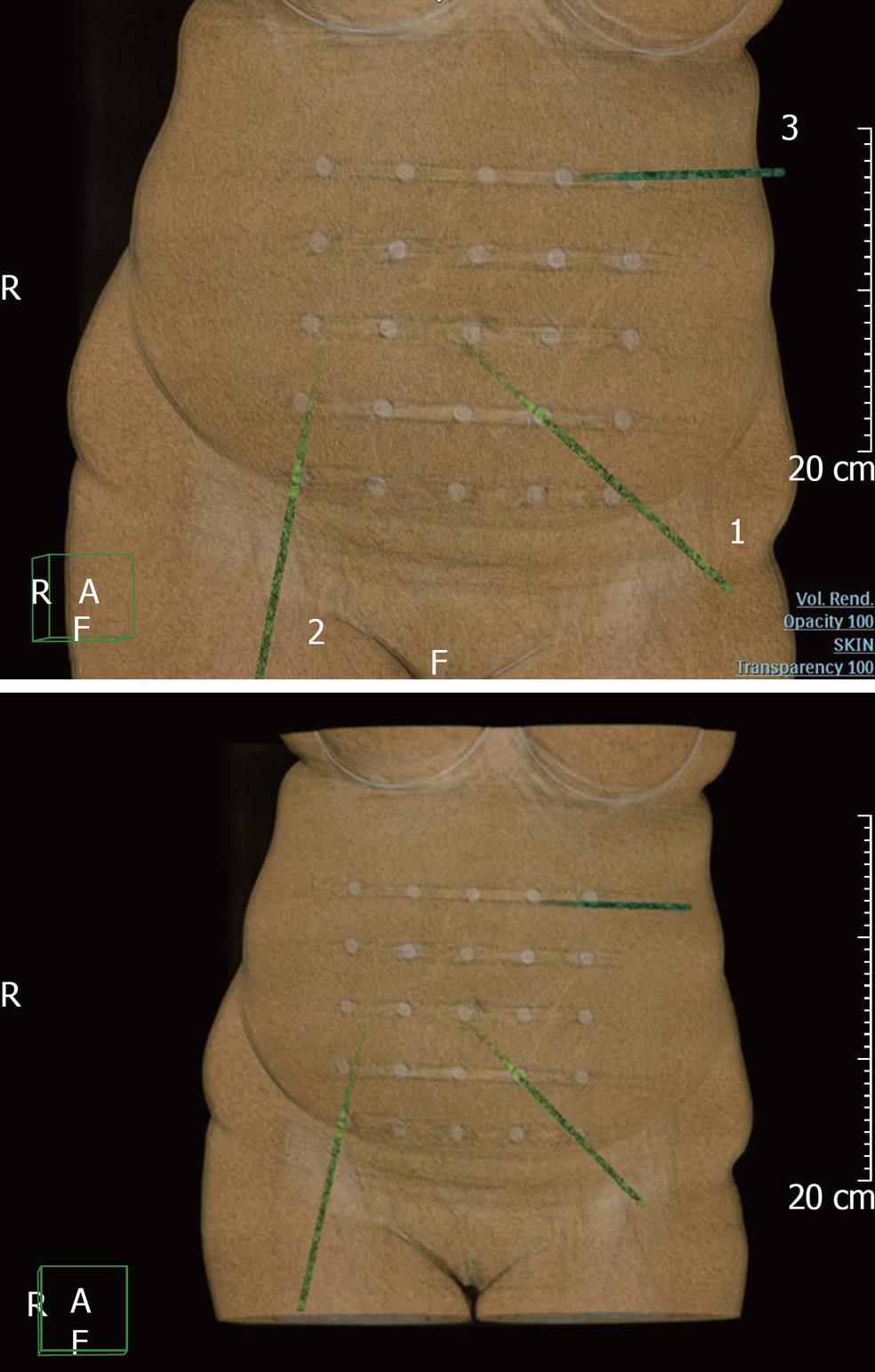
We used a self-constructed navigation net (Figure 2) for more accurate projection of the virtually installed trocar location points on the anterior abdominal wall of the patients. The net cells are 5 cm × 5 cm wide, and roentgen positive tags are installed at the corners of each square. Before starting the unenhanced phase of the multidetector computed tomography (MDCT) examination, we placed the net on the patient’s anterior abdominal wall; the central roentgen positive tag of the net was located on the umbilical area. After adequate virtual installation of the “vectors” on the navigation net background, we achieved the exact overlay of the robotic and assistant ports points onto the roentgen positive tags of the net.
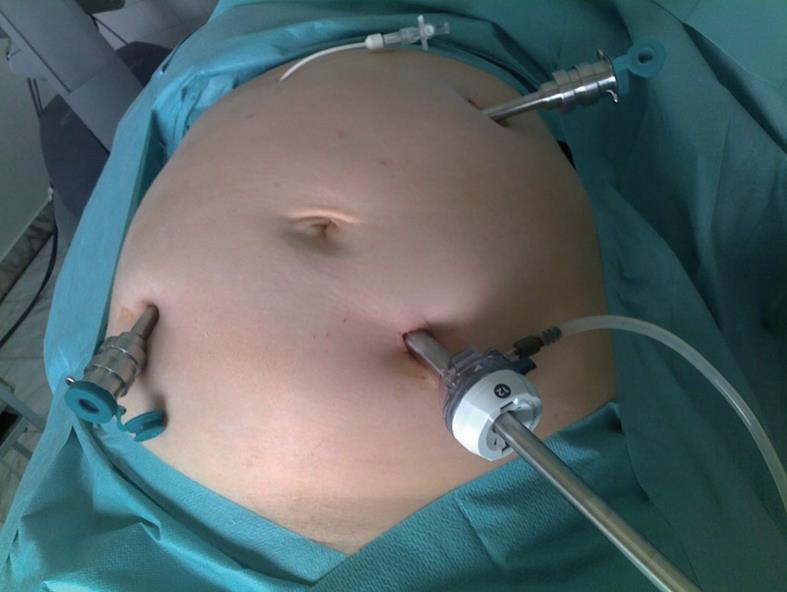
Just before the operation, the navigation net was placed on the anterior abdominal wall (centralized on the umbilical area, as previously described). Having full information about the location of the “virtual” trocar points, relative to the roentgen positive tags of the net, the places for the robotic ports injection were marked (Figure 3).
This method helps to model the main steps of the robot-assisted intervention, to optimize the manipulators’ work and to lower the risk of injury to internal organs. When compared to the open approach, robotic surgery has been shown to have comparable times with the additional benefits of minimally invasive surgery. It becomes possible to analyze the choice of necessary instruments and their arrangement in the robotic manipulators. The properly selected trocar injection sites, virtually planned before surgery, give the opportunity to avoid additional injury of the anterior abdominal wall associated with trocar disposition.
There were no complications registered during surgery or in the post-operative period. All of the interventions occurred without serious blood loss. Comparing two groups of patients with similar operations (Atypical liver resection for non-parasitic liver cysts). The mean duration of the operations completed with 3-D modeling shortened to 90 ± 15 min in comparison with those conducted without the 3-D method at 130 ± 15 min. We were able to reduce robotic arms interference during surgery. The surgical area was optimal for robotic and assistant manipulators without any need for reinstallation of the trocars. The mean duration of the postoperative period was 7 d.
A 56-year-old woman was admitted to the Vishnevsky Institute of Surgery in May 2010 for further examination of a liver cyst (VII-VIII segments) detected during a routine ultrasonographic examination in 2006. The cyst showed an increase in size (up to 8 cm) on a subsequent ultrasound examination. Vital signs where normal and physical examination showed no abnormalities. A laboratory screening including complete blood count, renal function and liver function tests, was normal. We did not observe any tenderness, discomfort or pain during palpation of the abdominal cavity.
Conventional ultrasound revealed a thin-wall fluid structure in the VIII segment of the liver, of irregular round form, with homogenous anechogenic content, 74 mm × 58 mm.
MDCT of the abdominal cavity showed a 8 cm lesion (7 НU), in the VII-VIII segments of the liver, with a homogenous structure and no contrast enhancement; no cyst capsule was observed. We performed preoperative 3-D computed modeling of the robot-assisted intervention. A robot-assisted atypical resection of the VII-VIII liver segments and drainage of the abdominal cavity was carried out during the robotic laparoscopic fenestration of the cyst. The duration of the surgery was 80 min. According to the urgent histological examination, the lesion was a non-parasitic cyst of the liver. In 3-4 h after the intervention the patient could walk unassisted and after 5-6 h water and soft foods were allowed. On the second day the patient was completely mobile, without any food limitations. On the 5th day the sutures were removed, and after the control ultrasound on the 7th day, the patient was discharged from hospital. Thus, the postoperative period was uncomplicated, so there was no need for administration of narcotic analgesics.
The introduction of a virtual modeling procedure into the diagnostic algorithm is a significant contribution to standard robot-assisted interventions, and a new step for its application in intra-abdominal surgery. This method helps to define the optimal location of the robotic ports, taking into consideration anatomical and technical features of individual cases.
With experience, will come standardized schemes for locating robotic and assistant ports on the anterior abdominal wall appropriate to the type of surgical manipulation.
In addition, unlike robot-assisted operations, virtual modeling can be easily provided and does not depend on the localization and the difficulty of surgery. The modeling stage helps to predict and evaluate potential difficulties of the robotic operation in an individual case and gives the opportunity to choose between robot-assistant surgery or another type of intervention.
Robotic surgery is an evolution of conventional laparoscopy potentially offering the surgeon increased dexterity and better vision. With accumulated experience, more and more interventions will be performed robotically, resulting in significant benefit to our patients. The combination of two high-tech methods such as MDCT with 3-D modeling and robot-assisted surgery can increase the safety of mini-invasive surgery and provide maximum use and widespread acceptance of robotics in abdominal surgery.
Robotic techniques are considered “gold standard” in many fields of surgery, including urology, nephrology, gynecology, oncology, etc. However, the appliance of robotic technology in abdominal surgery is limited. The infrequent use of robot-assisted interventions on the peritoneum and retroperitoneal space is due to limited instrument dexterity and the necessity to constantly manipulate in different regions of the abdomen during surgery; it is difficult to transpose the intestinal tract from one floor of the peritoneal cavity to using robotic manipulators. At the same time in abdominal surgery many operations require highly precise movements which can be achieved with the help of robotics, providing three-dimensional vision, tremor filtration, and motion scaling. Therefore, it is important to find a solution that includes the advantages of both methods and provides maximum use and acceptance of robot-assisted technologies in the surgical treatment of various abdominal lesions. The purpose of this study was to determine the effectiveness of using multidetector computed tomography (MDCT) data in the preoperative planning of robot-assisted surgery.
The introduction of a virtual modeling procedure into the diagnostic algorithm is a significant contribution to standard robot-assisted interventions, and a new step for its application in intra-abdominal surgery. This method helps to define the optimal location of the robotic ports, taking into consideration anatomical and technical features of individual cases.
Unlike robot-assisted operations, virtual modeling can be easily provided and does not depend on the localization and the difficulty of surgery. The modeling stage helps to predict and evaluate potential difficulties of the robotic operation in the individual case and provides the opportunity to choose in favor of robot-assistant surgery or any other type of intervention.
With experience standard schemes will be developed for locating robotic and assistant ports on the anterior abdominal wall appropriate to the type of surgery.
The paper describes the usage of MDCT with 3-D modeling to plan port placement for robotic surgery in complex cases. The authors define two groups, with and with out preoperative 3-D modelling and state that operating time was shorter in one group.
Peer reviewer: Tobias Keck, MD, Department of Surgery, Albert-Ludwigs-Universität Freiburg, Hugstetter Str 55, Freiburg 79106, Germany
S- Editor Wang JL L- Editor Hughes D E- Editor Zheng XM
| 1. | Sarle R, Tewari A, Hemal AK, Menon M. Robotic-assisted anatomic radical prostatectomy: technical difficulties due to a large median lobe. Urol Int. 2005;74:92-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Chang CM, Moon D, Gianduzzo TR, Eden CG. The impact of prostate size in laparoscopic radical prostatectomy. Eur Urol. 2005;48:285-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Boczko J, Erturk E, Golijanin D, Madeb R, Patel H, Joseph JV. Impact of prostate size in robot-assisted radical prostatectomy. J Endourol. 2007;21:184-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Chang IH, Byun SS, Hong SK, Lee SE. Assessing the body mass index of patients might help to predict blood loss during radical retropubic prostatectomy in Korean men. BJU Int. 2007;99:570-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Binder J, Kramer W. Robotically-assisted laparoscopic radical prostatectomy. BJU Int. 2001;87:408-410. [PubMed] |
| 6. | Camarillo DB, Krummel TM, Salisbury JK. Robotic technology in surgery: past, present, and future. Am J Surg. 2004;188:2S-15S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Advincula AP, Song A, Burke W, Reynolds RK. Preliminary experience with robot-assisted laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 2004;11:511-518. [PubMed] |
| 8. | Diaz-Arrastia C, Jurnalov C, Gomez G, Townsend C. Laparoscopic hysterectomy using a computer-enhanced surgical robot. Surg Endosc. 2002;16:1271-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Koh CH. A new technique and system for simplifying total laparoscopic hysterectomy. J Am Assoc Gynecol Laparosc. 1998;5:187-192. [PubMed] |
| 10. | Beste TM, Nelson KH, Daucher JA. Total laparoscopic hysterectomy utilizing a robotic surgical system. JSLS. 2005;9:13-15. [PubMed] |
| 11. | Reynolds RK, Advincula AP. Robot-assisted laparoscopic hysterectomy: technique and initial experience. Am J Surg. 2006;191:555-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Fiorentino RP, Zepeda MA, Goldstein BH, John CR, Rettenmaier MA. Pilot study assessing robotic laparoscopic hysterectomy and patient outcomes. J Minim Invasive Gynecol. 2006;13:60-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |