Published online Oct 27, 2012. doi: 10.4240/wjgs.v4.i10.234
Revised: August 15, 2012
Accepted: August 25, 2012
Published online: October 27, 2012
AIM: To examine the feasibility of prospective, real-time outcome monitoring in a United Kingdom oesophago-gastric cancer surgery unit.
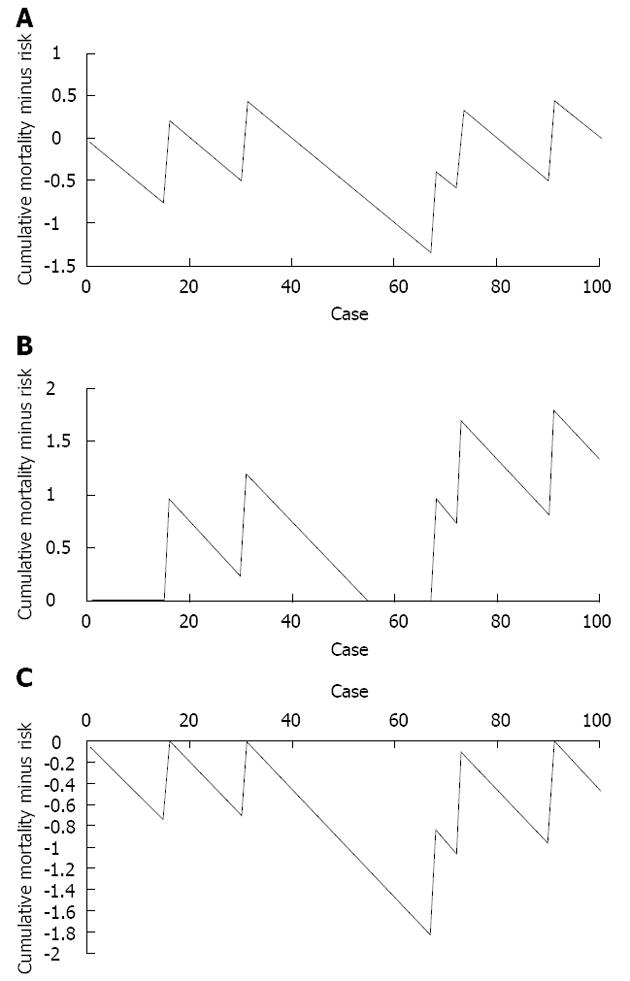
METHODS: The first 100 hybrid (laparoscopic abdominal phase, open thoracic phase) Ivor-Lewis oesophagectomies performed by a United Kingdom oesophago-gastric cancer surgery unit were assessed retrospectively using cumulative sum (CUSUM) techniques. The monitored outcome was 30-d post-operative mortality, with the accepted mortality risk defined as 5%. A variable life adjusted display (VLAD) was constructed by plotting a graph of cumulative mortality minus cumulative mortality risk on the y axis vs sequential case number on the x axis. This was modified to a zeroed VLAD by preventing the plot from crossing the y = 0 axis - essentially creating two plots, one examining trends where cumulative mortality was higher than mortality risk (i.e., worse than expected outcomes) where y > 0, and vice versa. Alert lines were set at y = ± 2. At any point where a plot breaches an alert line, it is felt that the 30-d post-operative mortality rate has deviated significantly from that expected and an internal review should be performed.
RESULTS: One hundred cases were assessed, with a mean age of 66.4 years, mean T stage of 2.1, and mean N stage of 0.48. Three cases were commenced using a laparoscopic technique and converted to open surgery due to technical factors. Median length of inpatient stay was 15 d. The crude 30 d mortality was 5% and the incidence of clinically significant anastomotic leak was 6%. The VLAD demonstrated a plot of cumulative mortality minus cumulative mortality risk (i.e., 5% per case) which remained in the range -1.4 to +0.5 excess mortalities. With the alert set at two greater or fewer than predicted mortalities, this method does not approach the point of triggering internal review. It is however arguable that a run of performance that is better than expected, causing the plot to be well below y = 0, would mask a subsequent run of poor performance by requiring a rise of greater than two excess mortalities to trigger the alert line. The zeroed VLAD removes this problem by preventing the plot that is examining above expected mortality from passing below y = 0, and vice versa. In this study period, no audit triggers were reached. It is therefore possible to independently assess runs of good, or poor performance and so target internal audit to the appropriate series of cases. It is important to note this technique allows targeted internal review, in response to both above and below average outcomes. This study has demonstrated the feasibility of prospective outcome monitoring using the above techniques, actual real-time implementation has the potential to pick up and reinforce good practices when performance is better than predicted, and provide an early warning system for when performance falls below that predicted. Further development is possible, including more patient specific risk adjustment using the oesophago-gastric surgery physiological and operative severity score for the enumeration of mortality and morbidity score.
CONCLUSION: CUSUM techniques provide a potential method of prospective, real-time outcome monitoring in oesophageal cancer surgery.
- Citation: Roberts G, Tang CB, Harvey M, Kadirkamanathan S. Real-time outcome monitoring following oesophagectomy using cumulative sum techniques. World J Gastrointest Surg 2012; 4(10): 234-237
- URL: https://www.wjgnet.com/1948-9366/full/v4/i10/234.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v4.i10.234
High risk surgery is, in the United Kingdom, undergoing a process of centralisation to high volume centres[1-6]. This has been driven by an increased focus on short and long term surgical outcomes and evidence showing better results from high volume centres. The authors have applied a real-time, risk-adjusted measure of outcomes after Ivor-Lewis oesophagectomy, with the aim of immediately triggering internal audit following a period of worsening outcomes.
In the United Kingdom, cardiac surgery has pioneered the development of real-time outcome monitoring, followed by transplant surgery, obstetrics, surgical education and most recently plastic and burns surgery[7-16]. Cumulative sum (CUSUM) techniques, incorporating risk-adjustment using variable life adjusted displays (VLADs), have been demonstrated to provide a valid, continuous measure of surgical outcomes[7]. They allow for ongoing assessment of outcomes against the acceptable standard, both better and worse, and early assessment when they are significantly different to those expected. It is crucial to note that this can pick up on periods of time when a unit is performing above average, as well as below average, and give the opportunity to reinforce those contributing factors. For periods of below average performance, the VLAD performs as an early warning system, allowing early recognition and management of contributing factors.
Collins has examined the role of VLADs and other CUSUM techniques in monitoring outcomes after oesophagectomy, utilising the Scottish audit of gastro-oesophageal cancer services data set[17]. That retrospective study has highlighted the feasibility of VLADs in monitoring the results of individual units.
The authors present the results of applying VLADs and zeroed-VLADs to monitoring outcomes in a single United Kingdom oesophago-gastric cancer centre and a mechanism for implementing this technique as a prospective, real-time measure.
The results of the first 100 hybrid (laparoscopic abdominal phase, open thoracic phase) were assessed using CUSUM techniques. A VLAD of 30 d post-operative mortality, using an acceptable mortality risk of 5%, was created as detailed below. The results from this analysis were used to validate a tool for monitoring prospective outcomes using zeroed-VLAD plots.
A VLAD is constructed by plotting the cumulative mortality minus predicted mortality on the y axis, against cumulative number of cases on the x axis (Figure 1A). The generated plot is extended every case and should follow the y = 0 line if the actual rate of the measured outcome matches that of the predicted rate. If the actual rate is higher than predicted, the plot rises, and if the actual rate is lower than predicted, it falls. “Alert” lines are set at y = 2 and y = -2, and when the plot crosses the alert line the outcome rate is regarded as having deviated enough to warrant review.
It is apparent that a good “run”, as seen by the large negative deflection in Figure 1A, could mask a subsequent poor run, as that run would start below y = 0 and require more than two excess mortalities to trigger a review. This problem can be eliminated by preventing the plot from becoming negative, so y > 0 at all times (Figure 1B). This is enabled by the simple expedient defined by the equation:
= if y < 0, y = 0
Essentially, only runs of worsening outcome are examined. Likewise, the mirror image plot, where y < 0 at all times, can be used to trigger review when results are significantly better than predicted (Figure 1C).
Data were collected and analysed using Microsoft Excel 2007. Construction of the relevant plots was performed as described elsewhere in this study.
The patient and disease load, and non risk-adjusted outcomes were as expected for a United Kingdom upper gastrointestinal tract (UGI) cancer centre (Table 1).
| Demographics | Data |
| Age, yr (mean ± SD) | 66.4 ± 9.2 |
| Mean T stage | 2.1 |
| Mean N stage | 0.48 |
| Median length of stay, d (25%-75%) | 15 (13-20) |
| Crude 30-d mortality | 5.4% |
| Anastomotic leak rate | 6.5% |
The zeroed-VLAD (Figure 1B) demonstrates two spikes in mortality, neither of which crosses the alert line at y = 2 excess mortalities. The zeroed-VLAD for better than average results (Figure 1C) demonstrates one “run” of good results which does not cross the alert line at y = -2 (i.e., two fewer mortalities than expected).
The authors demonstrate the first published implementation of VLADs to monitor oesophagectomy outcomes within an UGI cancer centre. While this technique has gained widespread acceptance in cardiac surgery, its uptake in other specialties is still taking place. The authors believe that this could provide a robust tool for monitoring outcomes in other high risk operations and are using this to monitor the outcomes in their unit.
The benefit of targeted real-time internal review, compared to the current practice of retrospective assessment, is in the opportunity to identify and address causes of poor results early. This clearly prevents potentially deleterious practices continuing longer than necessary and could improve patient outcomes. Likewise, the opportunity to flag up periods of better than expected performance allow for identification, standardisation and dissemination of exceptional practices.
This study has used a 5% mortality risk as the acceptable standard. This is however being further modified by the centre and the authors will be assessing risk on an individual basis using the oesophagogastric physiological and operative severity score for the enumeration of mortality and morbidity score[18]. It is thought that more accurate risk assessment, in a similar fashion to cardiac surgeons, will provide a further refinement to this technique.
While some within the profession may resist the concept of rigorous and open outcome monitoring, it is likely to become the standard as healthcare outcomes become more closely observed[19]. Indeed, this is not intended as a punitive measure, but as a means to identify early those random factors that have a significant impact upon outcome. This however raises the issue of how to manage the review process, particularly that when outcomes are poorer than desired. While not further elaborated here, the authors propose a checklist-based approach to review of contributing cases. This is due to recent work in healthcare checklists showing a benefit in removal of personal factors, and ensuring that all potential issues are covered in a standardized fashion[20-26].
This tool, if widely implemented, could well stimulate close monitoring and therefore improvement of oesophagectomy outcomes.
It is unusual in the field of healthcare for outcomes to be monitored in a prospective, real-time fashion. The potential of real-time outcome monitoring, with the early detection of both good and poor outcomes, is the improvement of medium and long term outcomes. Currently, in the United Kingdom, only cardiac surgery rigorously monitors surgical outcomes in this fashion, using cumulative sum (CUSUM) techniques. This study examines the feasibility of adapting CUSUM techniques to monitoring of 30 d mortality in oesophago-gastric surgery.
CUSUM techniques are gradually being introduced to healthcare, with increasing understanding of their role in outcome monitoring. This is being explored in many fields, particularly surgery, obstetrics and skills training.
Previous work has defined the role of risk-adjusted outcome monitoring using variable life adjusted displays (VLADs). These essentially track the outcome rate compared to the acceptable, or predicted outcome rate and trigger when the plot deviates to a significant extent. Other studies are examining the role of these techniques in transplant surgery, obstetrics, surgical education and other medical fields, although to date only cardiac surgery has successfully implemented a prospective CUSUM based means of monitoring surgical outcomes.
If feasible, the techniques outlined hear could form the basis for prospective, real-time outcome monitoring in oesophago-gastric surgery. This is potentially a significant improvement on current retrospective outcome assessment.
CUSUM is a statistical control technique which monitors cumulative outcome rate vs sequential cases, essentially creating a plot with the gradient being the outcome rate. VLADs are modifications of the basic CUSUM plot, plotting cumulative outcomes minus cumulative expected outcome rate against sequential cases. A VLAD for a process which has an outcome rate as expected should demonstrate a horizontal line, a process with above expected outcome rate is a rising line, and vice versa.
This is a good descriptive study in which authors examine the feasibility of prospective, real-time outcome monitoring in a United Kingdom oesophago-gastric cancer surgery unit. The results are interesting and suggest that CUSUM techniques provide a potential method of prospective, real-time outcome monitoring in oesophageal cancer surgery.
Peer reviewer: Liang-Shun Wang, MD, Professor, Vice Superintendent, Shuang-Ho Hospital, Taipei Medical University, No. 291 Jhongjheng Rd., Jhonghe City, New Taipei City 237, Taiwan, China
S- Editor Gou SX L- Editor A E- Editor Xiong L
| 1. | A policy framework for commissioning cancer services: A report by the Expert Advisory Group on Cancer to the Chief Medical Officers of England and Wales. London: Department of Health 1995; . |
| 2. | Bachmann MO, Alderson D, Edwards D, Wotton S, Bedford C, Peters TJ, Harvey IM. Cohort study in South and West England of the influence of specialization on the management and outcome of patients with oesophageal and gastric cancers. Br J Surg. 2002;89:914-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Dickson GH, Waters R, Bull J, Kaul V, Sitzia J. Should we continue oesophageal surgery in a district general hospital? A review of 200 consecutive cases. Ann R Coll Surg Engl. 2001;83:167-171. [PubMed] |
| 4. | Swisher SG, Deford L, Merriman KW, Walsh GL, Smythe R, Vaporicyan A, Ajani JA, Brown T, Komaki R, Roth JA. Effect of operative volume on morbidity, mortality, and hospital use after esophagectomy for cancer. J Thorac Cardiovasc Surg. 2000;119:1126-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 254] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 5. | Gillison EW, Powell J, McConkey CC, Spychal RT. Surgical workload and outcome after resection for carcinoma of the oesophagus and cardia. Br J Surg. 2002;89:344-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Branagan G, Davies N. Early impact of centralization of oesophageal cancer surgery services. Br J Surg. 2004;91:1630-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Lovegrove J, Valencia O, Treasure T, Sherlaw-Johnson C, Gallivan S. Monitoring the results of cardiac surgery by variable life-adjusted display. Lancet. 1997;350:1128-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 181] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Sherlaw-Johnson C, Gallivan S, Treasure T, Nashef SA. Computer tools to assist the monitoring of outcomes in surgery. Eur J Cardiothorac Surg. 2004;26:1032-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Axelrod DA, Kalbfleisch JD, Sun RJ, Guidinger MK, Biswas P, Levine GN, Arrington CJ, Merion RM. Innovations in the assessment of transplant center performance: implications for quality improvement. Am J Transplant. 2009;9:959-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Boulkedid R, Sibony O, Bossu-Salvador C, Oury JF, Alberti C. Monitoring healthcare quality in an obstetrics and gynaecology department using a CUSUM chart. BJOG. 2010;117:1225-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Steiner SH, Cook RJ, Farewell VT, Treasure T. Monitoring surgical performance using risk-adjusted cumulative sum charts. Biostatistics. 2000;1:441-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 137] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Novick RJ, Fox SA, Stitt LW, Forbes TL, Steiner S. Direct comparison of risk-adjusted and non-risk-adjusted CUSUM analyses of coronary artery bypass surgery outcomes. J Thorac Cardiovasc Surg. 2006;132:386-391. [PubMed] |
| 13. | Buchs NC, Pugin F, Bucher P, Hagen ME, Chassot G, Koutny-Fong P, Morel P. Learning curve for robot-assisted Roux-en-Y gastric bypass. Surg Endosc. 2012;26:1116-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Williams AK, Chalasani V, Martínez CH, Osbourne E, Stitt L, Izawa JI, Pautler SE. Cumulative summation graphs are a useful tool for monitoring positive surgical margin rates in robot-assisted radical prostatectomy. BJU Int. 2011;107:1648-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Okrainec A, Ferri LE, Feldman LS, Fried GM. Defining the learning curve in laparoscopic paraesophageal hernia repair: a CUSUM analysis. Surg Endosc. 2011;25:1083-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Bokhari MB, Patel CB, Ramos-Valadez DI, Ragupathi M, Haas EM. Learning curve for robotic-assisted laparoscopic colorectal surgery. Surg Endosc. 2011;25:855-860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 314] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 17. | Collins GS, Jibawi A, McCulloch P. Control chart methods for monitoring surgical performance: a case study from gastro-oesophageal surgery. Eur J Surg Oncol. 2011;37:473-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Tekkis PP, McCulloch P, Poloniecki JD, Prytherch DR, Kessaris N, Steger AC. Risk-adjusted prediction of operative mortality in oesophagogastric surgery with O-POSSUM. Br J Surg. 2004;91:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Levy SM, Senter CE, Hawkins RB, Zhao JY, Doody K, Kao LS, Lally KP, Tsao K. Implementing a surgical checklist: more than checking a box. Surgery. 2012;152:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 20. | Newkirk M, Pamplin JC, Kuwamoto R, Allen DA, Chung KK. Checklists change communication about key elements of patient care. J Trauma Acute Care Surg. 2012;73:S75-S82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Robb WB, Falk GA, Larkin JO, Waldron R, Waldron RP. A 10-step intraoperative surgical checklist (ISC) for laparoscopic cholecystectomy-can it really reduce conversion rates to open cholecystectomy? J Gastrointest Surg. 2012;16:1318-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Dhillon P, Murphy RK, Ali H, Burukan Z, Corrigan MA, Sheikh A, Hill AD. Development of an adhesive surgical ward round checklist: a technique to improve patient safety. Ir Med J. 2011;104:303-305. [PubMed] |
| 23. | Lamb BW, Sevdalis N, Vincent C, Green JS. Development and evaluation of a checklist to support decision making in cancer multidisciplinary team meetings: MDT-QuIC. Ann Surg Oncol. 2012;19:1759-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | van Klei WA, Hoff RG, van Aarnhem EE, Simmermacher RK, Regli LP, Kappen TH, van Wolfswinkel L, Kalkman CJ, Buhre WF, Peelen LM. Effects of the introduction of the WHO "Surgical Safety Checklist" on in-hospital mortality: a cohort study. Ann Surg. 2012;255:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 302] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 25. | Haynes AB, Weiser TG, Berry WR, Lipsitz SR, Breizat AH, Dellinger EP, Herbosa T, Joseph S, Kibatala PL, Lapitan MC. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3703] [Cited by in RCA: 3387] [Article Influence: 211.7] [Reference Citation Analysis (0)] |
| 26. | Berrisford RG, Wilson IH, Davidge M, Sanders D. Surgical time out checklist with debriefing and multidisciplinary feedback improves venous thromboembolism prophylaxis in thoracic surgery: a prospective audit. Eur J Cardiothorac Surg. 2012;41:1326-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |