Published online Aug 27, 2011. doi: 10.4240/wjgs.v3.i8.113
Revised: August 10, 2011
Accepted: August 16, 2011
Published online: August 27, 2011
AIM: To evaluate patients with proximal rectal cancer (PRC) (> 6 cm up to 12 cm) and distal rectal cancer (DRC) (0 to 6 cm from the anal verge).
METHODS: Two hundred and eighteen patients (120 male, 98 female, median age 58 years, range 19-88 years) comprised 100 with PRC and 118 with DRC. The proportion of T1, T2 vs T3, T4 stage cancers was similar in both groups (PRC: T1+T2 = 29%; T3+T4 = 71% and DRC: T1+T2 = -31%; T3+T4 = 69%). All patients had cancer confined to the rectum - those with synchronous distant metastasis were excluded. Surgical resection was with curative intent with or without pre-operative chemoradiation (c-RT). Follow-up was for a median of 35 mo (range: 12 to 126 mo). End points were: 30 d mortality, complications of operation, microscopic tumour- free margins, resection with a tumour-free circumferential margin (CRM) of 1 to 2 mm and > 2 mm, local recurrence, survival and the permanent stoma rate.
RESULTS: Overall 30-d mortality was 6% (12): PRC 7 % and DRC 4%. Postoperative complications occurred in 14% with PRC compared with 21.5% with DRC, urinary retention was the complication most frequently reported (PRC 2% vs DRC 9%, P = 0.04). Twelve percent with PRC compared with 37% with DRC were subjected to preoperative c-RT (P = 0.03). A tumour-free CRM of 1 to 2 mm and > 2 mm was reported in 93% and 82% with PRC and 88% and 75% with DRC respectively (PRC vs DRC, P > 0.05). However, local recurrence was 5% for PRC vs 11% for DRC (P < 0.001). Three and five years survival was 65.6% and 60.2% for PRC vs 67% and 64.3% for DRC respectively. No patient with PRC and 23 (20%) with DRC received an abdomino-perineal resection.
CONCLUSION: PRC and DRC differ in the rate of abdomino-perineal resection, post-operative urinary retention and local recurrence. Survival in both groups was similar.
- Citation: Wijenayake W, Perera M, Balawardena J, Deen R, Wijesuriya SR, Kumarage SK, Deen KI. Proximal and distal rectal cancers differ in curative resectability and local recurrence. World J Gastrointest Surg 2011; 3(8): 113-118
- URL: https://www.wjgnet.com/1948-9366/full/v3/i8/113.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v3.i8.113
The aims of treatment for rectal cancer are first, to achieve curative resection and second, to restore bowel continuity, thus avoiding a long term stoma. Compared with the developed world, in the developing world, most patients adjust less satisfactorily to an abdominal stoma because of socio-economic constraints and the lack of stoma care nurses in many parts[1]. Cancer of the left side of the colon and rectum constitutes the majority of large bowel cancer in Southern Asia. Of these, most cancers are to be found in the rectum, approximately 60% in the lower rectum between 0 and 6 cm from the anal verge[2].
Historically, surgical operation for cancer of the lower rectum has been abdomino-perineal excision of the rectum[3]. With development of stapling technology and reduction in the minimum safe distal resection margin to one centimetre in favourable tumours, the rate of restorative resection for distal rectal cancer (DRC) has increased[4,5]. More recent developments that have further enhanced the feasibility of restorative resection are the use of pre-operative chemoradiation (c-RT)[6] and the technique of intersphincteric resection[7].
Data from Japan have shown that cancers of the lower rectum, more than cancer of the proximal rectum, tend to spread to nodes of the inferior mesenteric group as well as drain via the internal iliac nodes[8,9]. Some have shown internal iliac nodal involvement in up to 15 percent of cancers of the lower rectum[8]. Compared with cancer of the proximal rectum, surgical operation for cancer of the lower rectum is likely to be associated with a greater rate of local recurrence in the pelvis because of untreated internal iliac nodes. Currently, the only available approaches to treatment of rectal cancer-involved internal iliac nodes are either pelvic lymphadenectomy or pre-operative c-RT. Thus, cancer of the proximal rectum is likely to be different from cancer of the distal rectum. The aim of our prospective study was to compare the rate of curative resection, local recurrence within the pelvis and survival in patients having surgical resection for proximal rectal cancer (PRC) (> 6 cm and up to 12 cm
from the anal verge) vs DRC (0 to 6 cm from the anal verge). We also assessed the proportion of permanent stomas that were received by patients having surgery for PRC and DRC.
From June 1995 to April 2008 two hundred and eighteen patients [120 male (55%), 98 female (45%); median age 58 years, range 19 to 88 years] with rectal cancer confined to the pelvis, without known distal metastasis, underwent surgical treatment at the North Colombo Teaching Hospital (Table 1). Some one hundred (46%; 54 male, 46 female) had cancer > 6 cm from the anal verge (PRC) compared with 118 (54%; 66 male, 52 female) with cancer between 0 and 6 cm from the anal verge (DRC). We chose a limit of 6 cm from the anal verge to determine DRC because rectal cancer at this level would require complete removal of the rectum with a distal tumour-free margin of one to two cm with total mesorectal excision in all cases, unlike in some high PRCs, where it would suffice to remove only a part of the mesorectum[4]. Also, in addition to mesorectal spread of rectal cancer as a cause for local recurrence, rectal cancer between 0 and 6 cm from the anal verge is likely to spread to internal iliac nodes as well as to mesenteric nodes, unlike in PRC which spreads proximally to the mesenteric group of nodes[8,9].
| Criteria | Proximal rectal cancer (n = 100) | Distal rectal cancer (n = 118) |
| Age (median, range, yr) | 60 (23 -88) | 57 (19 – 85) |
| Gender | ||
| Male | 54 | 66 |
| Female | 46 | 52 |
| ASA status | ||
| 1 | 26 | 32 |
| 11 | 50 | 68 |
| 111 | 20 | 16 |
| 1V | 4 | 2 |
| Type of operation | ||
| Elective | 80 | 113 |
| Urgent/emergent | 20 | 5 |
| Mean height of lower limit of tumor from anal verge (cm) | 10.5 | 4.5 |
All patients were evaluated by comprehensive history and physical examination. Digital rectal examination was performed to assess tumour fixity and distance of tumour from the anal verge was also measured by rigid proctoscopy. Clinical assessment of the anal sphincter was performed by digital assessment of resting and squeezing anal tone. The proximal colon was examined to exclude synchronous polyps, tumour or polyposis syndromes and a biopsy of the tumour was obtained, morphology of the tumour documented and endoluminal ultrasound performed at the time of colonoscopy. Further investigation consisted of standard haematology and biochemical evaluation. Radiological investigations consisted of chest X-ray and trans-abdominal ultrasound and, from 2003, combined computerized tomography (CT) or magnetic resonance imaging (MRI) of the abdomen and pelvis. All patients were counseled by a stoma care nurse, stoma sites were marked preoperatively and the operation was performed after bowel preparation using polyethylene glycol 24 h preoperation, except in those presenting with obstruction or perforation.
Patients with T3 or T4 tumours, as judged by endo-luminal US or CT/MR, were given preoperative irradiation which consisted of 5040 cGy delivered in fractions of 180 cGy per day, 5 d per week. 5-Fluorouracil was given concomitantly in a 120 h continuous intravenous infusion at a dose of 1000 mg/m2 of body-surface area per day during the first and fifth weeks of radiotherapy. Surgery was performed 6 wk from completion of chemo-radiation following restaging of the disease. Those with PRC received preoperative c-RT on a selective basis: bulky tumours observed to involve the circumferential margin (CRM) on magnetic resonance scan and tumours that involved the circumference of the lumen.
Operations were performed under general anaesthesia with intermittent positive pressure ventilation. Patients were positioned in the modified Lloyd- Davies position with a 15o to 30 reverse Trendelenberg tilt. Preoperatively, patients received prophylactic antibiotics, an urethral catheter was inserted and the rectum washed with 250 mL of 5% povidone iodine solution. The abdomen was incised in the lower midline to gain access to the peritoneal cavity. Proximal extension of the incision was necessary if mobilization of the splenic flexure was deemed essential at operation, particularly if the tumour was extra-peritoneal and an extended low anterior resection was planned, as in the case of most DRCs. We performed total mesorectal excision in all distal rectal tumours. Most tumours in the upper rectum, that is, rectum enveloped by peritoneum, were managed surgically by division of the rectum at least 2 cm distal to the tumour but with mesorectal excision 5 cm distal to the lower limit of the tumour. In all cases, we performed nerve sparing resections as described previously[10]. In anterior wall rectal tumours we incorporated Denonvillier’s fascia in men or a cuff of posterior vaginal wall in women to ensure a curative resection. Postoperatively, after stabilization of vital signs and satisfactory postoperative pain control was achieved, all patients were managed either in an intensive care or high dependency unit for 24 to 48 h before transfer to a general ward.
Inter-sphincteric resection was performed through the anus with the aid of a ‘Lone Star’ (Lone Star Medical Products, Inc., Stafford, Texas, USA) retractor. The lower limit of the tumour was visualized trans-anally and a distal margin of at least 1 cm was marked by electrocautery. The incision at this predetermined site was deepened to enter the inter-sphincteric space. Inter-sphincteric dissection, usually commenced at or below the dentate line and incorporated part of or, sometimes, the whole internal anal sphincter, approached the lowermost limit of anorectal mobilisation to reach the pelvic floor by abdominal dissection in the inter-sphincteric space, wide of the tumour. The mobilized rectum with the tumour was then delivered via the anal canal. Reconstruction was achieved by handsewn trans-anal, colo-anal anastomosis with 3/0 polyglactin 910 sutures. A diverting loop ileostomy was performed: in all patients with DRC who underwent restoration of intestinal continuity; in those with PRC, after pre-operative c-RT, where there was a positive air leak test during insufflation of the anastomosis under water in the pelvis; or where the surgeon deemed it necessary because of excessive bleeding during the operation.
The levels of resection employed in this study are as described previously[11]. Accordingly, high anterior resection is defined as resection where the level of anastomosis is proximal to 10 cm from the anal verge. Anterior resection is where the anastomosis is less than 10 cm from the anal verge but above the level of the pelvic floor where a part of the distal rectum is left in place. A low anterior resection is defined as an anastomosis at the level of the pelvic floor. It is an extended low anterior resection, when a colo-anal anastomosis followed inter sphincteric resection in which the anastomosis was within the anal canal. Thus, PRC was treated either by high anterior resection or anterior resection whilst all DRC patients received either a low anterior resection or an extended low anterior resection. A proportion received either Hartmann’s resection or an abdomino-perineal resection of the rectum.
All patients were followed up at the outpatient clinic at 2 wk, 4 wk and at 3 monthly intervals for 3 years. Subsequently, patients were followed at 6 monthly intervals up to 5 years and in the absence of recurrent cancer, annually thereafter. Serum CEA was measured at each follow-up visit. Chest X-ray, CT scan of the abdomen to evaluate the liver and colonoscopy were undertaken at the end of the first and the second year. Thereafter, patients were advised to follow standard colonoscopy protocols for those at average risk of colorectal cancer[12]. Those who had had restorative proctocolectomy with an ileal pouch were assessed by pouchoscopy.
Local recurrence in the pelvis was confirmed if there was histologically proven cancer present in the pelvis either by fine needle aspiration, trucut biopsy or histopathological examination of a resected specimen. Median follow up after operation was 35 mo (range 12 to 126 mo). In cases of loss to follow up, survival was evaluated up to the time of the last documented visit.
The endpoints of our study were: mortality at 30 d post-operation, morbidity (anastomotic leakage, pelvic sepsis, wound infection, chest infection and urine retention); curative resection, where all margins (proximal, distal and CRMs) were histologically free of tumour (R0) vs resection with at least one margin involved by tumour (R1); local recurrence in the pelvis; and overall survival. Concerning CRM of resection, we evaluated resection rates in a microscopic margin free of tumour for > 1 mm but <
2 mm and > 2 mm separately. Also, the rate of permanent stomas was compared between operations for PRC and DRC.
Data have been presented as either median and range or mean and standard deviation. Differences between PRC and DRC have been compared using the χ2 test and Fisher’s exact test in case of a number less than 5. Operative data have been compared with one way ANOVA using SPSS version 16 (SPSS, Chicago, USA). Significance was assigned to a P-value of less than 0.05. Survival was analysed using Kaplan-Meier curves. The study was approved by the National Research Council and the University of Kelaniya.
The mean distance of the lower margin of the tumour from the anal verge for PRC was 10.5 cm and for DRC, 4.5 cm. Overall, peri-operative mortality (deaths within 30 d of operation) was 6% [PRC 7 (7%), DRC 5 (4%)]. The most common complication encountered was urinary retention in 2 (2%) in PRC and 11 (9%) in the DRC group (P = 0.041, Fisher’s exact test). Surgical wound site infection, chest infections, anastomotic leakage and pelvic abscess formation were among other reported complications and were similar in both groups (Table 2). Mean operation time for PRC was 212 min (SD ± 48) vs 237 min (SD ± 43) for DRC (P = 0.011, one way ANOVA). Mean operative blood loss was 691 mL (SD ± 306) and 959 mL (SD ± 425) respectively for PRC vs DRC (P = 0.002, one way ANOVA). Use of preoperative chemo-radiation had no significant bearing on the operation time but was associated with greater operative blood loss [c-RT; 986 mL SD ± 438 vs no c-RT 803 mL SD ± 378 (P = 0.034, one way ANOVA]. Compared with PRC 12 (12%), significantly more patients with DRC 44 (37%) were subjected to pre-operative c-RT (χ2: 18.2, P = 0.034).
The type of operation performed is shown in Table 3. In all, 62 (28%) patients with DRC underwent inter-sphincteric resection and reconstruction. Abdomino-perineal excision with a permanent colostomy was undertaken exclusively in those with DRC, 23 (19%) (Table 3). A proximal diverting loop ileostomy was performed in 47 of 79 (59%) with PRC receiving a primary anastomosis and in 86 of 89 (96%) patients with DRC who received a primary anastomosis (P < 0.001, χ2 test). In the majority, PRC 33 (70%) and DRC 70 (81%), the ileostomy was reversed at 3 mo. The rate of permanent colostomy in our study is low; none in the PRC group received abdomino-perineal excision, while a colostomy following Hartmann operation was performed in sixteen with PRC and five with DRC, eighty percent of which have been reversed, leaving only four with a stoma likely to remain permanently. In patients with DRC, 23 (20%) received a permanent stoma consequent to abdomino-perineal excision. Thus 27 (12%) patients of 218 were left with a permanent colostomy.
| Operation | Proximal rectal cancer | Distal rectal cancer |
| High anterior resection of rectum | 23 (23) | 0 (0) |
| Anterior resection of rectum | 9 (9) | 3 (2.5) |
| Low anterior resection or extended low anterior resection | 44 (44) | 75 (63.5) |
| Abdomino-perineal excision of rectum | 0 (0) | 23 (19.5) |
| Hartmann’s operation | 16 (16) | 5 (4.2) |
| Paul Mickulicz operation | 4 (4) | 0 (0) |
| Subtotal colectomy | 1 (1) | 4 (3.4) |
| Restorative proctocolectomy with ileal anal pouch anastomosis | 2 (2) | 7 (6) |
| Transanal resection | 1 (1) | 1 (0.8) |
| Total | 100 (100) | 118 (100) |
Histological features of the resected specimen of rectal cancer are shown in Table 4. For CRM of clearance, a microscopic margin free of cancer greater than 2 mm, R0 resection rates were 82 (82%) for PRCs and 89 (75%) for DRCs. If a CRM of 1 to 2 mm was considered, the rate of margin-free resection was 93 (93%) for PRC and 104 (88%) for DRC. There was no significant difference between the proportion of R1 resections for PRC and DRC [PRC 18 (18%) vs DRC 29 (25%), P = 0.513, χ2].
| Histological criteria | Proximal rectal cancer (100) | Distal rectal cancer (118) |
| Differentiation1 | ||
| Well | 21 (21) | 17 (14) |
| Moderate | 69 (69) | 83 (70) |
| Poor | 8 (8) | 10 (9) |
| Presence of mucin | ||
| Mucinous | 5 (5) | 9 (8) |
| Signet ring | 0 (0) | 1 (1) |
| Tumour stage2 | ||
| T1 | 9 (9) | 8 (7) |
| T2 | 20 (20) | 28 (24) |
| T3 | 57 (57) | 66 (56) |
| T4 | 14 (14) | 16 (14) |
| Node stage3 | ||
| N0 | 58 (58) | 58 (49) |
| N1 | 15 (15) | 30 (25) |
| N2 | 21 (21) | 28 (24) |
| N3 | 3 (3) | 0 (0) |
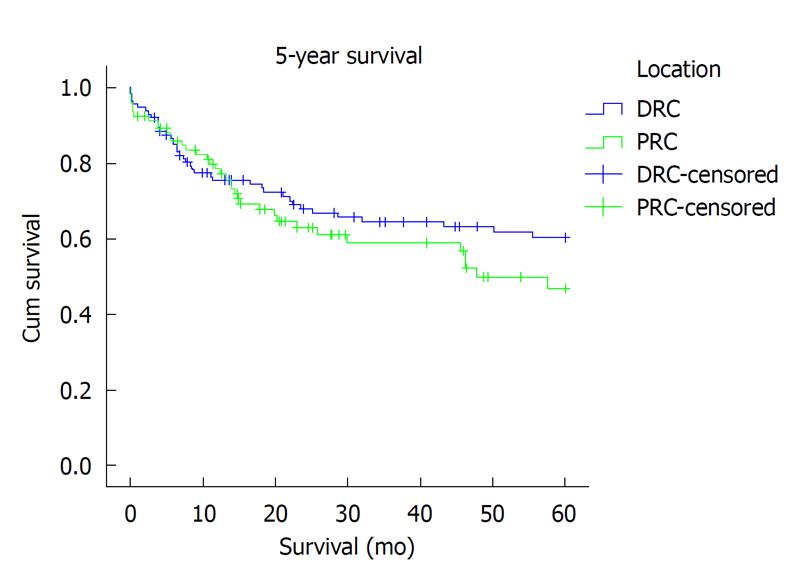
Overall, local recurrence was seen in 8% (18) of patients: 5% (5) PRC vs 11% (13) DRC, P = 0.001, χ2. Seventeen patients (92%) developed local recurrence within the first 3 years after operation and 1 (8%), after 5 years. Only 3 of eighteen (17%) developed anastomotic recurrence. In the remaining 15 (83%) local recurrence was extra-anastomotic. For PRC and DRC, metachronous liver and lung metastasis (> 6 mo after operation) was seen in thirteen (6%) and three (1.25%) patients respectively. Overall survival at 3 years (65.6% vs 67%; Kaplan-Meier P = 0.553) and at 5 years (60.2% vs 64.3%; Kaplan-Meier P = 0.254) was similar (Figure 1).
In this study, which compared patients with PRC and DRC, the overall mortality of 6 percent is comparable with a recent report of 8 percent from the United Kingdom[13]. Furthermore, data from this study examined local recurrence rates for rectal cancer during a period of transition, where, preoperative c-RT was used as adjuvant therapy on a selective basis. Thus in our study, those with T stage III or IV rectal cancer and those with DRC were more likely to receive pre-operative c-RT compared with similar tumours in the proximal rectum. Earlier stage tumours were treated by surgical resection of the rectum without pre-operative c-RT, employing total mesorectal excision, to achieve oncologically curative circumferential and distal resection margins as proposed by Heald et al[4]. All those with metastasis to the liver or lung at the time of operation were excluded from analysis. Despite similar rates of curative resection of PRC and DRC, our study has shown that the rate of local recurrence after curative surgical resection of PRC was significantly less than that following resection of cancer of the distal rectum (5% vs 11%), which is similar to data from the Swedish cancer registry[14]. The overall local recurrence rate of five percent for PRC is acceptable and is unlikely to be reduced further by irradiation. The disadvantages of pre-operative irradiation, such as postoperative anastomotic leakage[6] and the interval after completion of c-RT up to the time of surgical excision, are likely to outweigh the benefits of reducing local recurrence any further in these patients. Thus surgical resection alone will remain the key factor in minimising local recurrence in PRC.
By contrast, pre-operative c-RT which is followed by surgical resection is likely to be of greater benefit in patients with cancer of the distal rectum; the randomized controlled trial by Sauer et al[6] has shown the efficacy of this modality in reducing local recurrence rates for rectal cancer staged T3 or T4[6]. Furthermore, Fujita et al[8] have shown involvement of nodes of the internal iliac group in up to fifteen percent of patients with DRC. Conventional surgical resection does not remove internal iliac nodes and our data may be contributory to the suggestion of extra-rectal pelvic nodal recurrence in low rectal cancer since most local pelvic recurrences were extra-anastomotic. Thus, local recurrence in the pelvis may arise from either incomplete circumferential resection or cancer in iliac nodes, a factor that may be better controlled with pre-operative chemo-radiation. Abdomino-perineal resection was only required in those with DRC. Most, with cancer proximal to six centimetres from the anal verge, were managed surgically by restorative resection except in circumstances where a Hartmann’s procedure was deemed necessary. The overall rate of a permanent stoma was low. We believe that multi-disciplinary involvement in planning treatment before operation, protocol based management by high volume specialist teams and new techniques such as inter-sphincteric resection have contributed to a low stoma rate.
In conclusion, in our study, PRC differed from DRC. No patient with PRC required abdomino-perineal resection and, more importantly, local recurrence rate of cancer in the proximal rectum was significantly less than that of DRC. Urinary retention was more frequent after surgery for DRC compared with PRC. However survival was similar in both groups. In consideration of outcome trials, except for survival analysis, it would be useful to stratify rectal cancer as proximal and distal cancer.
Rectal cancer comprises the majority of large bowel cancers in the developing world. Local recurrence of rectal cancer after curative resection is the most dreaded complication. Surgical treatment has been based on anatomic division of the rectum as high, middle and low. However, local spread of proximal rectal cancer (PRC) has been to inferior mesenteric nodes and spread of distal rectal cancer (DRC) mostly to middle rectal and inferior rectal nodes, referred to as pelvic nodes. The latter, cannot be addressed by surgical resection alone. Pre-operative chemoradiation (c-RT), in addition to down-staging and downsizing rectal cancer, may have a role in treatment of surgically unresected pelvic lymph nodes.
In this study, the authors have considered rectal cancer as proximal or distal based on a point 6cm from the anal verge of the lowermost edge of the tumour. Despite the use of c-RT before operation in a majority of DRCs (0 to 6 cm from the anal verge), the authors have shown that curative, microscopic resection and consequently local recurrence of rectal cancer, remains significantly greater in DRC compared with PRC (6 to 12 cm from the anal verge).
New methods are required to better treat cancer of the distal rectum. Current studies are evaluating this by allowing for a longer time interval between completion of c-RT and surgical removal of the rectum, so as to enable apoptosis of rectal cancer cells to occur more completely than previously thought.
This study shows that in trained hands and with the use of a multi-disciplinary team comprising oncologic, radiological and pathologic specialists, surgeons in developing countries could achieve a remarkably low rate of permanent stomas and acceptable local recurrence.
Rectal cancer may be better addressed as involving the proximal and distal rectum. Based on this classification of the location of a rectal cancer, most DRCs and a smaller proportion of PRC are likely to warrant pre-operative c-RT, since we have shown lower rates of local recurrence for cancer of the proximal rectum. Furthermore, all DRCs and a proportion of PRCs, will require surgical resection of the rectum by total mesorectal excision.
It's always very interesting to evaluate the results of two different therapeutic strategies. This is a well written manuscript.
Peer reviewer: Gregory Peter Sergeant, MD, Department of General Surgery, University Hospital Leuven, Herestraat 49, Leuven B-3000, Belgium; Joseph M Plummer, MD, Department of Surgery, University of the West Indies, Kingston 7, Jamaica
S- Editor Wang JL L- Editor Hughes D E- Editor Zheng XM
| 1. | Silva MA, Ratnayake G, Deen KI. Quality of life of stoma patients: temporary ileostomy versus colostomy. World J Surg. 2003;27:421-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Perera T, Wijesuriya RE, Suraweera PH, Wijewardene K, Kumarage SK, Ariyaratne MH, Deen KI. Prevalence of colorectal cancer and survival in patients from the Gampaha District, North Colombo region. Ceylon Med J. 2008;53:17-21. [PubMed] |
| 3. | Miles WE. A method of performing abdomino-perineal excision for carcinoma of the rectum and of the terminal portion of the pelvic colon (1908). CA Cancer J Clin. 1971;21:361-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 193] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982;69:613-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 1937] [Article Influence: 45.0] [Reference Citation Analysis (1)] |
| 5. | Enker WE, Thaler HT, Cranor ML, Polyak T. Total mesorectal excision in the operative treatment of carcinoma of the rectum. J Am Coll Surg. 1995;181:335-346. [PubMed] |
| 6. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4342] [Cited by in RCA: 4466] [Article Influence: 212.7] [Reference Citation Analysis (1)] |
| 7. | Weiser MR, Quah HM, Shia J, Guillem JG, Paty PB, Temple LK, Goodman KA, Minsky BD, Wong WD. Sphincter preservation in low rectal cancer is facilitated by preoperative chemoradiation and intersphincteric dissection. Ann Surg. 2009;249:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 8. | Fujita S, Yamamoto S, Akasu T, Moriya Y. Lateral pelvic lymph node dissection for advanced lower rectal cancer. Br J Surg. 2003;90:1580-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 151] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 9. | Ueno M, Oya M, Azekura K, Yamaguchi T, Muto T. Incidence and prognostic significance of lateral lymph node metastasis in patients with advanced low rectal cancer. Br J Surg. 2005;92:756-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 255] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 10. | Perera MT, Deen KI, Wijesuriya SR, Kumarage SK, De Zylva ST, Ariyaratne MH. Sexual and urinary dysfunction following rectal dissection compared with segmental colectomy. Colorectal Dis. 2008;10:689-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Tytherleigh MG, Warren BF, Mortensen NJ. Management of early rectal cancer. Br J Surg. 2008;95:409-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol. 2009;104:739-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 981] [Cited by in RCA: 1059] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 13. | Borowski DW, Bradburn DM, Mills SJ, Bharathan B, Wilson RG, Ratcliffe AA, Kelly SB. Volume-outcome analysis of colorectal cancer-related outcomes. Br J Surg. 2010;97:1416-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Påhlman L, Bohe M, Cedermark B, Dahlberg M, Lindmark G, Sjödahl R, Ojerskog B, Damber L, Johansson R. The Swedish rectal cancer registry. Br J Surg. 2007;94:1285-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 281] [Article Influence: 15.6] [Reference Citation Analysis (0)] |