INTRODUCTION
Pancreatic adenocarcinoma remains the fourth leading cause of cancer-related death and is one of the most aggressive malignant tumors with an overall 5-year survival rate of less than 4%. It is the most common pancreatic exocrine neoplasm and accounts for 75%-85% of all pancreatic malignancies. Despite all the progress in the fight against other cancers in recent years, the prognosis for patients diagnosed with pancreatic cancer has remained extremely poor. It is characterized by rapid local spread, persistent invasion of surrounding structures and the early creation of distant metastases. Surgical resection remains the only potentially curative treatment but it is only possible for 15%-20% of patients with pancreatic adenocarcinoma. About 40% of patients have locally advanced nonresectable disease. The remaining patients have metastatic disease. Therefore, about 80%-85% of patients are treated palliatively or neoadjuvantly[1]. Consequently, accurate staging is absolutely necessary to differentiate the resectable patients from the unresectable and new imaging modalities play the critical role in making this differentiation. Multi-detector computed tomography (MDCT) has been widely accepted as the imaging technique of choice for diagnosing and staging pancreatic cancer[2].
In the absence of metastatic disease, which would preclude resection, determination of vascular invasion is an important parameter for estimating pancreatic cancer resectability. Current imaging modalities have improved and allow detection of vascular invasion more accurately. Vascular resection and reconstitution during pancreatic surgery offers a challenge for surgeons; excellent experience in surgical anatomy and vascular anastomosis is required[3]. With improved surgical techniques and advanced perioperative management, vascular resection and reconstruction are performed more frequently; patients thought once to be unresectable are undergoing radical surgery[4]. MDCT with volume-rendering helps surgeons to determine the operability, predict surgical difficulty and prognosis before the operation, but sometimes incorrect predictions lead to inappropriate therapy (Figure 1).
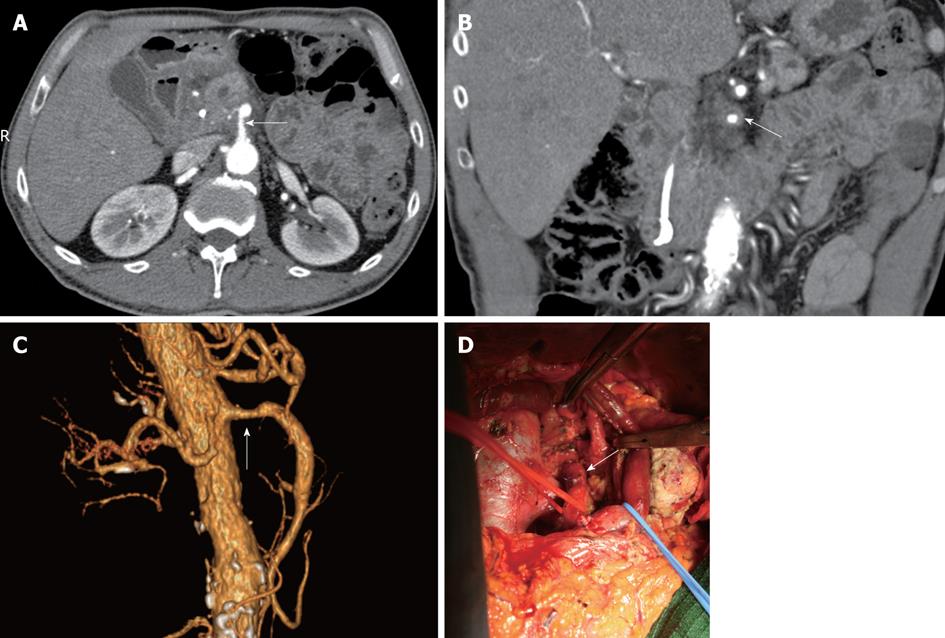
Figure 1 Pancreatic head carcinoma.
A: Axial image showed that the superior mesenteric artery (SMA) was surrounded by more than 50% of the vessel (white arrow) circumference by tumor and the vessel wall appeared infiltrated; B: Coronal oblique plane. Computed tomography image showed tumor encasement (white arrow) of the SMA (more than 180° of the vessel circumference surrounded by tumor); C: Volume rendering (VR) 3D images showed and a segment of the SMA stenosed (white arrow); D: Extended pancreaticoduodenectomy (Whipple procedure) with the pancreatic body excision and superior mesenteric vein resection. At surgical exploration, the common hepatic artery (white arrow) was found not to be invaded by tumor and tumor was successfully resected.
In order to prevent diagnostic mistakes and improve the accuracy in determining the resectability of pancreatic cancer, it is important to be able to distinguish the MDCT signs of local spread, vessel invasion and metastatic disease. The development of modern imaging techniques with improved resolution has allowed preoperative staging of tumors. With these opportunities, MDCT has the capability to improve selection of patients who may benefit from tumor resection, so that significant perioperative morbidity and mortality of unnecessary laparotomies can be avoided[5]. During surgical exploration, invaded vessels may be assessed only when the operation is already quite advanced (section of the pancreas, digestive transaction); therefore, detection of vascular invasion by MDCT is essential for preoperative staging of pancreatic cancer[6].
MARGIN ASSESSMENT
Margin assessment is necessary to determine the adequacy of resection following pancreaticoduodenectomy. Numerous studies have reported that a positive margin of resection is an independent predictor of poor long-term survival following pancreaticoduodenectomy for pancreatic adenocarcinoma[7]. All pancreatic resections should be classified according to the residual disease status (termed “R” factor): R0, no gross or microscopic residual disease; R1, microscopic residual disease (microscopically positive surgical margin with no gross residual disease); R2, grossly evident residual disease. The surgical margins for pancreaticoduodenectomy specimens routinely evaluated by histology include the pancreatic transection margin, the common bile duct (or hepatic duct) transection margin, the gastrointestinal transection margins and the soft-tissue margin adjacent to the proximal superior mesenteric artery (SMA), that is the mesenteric soft tissue and perineural tissue to the right of the proximal 3-4 cm of the SMA; some refer to this margin as the retroperitoneal, mesenteric or uncinate margin.
One of the key requirements for a successful surgery is a negative retroperitoneal soft-tissue margin[4]. While the pancreatic and bile duct margins may be re-resected if the intraoperative frozen section analysis suggests a positive margin, the SMA margin cannot be re-resected because, in general, surgeons do not resect the SMA for adenocarcinoma. Therefore, the most common margin found to be positive after pancreaticoduodenectomy is the SMA margin. Resection with a positive margin (R1 or R2) results in a median survival of 8-12 mo that is not significantly different from those who undergo palliative therapy[8]. А microscopically positive SMA margin is usually due to perineural and lymphatic invasion along the autonomic plexus surrounding the SMA and celiac axis and, for that reason, R1 resections may occur (and be unavoidable) in up to 10%-20% of patients following a grossly negative tumor resection. On the other hand, most R2 resections can be avoided by accurate preoperative MDCT staging.
EXTRAPANCREATIC PERINEURAL AND LYMPHATIC INVASION
Peripancreatic lymphatic networks are frequently involved in pancreatobiliary carcinoma, affecting the prognosis. Nevertheless, little attention has been paid to CT imaging of normal and pathological conditions of peripancreatic lymphatic networks. Sai et al[9] evaluated MDCT images of peripancreatic lymphatic networks invaded by pancreatic carcinoma and compared them with those of normal peripancreatic lymphatic networks using imaging reconstruction with a multiplanar reformation technique. Apart from the region around the pancreatic body and tail, normal peripancreatic lymphatic networks were detected as “linear structures” on MDCT. However, peripancreatic lymphatic invasion by peripancreatic carcinoma was frequently identified as “reticular”, “tubular” or “soft tissue mass” appearance in the peripancreatic fat tissues. Peripancreatic lymphatic invasion by pancreatic carcinoma is more frequently detected around the common hepatic artery (HA), celiac artery, SMA and left para-aortic area. Depending on the tumor location, positive peripancreatic lymphatic invasion is detected most frequently at the area around the common HA in the head region and at the area around the celiac artery in the body and tail regions. Knowledge of MDCT imaging of normal and pathological peripancreatic lymphatic networks is necessary for determining the accurate staging of pancreatic carcinoma.
Radiologists must be familiarized with the common pathways of extrapancreatic perineural invasion of pancreatic adenocarcinoma[10]. 3D volume-rendered MDCT plays a great role in its diagnosis. The perineural plexuses closely follow peripancreatic vessels, which are well depicted by contrast-enhanced 3D volume-rendered imaging, thus facilitating the diagnosis of extrapancreatic perineural invasion of pancreatic cancer.
ASSESSMENT OF TUMOR RESECTABILITY
The most important aim in initial patient evaluation is determining resectability of the primary tumor. High-quality MDCT scanning can be used to classify pancreatic tumors into resectable (Stage I or II), locally advanced, surgically unresectable (Stage III), or metastatic disease (Stage IV). Institutions disagree about the criteria used to classify patients. There is an agreement that patients with a patent portal vein (PV), a superior mesenteric vein (SMV) and a fat plane between the tumor and the superior mesenteric and celiac arteries without distant metastasis are potentially resectable. These patients must be scheduled for immediate surgical resection. Patients who have encasement of the SMA, aorta, celiac artery or inferior vena cava (IVC) are classified as unresectable. The term “encasement” characterizes a tumor-vessel relationship in which a tumor is inseparable from the vessel for > 180° (> 50%) of the circumference of the vessel. The term “abutment” describes a tumor-vessel relationship in which a tumor is inseparable from the vessel for ≤ 180° (< 50%) of the circumference of the vessel[11]. An encasement of the PV or SMV by more than 180° (50%) over an extended segment is also classified as unresectable. It has been verified that the extent of vessel circumference that is in contact with the tumor correlates with the probability of vessel invasion. With ≤ 180° (≤ 50%) abutment, the likelihood of vessel invasion is overall about 40%. With > 180° (> 50%) abutment, the likelihood of invasion is 80%. When encasement by > 270° is present, vessel invasion is present in almost 100% of cases[12].
With recent advances in pancreatic imaging, the distinction between resectable (Stage I or II) and locally advanced (Stage III) disease may be quite hard in selected cases and the term “borderline resectable” is emerging to define these tumors[7]. There is no general agreement in the reported study on the definition or management of borderline resectable pancreatic tumors. The National Comprehensive Cancer Network defined borderline resectable tumors of the pancreas as those with severe unilateral SMV/PV impingement, tumor abutment on SMA, gastroduodenal artery (GDA) encasement up to its origin from the HA, tumors with limited IVC involvement, short-segment SMV occlusion with proximal and distal vein patency and colon or mesocolon invasion[13]. Over the past several years, Varadhachar GR and colleagues from the M. D. Anderson Cancer Center have attempted to develop objective radiographic criteria that define tumors of borderline resectability. These include: those with tumors that exhibit encasement of a short segment of the HA (without evidence of tumor extension to the celiac axis and that is amenable to resection and reconstruction); abutment of the SMA involving ≤ 180° (≤ 50%) of the arterial circumference or short-segment occlusion of the SMV, PV or the SMV-PV confluence with a suitable option available for vascular reconstruction using patent veins above and below the area of tumor involvement[14].
Chun and colleagues from Fox Chase Cancer Center add new data to the work of Varadhachary and pay special attention to the relationship of the tumor to the SMV and SMV-PV confluence using the Ishikawa classification system[15]. They suggest that a unilateral shift or narrowing of the SM-PV confluence, caused by tumor, should be considered as a borderline resectable case.
Recently, an expert consensus statement defined the following criteria for borderline resectable PV-SMV involvement: tumor-associated deformity, ≥ 180° (≥ 50%) encasement or reconstructible short segment occlusion[16]. Therefore, there is no consensus on what degree of venous impingement constitutes borderline resectability. A generally accepted definition of borderline resectable pancreatic cancer is needed and must be consistently applied. Not only one that would give an opportunity to compare data between institutions, but most importantly, would lead to better patient management.
At present, there are various definitions of borderline resectable pancreatic cancer; however, a working definition includes those tumors at high risk for having microscopic residual disease after surgical resection (R1). Therefore, operating on these patients implies close proximity of tumor to the retroperitoneal margin.
NEOADJUVANT TREATMENT
To increase the chance of achieving a margin-negative resection (R0) as well as negative lymph node involvement, both of which have been shown to have positive prognostic value on long-term outcome following surgery, Varadhachary et al[14] advocate neoadjuvant treatment with systemic chemotherapy followed by chemoradiation in patients with borderline resectable tumors defined by the extent of local tumor growth on MDCT. Preoperative chemoradiotherapy is used to treat potentially positive margins - that is, downstage from an R1 margin to R0 and to provide early treatment of micro-metastatic disease, which often exists in locally advanced tumors. Brown et al[17] reviewed the outcomes of BR patients who underwent neoadjuvant therapy followed by surgery and found an 85% rate of R0 resection. Katz et al[18] found that 41% of their BR patients treated with neoadjuvant therapy ultimately underwent surgery with grossly negative margins and 94% of these had microscopic negative margins. Median survival was 40 mo for patients undergoing resection, compared to 13 mo for patients not receiving surgery. Chemoradiotherapy also allows a period of time, usually 6 wk to 3 mo, to ascertain the tumor aggressiveness and exclude patients who have unresponsive and aggressive disease from surgery[14]. Preoperative therapy is associated with fewer positive lymph nodes in the pathological specimen despite larger tumor size on preoperative imaging[19]. Thus, preoperative therapy may convert nodes that were originally positive to negative or undetectable. In addition, chemotherapy with or without radiation therapy, delivered before surgery, is often better tolerated, as surgical recovery does not complicate the delivery of treatment. Preoperative neoadjuvant therapy moves surgery to the last step in the management of pancreatic cancer; however, surgery retains a fundamental role[20]. In the past, pancreatic surgery was associated with a high mortality, raising questions about the convenience of that approach. Improvements in perioperative management, surgical technique and postoperative care have led to low rates of perioperative mortality with pancreaticoduodenectomy. Modern surgical approaches include the involvement of vascular surgeons in the process to assist with venous resection and reconstruction, reducing complications and allowing for margin-negative resection even in cases of vessel abutment[21]. Optimally, these operations should be performed at high-volume centers, which have lower operative mortality and morbidity rates. Research has shown that the risk of major complications and mortality are significantly reduced if the pancreatic surgery is performed at a center that performs a high volume of these procedures. In the absence of other information about the quality of surgery at the hospitals near them, patients undergoing pancreatic surgery can significantly reduce their risk of operative death by selecting a high-volume hospital[22].
VASCULAR INVOLVEMENT
Seeking for a radical resection MDCT may prevent surgical exploration. It is regarded as the most important non-invasive staging technique[23]. However, MDCT criteria for vascular ingrowth differ between medical centers. Lu et al[24] (1997) reported a CT grading system for vascular involvement. The authors suggest a threshold of 180° (50%) circumferential contiguity of tumor to vessel as a criterion of unresectability for pancreaticoduodenectomy. Despite the fact that this analysis was carried out mainly for venous vessels, more recent studies propose to use the same criterion described by Lu et al[24] for the detection of arterial involvement[25]. Horton and Fishman gave priority to visualization of any alteration of artery caliber with associated tumor, more than to the percentage of vessel wall surrounded by tumor[26]. At the same time, Valls et al[2] considered any grade of artery to tumor contiguity as criterion of unresectability.
To improve the accuracy of MDCT in estimating invaded vessels, it is necessary to evaluate the MDCT signs of arterial and venous invasion separately because the particular features of peripancreatic arterial and venous invasion on MDCT are different[27]. The main reason for the different MDCT features of arterial and venous invasion is that the wall of the vein is much thinner and weaker than the wall of the artery. When veins are surrounded and infiltrated by tumor, the wall is influenced to be irregular and the caliber becomes narrowed. Simultaneously, the flow rate in veins is slower and the tumor often penetrates the wall of the vein to form a cancer thrombus, causing vascular occlusion. As the wall of the artery is significantly thicker and more flexible than the wall of the vein and the caliber of the artery is smaller by itself, even when the arteries are encased in tumor, the wall remains regular. Li et al[27] reported that in their study, some arteries were found not to be invaded at surgical exploration, although they were surrounded by more than 50% of the vessel circumference and their caliber remained unchanged and the wall was regular. Hough et al[28] found that tumors in the head of the pancreas could cause a teardrop appearance of the SMV in axial images. They believed that the teardrop SMV sign was a reliable indicator of irresectability. In their retrospective study, teardrop SMV was the only sign of irresectability in 13 of 17 patients. Apparently, the teardrop SMV sign is a consequence of either direct tumor infiltration or peritumoral fibrosis, which changes the vessels normal round shape. Invaded arteries may appear stretched on MDCT images because of the presence of focal tissue fibrosis, which may accompany pancreatic cancer[26]. Lepanto et al[29] found that CT-angiography significantly increased the ability to identify venous invasion but did not improve the detection of arterial invasion. Based on our study, the image quality of MDCTA is significantly higher than CTA since it gives the opportunity for multiplanar reconstructions, volume-rendering with three-dimensional visualization of the relationship between tumor and vessel. However, it is important to review axial images as well as they are more capable of demonstrating the contiguity of tumor to vessel and change in caliber of the vessel wall.
Regarding isolated venous involvement, most pancreatic surgeons do not consider it as a contraindication for surgery as they perform partial venous resection with end-to-end anastomosis or using bypass grafts. Venous resections and reconstructions are increasingly performed as the technique is feasible and reliable, with а morbidity and mortality similar to pancreaticoduodenectomy without vascular reconstruction[4]. The invasion of the superior mesenteric or PV is not itself a criteria of unresectability[30]. Contrary to venous involvement, arterial invasion has traditionally been considered to be inoperable in patients with pancreatic adenocarcinoma because of the high morbidity and mortality rates associated with arterial resection and reconstruction. Not to mention, arterial invasion usually includes extensive spread with involvement of the mesenteric neural plexus, making radical resection oncologically insubstantial because of the frequent finding of positive margins[31]. As surgical techniques for the treatment of pancreatic cancer evolve and with the improved accuracy of MDCT in detecting vascular invasion, resection of invaded arteries has become a questionable issue. Many authors regard this invasion as a contraindication to surgery[31,32] and yet, in carefully selected cases, early arterial invasion is considered resectable[14]. Furthermore, some surgeons attempt extirpations, including resections of arteries and veins with vascular reconstructions if vascular invasion is present[33].
PANCREATIC SURGERY
In the 1970s, regional pancreatectomy advocated by Fortner was associated with extremely high morbidity and mortality rates, with no influence on long-term survival[34]. With the installation of a multidisciplinary approach, improvements in imaging modalities, surgical proficiency and perioperative care reduced mortality rates and improved 5-year survival rates are now accomplished following resection with major vessel reconstruction in high-volume centers[22]. Since the 1970s, there have been considerable developments in radiological capabilities and surgical techniques, resulting in improved preoperative staging, better patient selection and reduced surgical morbidity and mortality[14,35]. Perioperative mortality rates of less than 4% following pancreaticoduodenectomy are now achieved in high-volume centers[36].
Involvement of the common HA and the celiac trunk are the most prevailing forms of vascular invasion by tumors of the distal pancreas and for a long period of time this was considered as a contraindication to surgery. In 1953, Appleby proposed en bloc resection of the celiac trunk with distal pancreatectomy and total gastrectomy for the treatment of locally advanced gastric cancer[37]. In 1976, this operation was first adapted to the resection of cancer of the body and tail of the pancreas by Nimura et al[38]. Patients with cancer of the body and tail of the pancreas present with more advanced disease than patients with adenocarcinoma in the head of the pancreas. Most of these patients remain asymptomatic until they have unresectable locally advanced or metastatic disease. Liu et al[39] recently reported that two times as many patients with left-sided tumors (53%) as right-sided tumors have unrecognized distant disease. On the contrary, even patients with locally advanced disease may be candidates for surgery.
In 1991, Hishinuma performed two distal pancreatectomies with resection of the celiac axis with gastric preservation, named the modified Appleby procedure[40]. Since the report by Nimura et al[38], about 40 cases of the stomach-preserving distal pancreatectomy with en bloc resection of the celiac axis have been reported. Hirano et al[41] reported a high R0 resectability rate (91%) with distal pancreatectomy with en bloc celiac axis resection, accompanied by a good 5-year survival rate (42%). Undoubtedly, this kind of surgery should be performed in the absence of distant metastasis when it is likely to control retroperitoneal invasion and blood flow from the SMA to the HA is ensured. Confirming blood flow to the stomach and liver after celiac artery resection has the highest value when performing the operation. Blood flow to the stomach and liver is maintained using an arcade through the inferior pancreaticoduodenal artery, GDA and proper HA from the SMA. Therefore, examination and confirmation of blood flow before, during and after the operation is important, to prevent postoperative hepatic failure and stomach necrosis. MDCT angiography or abdominal angiography can be useful in preventing postoperative hepatic failure and stomach necrosis, by examination and confirmation of blood flow before, during and after the operation.
Celiac or hepatic invasion, detected during surgery, can resected and reconstructed, either by direct anastomosis, by interposition of a venous graft (for example, reverse saphenous or internal jugular vein) or with a prosthesis. An arterial graft (for example, the splenic artery) can also be used[42]. These techniques seem feasible and relatively reliable, with a mortality of 5%[33].
Concerning the modified Appleby’s operation (en-bloc resection of the celiac trunk with distal pancreatectomy) for locally advanced pancreatic body and tail cancers, several Japanese groups recommend an extended resection of the celiac trunk, splenic artery, common HA and/or SMA, with an overall 5-6 mo survival. To avoid an acute hepatic insufficiency, hepatic vascularization must be constantly evaluated during surgery and, if necessary, compensated[43]. Moreover, different studies have reported that R0 resections are associated with considerable improvement in survival rates as opposed to palliative therapy and that patients directed to expanded vascular resections almost equals the survival rates of patients undergoing standard pancreatectomy[41,44].
Gagandeep et al[45] (2006) presented their experience with a modification of the Appleby operation in central pancreatic cancers involving the celiac trunk. The authors conclude that extended pancreatectomy with celiac axis resection can result in prolonged survival and should be considered in central and distal pancreatic cancers invading the celiac trunk.
Concerning invasion of the SMA and with an isolated arterial jejunal branch, the artery is reconstructed either by direct anastomosis, or by anastomosis to the aorta, after the clamping of the SMA is ensured by injecting heparin there.
Regarding an invasion of the HA, techniques of reconstruction require a venous graft (jugular, reverse saphenous, gonadic veins) or prosthesis, or an arterial graft (splenic, gastro-epiploic, gastroduodenal)[42].
Kondo et al[46] reported cases of distal pancreatic cancer with invasion of the celiac trunk and the common HA. In order to ensure a distal pancreatectomy with en bloc resection of the celiac trunk, without hepatic ischemia, the authors suggest obtaining a collateral pathway from the SMA by embolization of the HA.
Makary et al[47] (2005) described more conventional resection and reconstruction manipulations, using the GDA. Aiming for a complete resection, distal celiacopancreatectomy remains the only radical option for locally advanced distal pancreatic cancer. Moreover, the operation offers possible pain relief due to the resection of the celiac plexus and preserves the entire stomach since there is no need for gastroenterostomy[47]. Accurate staging is required and is successfully accomplished by MDCT. CT and MRI reliably display relevant anatomic variants of the arteries and their relationship to the tumor, which is essential for preoperative management of patients with pancreatic cancer[48].
VARIANTS OF VASCULAR ANATOMY
It is important to recognize arterial variants in the preoperative planning of extended pancreatic resections. The celiac and mesenteric arterial anatomy variants are fairly common and are of great significance in planning extended pancreatic resections. The importance of such awareness is conditioned by the necessity of wide periaortic and periarterial dissection and extensive vessel skeletonization during extended pancreaticoduodenectomy and extended distal pancreatectomy. This knowledge also facilitates upper abdominal surgery and helps avoid iatrogenic injury when the organ relationship is changed, visualization is limited or when the organs are too susceptible to ischemia, even if temporary[49].
In different fields of surgery, knowledge of celiac and mesenteric arterial variants is of great significance. MDCT examination not only clearly delineates the course of the aberrant vessels, but also reveals the arterial stenosis and occlusions which may be critical if undiagnosed or if diagnosed during or after surgery. Knowledge of the vascular anatomy is of great value because the organ relationship is usually changed and direct visualization of the surgical field is often limited in patients with large pancreatic tumors, in borderline resectable cases, in obesity, prominent local inflammation after biliary stenting and dense adhesions after prior surgery[50]. Preoperative knowledge of variant arterial anatomy may obviate extensive dissection to identify the vessels and avert vascular damage or, vice versa, allows the surgeon to excise vessels infiltrated by a tumor knowing in advance that it is accessible, thus avoiding an erroneous judgment about tumor unresectability.
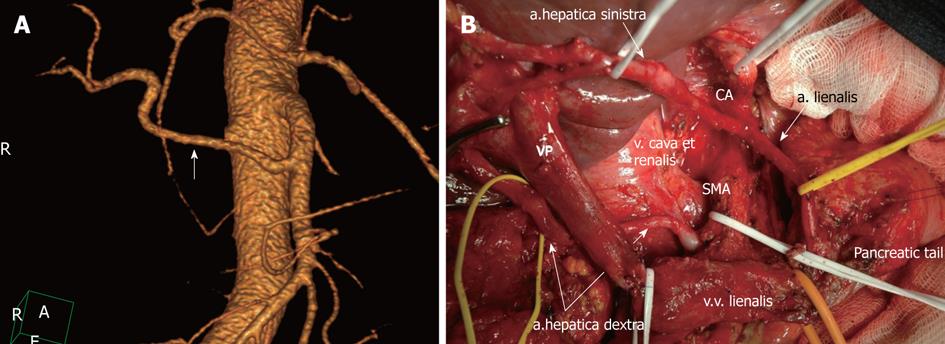
The most common variation in the hepatic arterial anatomy is the presence of a replaced right HA originating from the SMA (Figure 2). This has been reported to occur in 11%-21% of patients[51].
Figure 2 Michel’s type II celiac-mesenterial anatomy.
A: 3D computed tomography angiographic image. Replaced right hepatic artery (white arrow); B: View of the operating field after the extended pancreaticoduodenectomy. Note a the presence of a replaced right hepatic artery originating from the superior mesenteric artery (SMA). CA: Celiac artery.
There were reported cases of the hepatomesenteric trunk and replaced right HA passed laterally behind the PV and behind or above the pancreatic head and entered the hepatoduodenal ligament posterolaterally to the bile duct[49]. However, there have been reports of such vessels traveling behind or through the head of the pancreas, in which case they are susceptible to damage[52]. A replaced hepatomesenteric trunk and right HA should be recognized and preserved.
In general, 3D CT angiography makes the surgeon more confident when working in the perivascular and periaortal spaces. 3D rendering and multiplanar reformations help in determining the exact site and extent of vascular invasion. It is also very important for the radiologist to identify variant vascular anatomy that may increase or decrease the chances of successful surgical resection. There are venous and arterial anatomic variants that may make vessel reconstruction impossible (for example, multiple jejunal branches that insert high on the SMV close to the SMV-PV confluence; a low takeoff of the common HA from the celiac axis with an aberrant course inferior in relation to the PV; a completely replaced HA from the SMA coursing through the retroperitoneal soft-tissue margin). Again, there are venous and arterial anatomic variants that may make resection more attainable (for example, the HA arising separately from the aorta with the celiac artery only supplying splenic and GDAs; accessory right and left hepatic arteries arising from the superior mesenteric and left gastric arteries, respectively)[4].
LOCAL RECURRENCE
Despite the development of modern imaging techniques, radical surgery and adjuvant chemotherapy, hepatic and peritoneal tumor recurrence is frequent and considerably affects patient survival[40,41]. Recurrence of pancreatic cancer is detected in up to 85% of patients within 2 postoperative years after a potentially curative (R0) resection. The most frequent cause of relapse is retroperitoneal tissue infiltration, including neural and lymphatic invasion[53]. A close follow-up of patients with pancreatic resections (MDCT 3 and 6 mo after surgery) and aggressive management of local recurrences are recommended[54]. MDCT is the imaging modality of choice in determining postoperative local and distant abdominal recurrence of pancreatic adenocarcinoma, as well as metastatic lymphadenopathy. Detection of an irregular mass or infiltrations of the soft tissue in the area of resection, extending along the superior mesenteric vessels or the PV, are lesions highly suggestive of local tumor recurrence. The key point in evaluating these patients is to differentiate postoperative inflammation from a tumor relapse. Kim et al[55] suggest that encasement of the peripancreatic vessels, the loss of a distinct fat plane between mesenteric vessels, and the presence of adjacent bowel obstruction are indicators of recurrent tumors rather than postoperative inflammation. An increase in the size of the mass on serial follow-up imaging is used to differentiate local tumor relapse from postoperative inflammatory change or pseudotumor[55]. It is also recommended to correlate the findings on MDCT with the clinical findings of increased carbohydrate antigen 19-9[56].
PITFALLS
Classifying patients by tumor resectability on CT helps to estimate the tumor stage and to prognosticate survival rates of these patients more precisely[57]. The main limitation of CT is its low sensitivity for low-volume hepatic or peritoneal metastases. Despite the fact that the use of MDCT with thinner slices collimation led to the better visualization of liver lesions and improved their detection rate, the detection of an early metastatic disease to the liver is usually difficult[56]. In preoperative staging of the patients with suspected or biopsy-proven pancreatic cancer, it is recommended to include CT imaging of the pelvis to evaluate for peritoneal disease[7]. Discovery of early peritoneal involvement continues to be a difficult part in determining the resectability of pancreatic cancer. Small peritoneal metastases, as well as local infiltration of the peritoneum are difficult to distinguish because of their small size. The study of Valls et al[2] reported no cases of peritoneal metastases. Diehl et al[58] disclosed one case of missed peritoneal involvement by MDCT. Multiple studies show that peritoneal metastases are discovered approximately in 5%-7% of patients at surgery[26]. About 20%-35% of patients deemed resectable before surgery actually have missed on MDCT peritoneal involvement discovered at laparoscopy or laparotomy[59]. To avoid unnecessary laparotomy, many centers include laparoscopic staging in the algorithm for managing patients with radiological resectable pancreatic cancer. Biopsy of suspicious liver or peritoneal metastases missed or not differentiated by MDCT can be performed. Peripancreatic spread of tumor with involvement of the mesocolon can be determined. The development of modern imaging techniques with improved resolution has reduced the benefit of laparoscopy in staging patients with pancreatic cancer. Nevertheless, laparoscopy continues to upstage patients with preoperative MDCT confirmed resectable pancreatic cancer with a benefit in determining resectability of 15%-20%[60]. Peritoneal cytology increases the sensitivity of laparoscopy in preoperative staging, upstaging an additional 8% of patients with positive cytology and advanced unresectable pancreatic adenocarcinoma[61].
Previous studies using standard spiral CT reported low accuracy in detecting lymph nodes metastases, ranging from 16.7%[2] to 54%[58]. The use of MDCT with improved resolution did not result in a significantly heightened detection rate. This may be due to the absence of specific signs of malignancy at CT (MDCT) because lymph node size does not always determine its malignancy. Zamboni et al[53] advocates considering metastatic lymph nodes if the short-axis diameter is 1 cm or larger. Typically in clinical practice, the low detection rate of metastatic regional lymph nodes has a limited value since the peripancreatic lymph nodes can be resected en bloc with the tumor.
CONCLUSION
In the past, determination of pancreatic cancer resectability was made at surgical exploration. The development of modern imaging techniques has allowed preoperative staging of patients.
With improved surgical techniques and advanced perioperative management, vascular resection and reconstruction are performed more frequently; even major vessel involvement is not always considered a contraindication to surgery of borderline resectable pancreatic adenocarcinoma when a radical (R0) resection is likely. Nonetheless, there is much room for improvement in all aspects of treatment for pancreatic cancer. This refers to the absence of a unified definition of borderline resectable pancreatic adenocarcinoma and to the need of an optimal treatment algorithm for this distinct stage of disease, including neoadjuvant treatment to all patients with borderline resectable tumors when an incomplete (R1 or R2) resection is anticipated. The potential for a positive margin resection (R1, R2) can be minimized by careful attention to patient selection and operative technique. When attempting heroic surgery, a realistic approach concerning the patient’s age and health status, probability of recovery after surgery, perioperative morbidity and mortality and life quality after tumor resection is necessary.
Peer reviewer: Calogero Iacono, MD, Professor, Department of Surgery, University Hospital “GB Rossi”, Verona 37134, Italy
S- Editor Wang JL L- Editor Roemmele A E- Editor Zheng XM