Published online Jul 27, 2011. doi: 10.4240/wjgs.v3.i7.89
Revised: July 9, 2011
Accepted: July 15, 2011
Published online: July 27, 2011
Over the past two decades, transcatheter arterial embolization has become the first-line therapy for the management of upper gastrointestinal bleeding that is refractory to endoscopic hemostasis. Advances in catheter-based techniques and newer embolic agents, as well as recognition of the effectiveness of minimally invasive treatment options, have expanded the role of interventional radiology in the management of hemorrhage for a variety of indications, such as peptic ulcer bleeding, malignant disease, hemorrhagic Dieulafoy lesions and iatrogenic or trauma bleeding. Transcatheter interventions include the following: selective embolization of the feeding artery, sandwich coil occlusion of the gastroduodenal artery, blind or empiric embolization of the supposed bleeding vessel based on endoscopic findings and coil pseudoaneurysm or aneurysm embolization by three-dimensional sac packing with preservation of the parent artery. Transcatheter embolization is a fast, safe and effective, minimally invasive alternative to surgery when endoscopic treatment fails to control bleeding from the upper gastrointestinal tract. This article reviews the various transcatheter endovascular techniques and devices that are used in a variety of clinical scenarios for the management of hemorrhagic gastrointestinal emergencies.
- Citation: Loffroy RF, Abualsaud BA, Lin MD, Rao PP. Recent advances in endovascular techniques for management of acute nonvariceal upper gastrointestinal bleeding. World J Gastrointest Surg 2011; 3(7): 89-100
- URL: https://www.wjgnet.com/1948-9366/full/v3/i7/89.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v3.i7.89
Upper gastrointestinal bleeding (UGIB) is defined as originating in the distal esophagus, stomach and duodenum (proximal to the ligament of Treitz). The most common cause of nonvariceal UGIB is peptic ulcer disease (PUD). Other less common causes include benign and malignant tumors, ischemia, gastritis, arteriovenous malformations such as Dieulafoy lesions, Mallory-Weiss tears, trauma and iatrogenic causes[1,2]. Effective treatment requires timely and accurate diagnosis (location and etiology) and, unlike lower gastrointestinal bleeds, most patients have undergone endoscopic examination and treatment prior to their referral to interventional radiology. Of the small group of patients who fail endoscopic therapy, some are treated surgically[3] but, increasingly, the majority are referred for embolotherapy[4]. Transcatheter arterial embolization (TAE) has been performed for at least two decades and has been shown to be effective at controlling hemorrhage and decreasing mortality[5-10]. Embolization techniques have evolved with the use of microcatheters and new embolic agents. This review presents the clinical evaluation and causative mechanisms of hemorrhagic upper gastrointestinal emergencies. It also discusses the advances in endovascular embolization in their management, including embolic agents, therapeutic techniques, results and limitations.
Indications that require angiogram and embolization are described below. Several population-based and prospective studies support PUD being the most common cause of acute UGIB[11]. PUD refers to either gastric or duodenal ulcers but under a broad heading of “ulcers” some investigators also include esophageal ulcers. Approximately 50% of all cases of acute UGIB are attributed to PUD[11].
Another recently reported trend in acute UGIB involves cyclooxygenase-2 inhibitors. Cyclooxygenase-2 inhibitors, known for their decreased gastrointestinal toxicity, are extensively used for both their anti-inflammatory and analgesic properties. This class of anti-inflammatory drugs is associated with both decreased endoscopic lesions and episodes of UGIB when compared with their nonselective nonsteroidal anti-inflammatory drug counterparts[12,13]. Population-based studies evidence suggests that the introduction of cyclooxygenase-2 inhibitors may be associated with an overall increased nonsteroidal anti-inflammatory drug use and UGIB[14]. Gastrointestinal hemorrhage can certainly be facilitated when a patient has an endogenous coagulopathic state or is taking anticoagulation therapy.
Classically, Mallory-Weiss tears are mucosal lacerations at the gastroesophageal junction or in the cardia of the stomach[15]. These lesions can be associated with repeated retching or vomiting and are another important cause of nonvariceal UGIB. It is estimated that 5% to 15% of all cases of acute UGIB are secondary to Mallory-Weiss tears[15,16]. Most bleeding episodes caused by Mallory-Weiss tears are self-limited, requiring no endoscopic hemostasis[17]. Nevertheless, some cases are severe enough to require blood transfusions or therapeutic interventions such as endoscopic hemostasis, endovascular embolization or surgery.
Dieulafoy’s lesion is a rare etiology in acute UGIB. Dieulafoy’s lesions are difficult to identify endoscopically because they often retract. Their histopathological description is a “caliber-persistent artery” in the submucosal tissue[18]. These lesions are responsible for less than 5% of all nonvariceal UGIB[18].
Neoplasms, both malignant and benign, are another infrequent cause of non-variceal UGIB, contributing to less than 5% of all UGIB cases[19]. Although only a small fraction of UGIB is of neoplastic etiology, this may be the only presenting symptom of a neoplasm, which should be included in the differential diagnosis[19].
Other rare causes of nonvariceal UGIB should also be considered in any differential diagnosis. Hemobilia is a rare cause of UGIB that should be considered in the setting of recent hepatobiliary tree instrumentation, such as with endoscopic retrograde cholangiopancreatography or laparoscopic cholecystectomy. Bile duct and hepatic artery injuries are possible complications of these procedures, and patients can ultimately present with signs of UGIB[20]. In patients with chronic pancreatitis who present with acute UGIB, hemosuccus pancreaticus should also be excluded. Although it is an uncommon cause of UGIB overall, bleeding in these patients can be secondary to a pseudoaneurysm (PA) in peripancreatic blood vessels as a complication of pancreatic pseudocysts[21]. Finally, iatrogenic injuries secondary to biopsies or endoscopic procedures, such as percutaneous endoscopic gastrostomy tube placement, are also rare but documented causes of nonvariceal UGIB[22].
The vascular supply to the stomach and duodenum is quite rich, with avid redundant supply. This can make successful embolization more challenging; however, it decreases the incidence of postembolization ischemia[23]. The likelihood of successful embolization reflects prior knowledge of the location of the bleed. The left gastric artery (LGA) runs along the lesser curve of the stomach and supplies the stomach and distal esophagus. The LGA is most often the first branch of the celiac trunk (90%) but may arise directly from the aorta, as a lienogastric trunk or hepatogastric trunk[24]. It anastomoses with the right gastric artery (RGA). Small distal branches anastomose with short gastric arteries (from the splenic artery) and the left inferior phrenic artery. The RGA most often originates from the proper, left or middle hepatic artery but may also arise from the gastroduodenal artery (GDA) or the right hepatic artery (RHA). It is typically a small vessel that runs in the gastrohepatic ligament and supplies the distal lesser curve of the stomach and the pylorus. The greater curvature of the stomach is supplied by the gastroepiploic arcade that courses along the greater curvature of the stomach and is supplied by the right gastroepiploic artery (RGEA), the terminal branch of the GDA and the left gastroepiploic artery, a branch of the distal splenic artery (SA). A complete arcade (rather than incomplete or weak) is present in about 65% of patients. The duodenum is supplied by the pancreaticoduodenal arcade, supplied by superior and inferior, posterior and anterior pancreaticoduodenal arteries, branches of the GDA and superior mesenteric artery (SMA), respectively. The GDA arises from the common hepatic artery in a large majority of patients but may also arise from the RHA, a replaced RHA branch of the SMA, or directly from the celiac axis.
Patients undergoing a transcatheter procedure for the evaluation and management of hemorrhage are often poor surgical candidates. They are more often than not hemodynamically unstable and often have other clinical issues, such as an electrolyte imbalance or a coagulopathy. Whenever possible, the hemodynamic status of the patient should be stabilized by fluid resuscitation. Any pertinent laboratory abnormalities should be corrected by the time the patient reaches the interventional suite. The preprocedural laboratory data should include complete blood count, renal function and coagulation parameters. Optimal laboratory parameters include a serum creatinine < 1.5 mg/dL with an estimated glomerular filtration rate > 60, an international normalized ratio (INR) < 1.5 and a platelet count > 50 000/dL. If necessary, blood products such as fresh frozen plasma, platelets or packed red blood cells should be transfused before the procedure. They may also be given intra-procedurally. It is also desirable to correct any coagulopathy before embolization because achieving hemostasis depends on technically successful embolization as well as the patient’s ability to clot properly. Any history of prior drug or contrast medium allergies must be documented. Any pertinent prior diagnostic cross-sectional imaging studies should be reviewed as these may provide valuable information that may direct and potentially affect the outcome of the intervention. Virtually all patients will also have undergone upper endoscopy in an attempt to identify and treat the source of bleeding.
The typical candidate patient presents with the following: (1) massive bleeding (requiring transfusion of at least 4 U of blood/24 h) or hemodynamic instability (hypotension with systolic pressure < 100 mmHg and heart rate of 100 min or clinical shock secondary to blood loss); (2) bleeding that has failed to respond to conservative medical therapy, including volume replacement, antacids, H2-receptor blocking agents or proton pump inhibitors; and (3) bleeding that has failed to respond to at least one, and sometimes two, attempts at endoscopic control[25]. Endoscopy is performed before angiography. Performance of angiography before endoscopy leads to an unacceptably high frequency of unnecessary angiography. Endoscopic diagnosis and therapy can render angiography unnecessary. Endoscopy also helps in planning the timing and approach of angiography. For example, inability to determine the cause of bleeding at endoscopy because of severe bleeding should prompt urgent angiography. Even endoscopic localization of the bleeding site without determination of the cause helps guide which artery to cannulate first at angiography[26]. Negative endoscopic information, such as excluding esophageal bleeding, is valuable to the angiographer. On the other hand, Walsh et al[27] and Loffroy et al[25] found longer time to angiography to be predictor of early rebleeding after TAE. They concluded that every effort should be made to perform angiography with embolization early after bleeding onset. Thus, the ability to achieve bleeding control in critically ill patients seems to depend chiefly on early intervention.
There is much discussion about the necessity of diagnostic studies preceding angiography. Certainly, endoscopy has a diagnostic and therapeutic role and can completely replace the need for angiography of the upper gastrointestinal tract in more than 90% of patients[26]. However, most of the controversy involves the need for nuclear scintigraphy before diagnostic angiography in the patient with lower gastrointestinal bleeding. Scinitgraphy has been notoriously inaccurate when locating lesions for surgical resection; however, scintigraphy is certainly more sensitive, requiring reportedly 10-fold less hemorrhage than angiography to achieve a positive study[28,29]. As an adjunct to angiography, the probability of a positive diagnostic angiogram is increased by a preceding positive nuclear scintigraphic study. In general, the decision to proceed directly to angiography without nuclear scintigraphy depends on the clinical situation and the degree of hemorrhage. The clinical presentation of lower gastrointestinal bleeding may be episodic or continual. The former ranges from minor episodes that resolve, to chronic intermittent bleeding, to severe life-threatening hemorrhage. Angiography is warranted in the latter group and may, with provocation, play a diagnostic role in the second. Usually, nuclear scintigraphy is recommended before angiography in all cases of episodic bleeding. Angiography remains the primary diagnostic imaging tool in those patients with continual active hemorrhage, however, and delaying angiography for scintigraphy is not warranted. Intermittent lower gastrointestinal hemorrhage deserves special attention. This difficult group of patients often bleeds, frequently to low hemoglobin and hematocit levels, yet a site cannot be localized. Bleeding has been successfully provoked using a variable combination of anticoagulants, vasodilators and fibrinolytics. Wireless capsule endoscopy is now being used as a diagnostic tool for a variety of gastrointestinal disorders including Crohn’s disease, celiac disease and obscure gastrointestinal bleeding[30,31]. At the present time, however, it is not a practical modality to use in acute UGIB.
Finally, the amount of bleeding may affect treatment strategy, continued active bleeding demands for emergency angiography primarily without pre-procedural testing. Defreyne et al[32] and Whitaker et al[33] independently indicated that the most important determinant of the timing of angiography may be the findings of an experienced endoscopist when faced with nontractable bleeding.
In the advent of UGI bleeding, the source for hemorrhage is usually identified by endoscopy. Therefore, angiography is most often performed only as a precursor to TAE based on the knowledge of the vascular supply to the abnormal area. Diagnostic angiography for UGI bleeding is straightforward and is centered on the anatomy of the celiac artery and the SMA. A trans femoral approach is usually used and involves placement of a 5F introducer sheath in the common femoral artery. A variety of introducers and selective catheters with a smaller caliber can be used to cannulate the celiac artery and obtain access to the common hepatic artery. For selective catheterization by way of the femoral route, the most widely used catheters are the cobra, hook and short- and long-curve sidewinder with a 4F diameter. Once access is secured, arteriogram is performed to delineate the anatomy. Localization of contrast extravasation into the bowel lumen is considered as a direct angiographic sign of active GI bleeding. Commonly encountered indirect angiographic signs of GI bleeding include visualization of an aneurysm, PA or a submucosal vessel and early venous drainage of angiodysplasia. Other angiographic signs include neovascularity, mucosal or extramucosal hyperemia, intramural pooling of contrast or arterial wall abnormalities[34]. Selective catheterization for UGI bleeding includes the celiac and superior mesenteric arteries. The initial artery catheterized is the one most suspected of bleeding based on previous imaging or endoscopy, which is, of course, the celiac artery for UGI bleeding. If no extravasation is seen, then superselective angiography is advised. Depending on endoscopic findings that offer information on the likely location of the bleeding source, superselective catheterization of the GDA, LGA or SA may be performed. A microcatheter is always necessary and recommended for a distal superselective approach to the bleeding vessel so as to avoid the spasm or occlusion caused by larger 4F or 5F catheters. Longer injection durations or use of carbon dioxide for contrast medium can also improve sensitivity for small bleeds. In the absence of hemodynamic instability, computed tomographic angiography is a good and reliable method to highlight the presence of a bleed in progress and should be performed before any invasive procedure. Arteriography after superselective cannulation may show extravasation that could have been missed during contrast injection in the main hepatic artery. When a dual supply of the bleeding area is suspected, both arterial sources must be embolized to assure that all of the inflow ceases. This is typically noted in bleeding secondary to an ulcer that erodes into the GDA. Embolization in this case must start distally to prevent persistent “backdoor” hemorrhage from the right gastroepiploic and superior pancreaticoduodenal arteries and then proceed to the proximal side of the erosion.
We do not believe there is enough data to provide definitive guidelines to perform provocative mesenteric angiography or pharmacoangiography. Furthermore, this technique is mainly used to provoke lower GI bleeding, which is more challenging than UGIB. Indeed, contrary to lower GI bleeding, almost all upper gastrointestinal bleeders have undergone endoscopy in an attempt to identify, localize and treat the source of bleeding. Several prior studies have shown that empiric embolization based on endoscopic findings, in the absence of contrast extravasation, can be performed safely and successfully[25,35,36]. So the absence of angiographic extravasation is less problematic at the level of the upper gastrointestinal tract and does not prevent from embolizing the artery that supplies the bleeding site.
However, this technique can be applied in situations where conventional angiography is nondiagnostic. The addition of pharmacological agents to standard angiographic protocols in order to increase the diagnostic yield has been reported in case reports and small series. Provocative mesenteric angiography is the use of thrombolytic, vasodilating and anticoagulation medications to elicit active bleeding from a source that may have recently ceased hemorrhaging. Unfortunately, the available literature on this topic is limited. This technique was first reported in the literature in 1982[37]. Since then, our review of the literature yielded only seven small case series and two case reports totaling 93 patients[37-45]. Koval et al[38] doubled the rate of extravasations visualized by angiography from 32% to 65% with the introduction of pharmacologic angiography. Malden et al[39] and Ryan et al[41] separately showed a provoked bleeding rate of nearly 40% when previous angiograms had been normal with a minimal complication rate. Although reasonably successful and without any reported major hemorrhagic complications, provocative mesenteric angiography is not a commonly used examination. The precise reasons for this are unknown but likely relate to fear of potential uncontrollable hemorrhagic complications and lack of familiarity with this procedure by referring clinicians and interventional radiologists. Both of these are likely a reflection of the sparse data supporting provocative mesenteric angiography in the literature.
Basically, provocative angiography may increase the diagnostic ability when a normal angiogram is encountered for nonvariceal UGIB. But several procedure-related factors may affect the diagnostic yield of provocative studies; these include timing of the procedure, the type and dosage of provocative medications and the expertise of the operators. The types of pharmacological provocation reported in the literature have been variable[38-46]. Based on these data, an idealized protocol could include intra-arterial tolazoline at a dose of 25 mg, intra-arterial heparin at a dose targeted to double the patient’s baseline activated clotting time and intraarterial urokinase in aliquots of 250 000 U given over 15 min and monitored by diagnostic angiography. Endpoints are either hemorrhage or a total of 1 000 000 U of urokinase over an interval of approximately 1 h. However, it should be emphasized that the specific agents and their total doses in literature are largely arbitrary. Currently, provocative angiography is rarely needed for the diagnosis of GI bleeding and only limited reports for use in difficult and recurring nonvariceal UGIB have shown to be beneficial[37,41]. We believe that further prospective studies are required to provide a better understanding of optimal patient selection and optimal pharmacological provocation. Only with these kinds of data can provocative GI bleeding studies be appropriately placed in a diagnostic algorithm for evaluation of patients with GI hemorrhage of obscure origin.
Over the past two decades, angiographic interventions have shifted from playing a purely diagnostic role to being a major therapeutic option in the management of nonvariceal UGIB. Transcatheter intervention to control GI bleeding takes two forms: the infusion of a vasoconstricting medication and the mechanical occlusion of the arterial supply responsible for the hemorrhage.
Selective infusion of intraarterial vasoconstrictors was one of the first angiographic treatments for GI bleeding. Vasopressin, a posterior pituitary hormone, elicits smooth muscle contraction in the mesenteric bed, thereby decreasing the perfusion pressure to the bowel and potentially resulting in thrombosis of the bleeding site. Vasopressin infusion is easy to perform, most often by placing a 5F diagnostic catheter into the artery most suspected of bleeding[47]. Vasopressin is then infused at a rate of 0.2 to 0.4 U/min until successful control of bleeding is observed on angiography. Then, the mesenteric intraarterial infusion is continued for 12-48 h. Vasopressin infusion has lost favor for two main reasons: necessary catheterization times can require several days and, more importantly, the emergence of embolotherapy. For nonvariceal UGIB, the offending arteries were easily accessible even with large and crude catheter systems and embolic agents of the past. In addition, given the rich arterial collateral network and proximal site of embolotherapy, ischemia was not thought to be problematic. Given that embolotherapy replaced vasopressin infusion early in the treatment of UGIB, there is little recent data on the use of this technique. In addition, much of the data includes treatment of variceal bleeding. A review of four of the more recent studies consisting of 267 patients demonstrated an initial 70% to 80% success rate. They observed approximately 20% rate of rehemorrhage with bleeding refractory to infusion in up to 40% of patients[48-50]. Failures of vasopressin are thought to be due to the rich collateral supply to the upper GI tract and the inability to treat potential collateral supply pathways to the bleeding site, but this is unproven. Finally, the use of vasopressin in the treatment of nonvariceal UGIB is empiric as there is no substantial data to support its use[51].
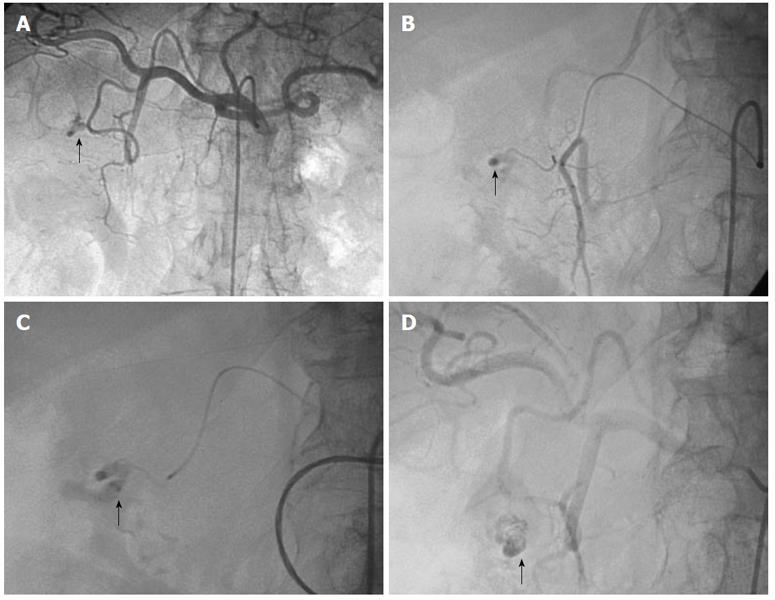
Standard technique: Because of the high rebleeding rate with infusion therapy, other angiographic interventions were developed to treat nonvariceal UGIB. As is true of most other minimally invasive and image-guided interventions, embolotherapy has supplanted surgery in most centers as the treatment of choice for endoscopy-refractory UGIB. This method is associated with an initial bleeding control rate of 89%-98%. Clinical success rates range from 52%-98% with most reports showing success rates of 70%-80%[23,46,52,53]. The role of TAE is to selectively reduce blood supply at the source of bleeding while maintaining enough collateral blood flow to maintain intestinal viability. Typically, in cases of active hemorrhage with extravasation of contrast, the bleeding vessel is identified by superselective catheterization using a microcatheter and embolized with microcoils, particles or glue if arterial flow is not blocked by the microcatheter (Figure 1). Superselection and embolization of short segments of visceral arteries can be performed with newer advances in hydrophilic steerable wires, microcatheters and embolic agents. Coaxial systems allow 2 to 3F catheters to pass through larger primary catheters to allow access to distal arterial branches[52]. In general, bleeding in the esophagus and fundus of the stomach is treated by embolization of the LGA (Figure 2). Bleeding in the body and antrum of the stomach may be controlled by embolization of either the gastroepiploic, right gastric or gastroduodenal arteries depending on the source of bleeding.
Choice of embolic agent: As is the case with microcatheters and guide wires, embolic agents too have evolved over time. Many embolization agents have been used successfully: coils, particulate material such as resorbable gelatin sponge, and nonresorbable polyvinyl alcohol (PVA) or trisacryl gelatin particles. Liquids such as N-butyl 2-cyanoacrylate (NBCA) glue or ethylene-vinyl alcohol copolymer (Onyx®, Micro Therapeutics, Inc., Irvine, CA, USA) are less popularly used[25,46,52,54]. The choice of the best embolic agent is still debatable. Embolotherapy in these emergency patients has to be fast, easy to perform and effective. Success is achievable with different materials in experienced interventional radiologist’s hands. Embolic agents have different constraints depending on the material used.
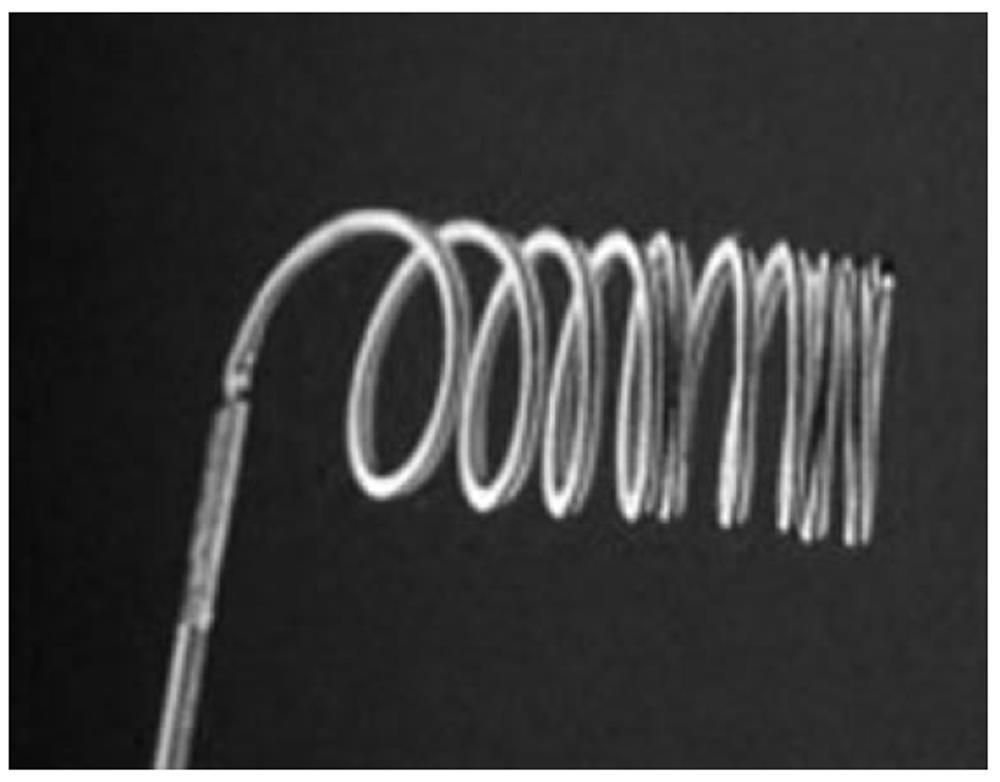
Coils alone inserted in the GDA or superselectively in the pancreaticoduodenal arteries have been used with success by several investigators[55-59]. When used in the setting of UGIB, coils are usually used to occlude or reduce flow into a major vessel, which can also be treated at a more distal level with a particulate agent (usually gelatin sponge) to aid in hemostasis. The main advantages of using coils are that they can be delivered in a very precise fashion and carry low risk of infarction because of the preservation of the distal microvasculature. Coils and microcoils of different size and length can be used (Figure 3). They may have thrombogenic fibers to facilitate occlusion of the vessel and they may offer the option of detachability and retrievability. The main drawback is that they are permanent and may preclude re-accessing the vessel in the future should it prove necessary. Another disadvantage is that coil application is dependent upon vessel diameter and intrinsic blood clotting. That is probably why Aina et al[35] and Loffroy et al[25] showed an association between the use of coils alone and the incidence of bleeding recurrence, especially in patients with coagulopathy. The advantages of use of PVA or gelatin sponge in association with coils when choosing a strategy for this subgroup of patients cannot be over stressed.
Gelatin sponge is the main temporary embolic agent used worldwide (Figure 4). It has the advantage that after resorption, flow will be restored weeks after embolization. Furthermore, it is readily available, cheap and unlikely to cause ischemia. The disadvantages are that it requires some time to prepare appropriate-sized particles and the pace of recanalization is unpredictable. Lang[60] compared several embolic agents in a series of 57 patients. They reported that a high rate of bleeding recurrence was observed when gelatin sponge was used alone. Similarly, Encarnacion et al[7] achieved a low success rate (62%) in their series, which included mostly patients embolized with gelatin sponge alone. These data confirm that the use of gelatin sponge as the only embolic agent guarantees only short-term results and should probably be avoided.
Particles such as PVA particles and tris-acryl gelatin microspheres may be of advantage when a flow-directed strategy is favorable, e.g. when diffuse tumor vascularization is to be excluded from the arterial supply (Figure 5). These agents have been used successfully in treating gastrointestinal hemorrhage, usually through a microcatheter and at a site distal to major vessels[60]. Only larger particles (> 500 μm) should be used to decrease the risk of ischemia from normal tissue devascularization.
Good results have also been reported with NBCA[25,61-63]. Toyoda et al[63] reported that the time required for TAE using NBCA was significantly lower than for TAE procedures that do not use NBCA. This is important especially in cases of massive bleeding that require urgent hemostasis. Indeed, the use of NBCA glue is particularly of interest in hemodynamically unstable patients and in cases of underlying coagulopathy. A number of institutions have adopted selective embolization using glue as the only embolic agent as the salvage treatment of choice in UGIB cases. However, the use of NBCA glue requires training and considerable experience, given the risk of bowel infarction and glue reflux into other vessels. Reflux of NBCA may also result in its polymerization to the catheter tip (Figure 6). This bit of NBCA may then be stripped from the catheter during catheter retraction, resulting in nontarget embolization. The use of a proper technique, including prompt removal of the catheter after injection as well as aspiration of the guide catheter after microcatheter removal, can significantly reduce this risk[62]. Another drawback is the potential risk of bowel stenosis over the long-term. Lang[60] found a 25% duodenal stenosis rate in a study of 28 patients that were followed up for at least 5 years after embolization for bleeding duodenal ulcers, even if the link between glue embolization and duodenal stenosis is difficult to evaluate.
Onyx® seems to have great potential as a liquid embolic agent in embolotherapy of acute arterial gastrointestinal bleeding. Lenhart et al[54] recently reported their experience with the use of Onyx® in such a setting. Their study represents the first series to date reporting results on arterial embolotherapy with Onyx® as an embolic agent in the gastrointestinal tract. The success rate was high (81%) and the complication rate was almost nil. Onyx® is a new liquid embolic agent composed of ethylene-vinyl alcohol copolymer dissolved in dimethyl sulphoxide (DMSO), with emerging applications in neurovascular procedures, predominantly embolization of cerebral aneurysms and arteriovenous malformations[64]. The main advantages of Onyx® are its nonadhesive properties, high radiopacity and long solidification periods (Figure 7). These properties of Onyx® compared to acrylic glues make the embolization procedure more controllable and predictable[65]. However, Onyx® has some disadvantageous characteristics. Firstly, DMSO can cause severe vasospasm if injected rapidly. Secondly, DMSO is volatile and is excreted via respiration and sweat. This has a typical smell not unlike that of diabetic ketoacidosis and may last a few days. The patient and ward staff should be warned to expect this. Lastly, the use of Onyx® has cost implications as it is much more expensive than alternative embolic materials and requires specific DMSO-compatible microcatheters. These factors explain its restricted use in neuroradiology in most institutions around the world.
Finally, it is not clear if careful selection of the embolic agents according to the bleeding vessel may play a role in a successful outcome. It would be worth comparing the different embolic agents for arterial embolotherapy in the gastrointestinal tract in a prospective randomized multi-center trial. However, data from the literature suggest that coils probably should not be used as the only embolic agent but rather in association with gelatin sponge, particles or glue for the treatment of gastroduodenal hemorrhage. Furthermore, surgical glue should probably be used more often, especially in patients with coagulopathy, because it provides a better and faster hemostasis and does not cause ischemia. Otherwise, the choice of embolic agent does not seem to affect clinical response or recurrence rates.
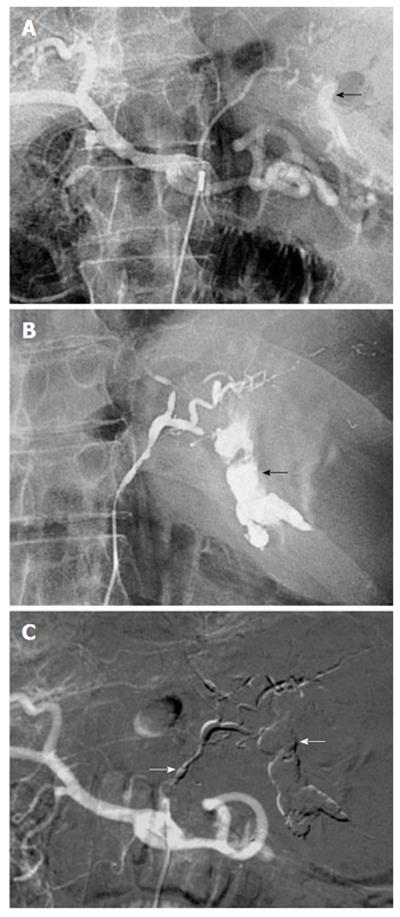
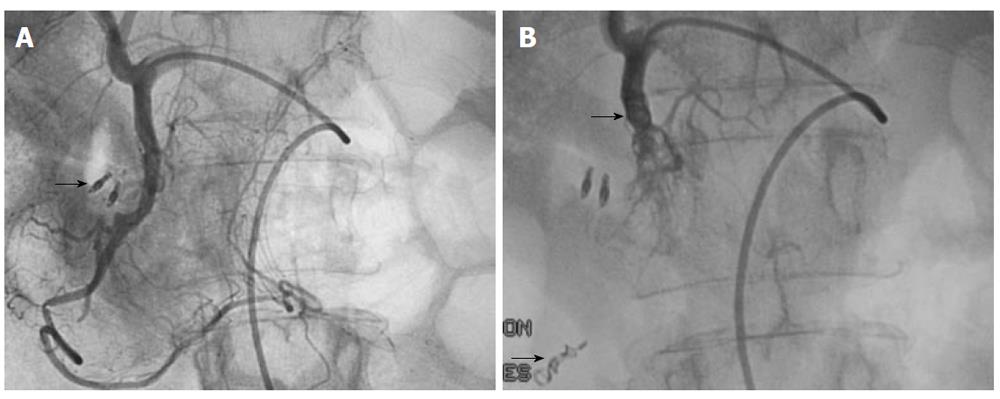
Specific techniques of interest: The upper gastrointestinal tract contains a rich collateral blood supply that often requires embolization of multiple arteries for successful outcomes. Careful angiographic assessment of the collateral pathways prior to embolization is essential, especially in patients with severe atherosclerotic occlusive disease or previous extensive upper gastrointestinal surgery. If the bleeding is from a duodenal ulcer, the GDA needs to be catheterized and embolized using the so-called sandwich technique: the catheter is pushed to the origin of the RGEA and coils are introduced as the catheter is withdrawn to the proximal GDA. Complete embolization of the GDA, which includes proximal and distal embolization and exclusion of its two side branches, is the end point in this case (Figure 8A and B)[25,55]. A selective superior mesenteric arteriogram is performed after embolization to ensure that no collateral supply to the bleeding site is present. If extravasation is identified, superselective catheterization of the inferior pancreaticoduodenal artery and the side branch responsible for the collateral circulation is performed with the microcatheter. Embolization with microcoils of the bleeding site is performed as distal as possible. This method can also be used at the level of the SA for treatment of pseudoaneurysmal lesions[21]. As such, the embolization needs to take place both distal and proximal to the lesion.
If no evidence of bleeding is found on pre-embolization arteriography, then empiric or blind embolization is advised. Blind or empiric is defined as embolization without angiographic proof of extravasation and is typically guided by endoscopic information regarding the location of the bleeding vessel. Coils and gelatin sponge are then often used in such a situation (Figure 8). Another useful maneuver in this scenario requires clips to be placed around the area of bleeding during pre-embolization endoscopy. The clips remain in position for several hours and allow for an educated guess of the location of the culprit vascular branch[56]. If no extravasation is seen despite the injection of contrast, then the branches terminating to the clip are superselected using microcatheter techniques and embolized. Arteriography at multiple projections is necessary at this step to ensure the relation between the clip and the adjacent branches.
Blind embolization is controversial. Because massive bleeding is often intermittent, most groups have adopted a policy to embolize on the basis of endoscopic findings even in situations where no extravasation is seen angiographically[36]. In the independent series from Aina et al[35], Loffroy et al[25] and Padia et al[36] there was no difference in outcome between patients who underwent blind embolization and those who underwent embolization after a bleeding site had been demonstrated angiographically. Other researchers have also advocated the practice of endoscopy-directed blind embolization[57,58]. Based on the findings from the literature and our own experience, we believe that blind embolization is appropriate. The GDA should be embolized using the sandwich technique, in which both ends of the artery are filled with coils to avoid retrograde bleeding from the superior mesenteric circulation. If there is suspicion that smaller muscular branches terminating to a clip are the culprits, then those should be embolized with any of the materials available.
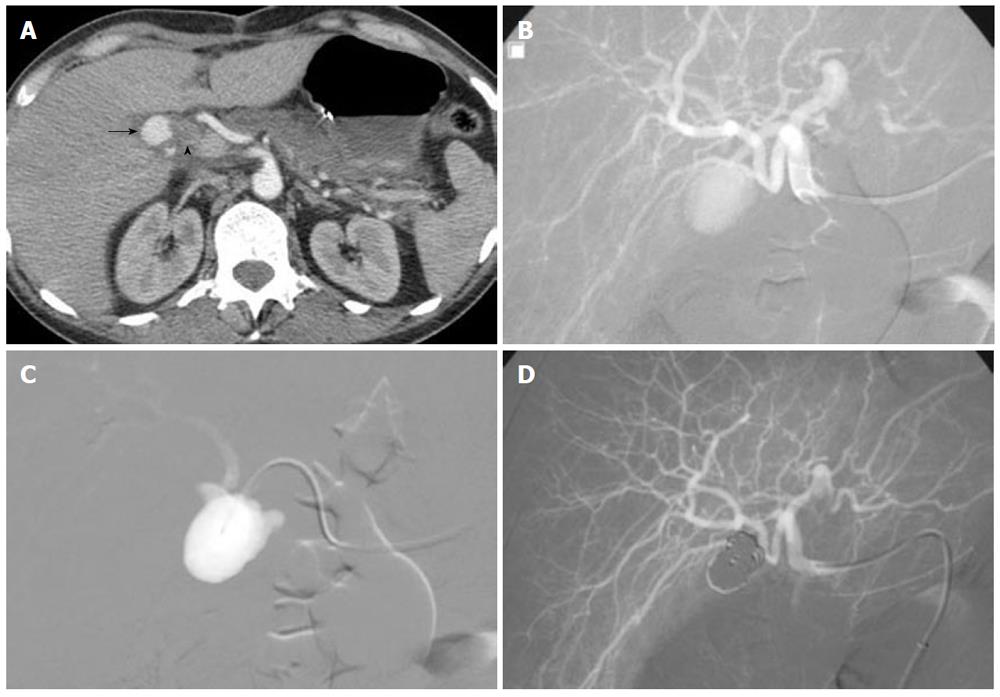
PA in peripancreatic or perihepatic blood vessels as a complication of pancreatic pseudocysts or in the setting of recent hepatobiliary tree instrumentation, respectively, may be responsible for UGIB. Recently, TAE has generated considerable interest as the first-line therapeutic method for PAs. The success rate is high, ranging from 62% to 100% in visceral PAs and the morbidity and mortality rates are low[21]. Most investigators agree that coils are the most appropriate embolic material[21,66]. However, the traditional technique for PA embolization includes isolating the lesion by deploying coils in the parent artery, covering both sides of the PA neck to prevent back-bleeding via collaterals (sandwich technique)[21,66]. Embolization of PAs using a combination of gelatin sponge and coils, NBCA and coils, coils alone or NBCA has also been described[66,67]. The main drawback of these techniques is the compromised patency of the parent vessel with potential ischemic complications. Modification of the embolization technique can help preserve the patency of parent artery while achieving complete embolization. Superselective arterial embolization is achieved by three-dimensional coil-packing of the PA sac using controlled, detachable microcoils placed in a concentric fashion (Figure 9). This endovascular technique is generally used for the treatment of intracranial aneurysms[68], often with the remodeling technique to avoid coil protrusion into the parent artery because of an unfavorable neck-to-sac ratio[69]. When the PA neck is thin, balloon-remodeling is unnecessary. However, this method has several potential limitations. The main technical drawback is that the microcatheter must be placed in the PA sac. Considerable experience is required to pass through the neck, which is typically slender in peripheral PAs. The other limitation concerns the number of coils required for complete occlusion of the PA sac, especially those of large size, and consequently the cost of the procedure. Compared to non-fibered coils, fibered coils have greater occlusive power, allowing occlusion with a smaller number of coils. Controlled, detachable fibered microcoils are now commercially available. Packing 80%-90% of the sac usually provides complete sac exclusion while avoiding complications such as secondary rupture. Few centers combine the use of detachable microcoils with an injection of Onyx® into the pseudo-aneurysmal sac to reduce the amount of coils and the risk of over-packing[70]. The coil mesh has proven to be very effective for capturing Onyx® and facilitated complete occlusion of a challenging lesion. However, a limitation to the selection of Onyx® for peripheral indications is its relatively high cost compared with microcoils, as previously said. In addition, its use may add to the complexity of embolization procedures because a balloon-assisted remodeling technique is often necessary.
Nonvariceal UGIB remains a common and often serious clinical dilemma. The past two decades have seen enormous advances in endovascular device development, including lower-profile catheter systems and better embolic agents, and treatment of a wide variety of hemorrhagic conditions. These all have made embolotherapy safer and more efficient and has led to its wider acceptance. A combination of newer improved techniques, materials and embolic agents has seen TAE replace surgery to become the gold standard for the treatment of life-threatening UGIB refractory to endoscopic hemostasis.
Peer reviewer: Rungsun Rerknimitr, MD, Division of Gastroenterology, Internal Medicine, King Chulalongkorn Memorial Hospital, Rama IVC Rd Lumpini, Bangkok 10310, Thailand
S- Editor Wang JL L- Editor Roemmele A E- Editor Zheng XM
| 1. | Huang CS, Lichtenstein DR. Nonvariceal upper gastrointestinal bleeding. Gastroenterol Clin North Am. 2003;32:1053-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Rollhauser C, Fleischer DE. Nonvariceal upper gastrointestinal bleeding. Endoscopy. 2004;36:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Schoenberg MH. Surgical therapy for peptic ulcer and nonvariceal bleeding. Langenbecks Arch Surg. 2001;386:98-103. [PubMed] |
| 4. | Defreyne L, De Schrijver I, Decruyenaere J, Van Maele G, Ceelen W, De Looze D, Vanlangenhove P. Therapeutic decision-making in endoscopically unmanageable nonvariceal upper gastrointestinal hemorrhage. Cardiovasc Intervent Radiol. 2008;31:897-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Rösch J, Dotter CT, Brown MJ. Selective arterial embolization. A new method for control of acute gastrointestinal bleeding. Radiology. 1972;102:303-306. [PubMed] |
| 6. | Funaki B. Endovascular intervention for the treatment of acute arterial gastrointestinal hemorrhage. Gastroenterol Clin North Am. 2002;31:701-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Encarnacion CE, Kadir S, Beam CA, Payne CS. Gastrointestinal bleeding: treatment with gastrointestinal arterial embolization. Radiology. 1992;183:505-508. [PubMed] |
| 8. | Lang EV, Picus D, Marx MV, Hicks ME. Massive arterial hemorrhage from the stomach and lower esophagus: impact of embolotherapy on survival. Radiology. 1990;177:249-252. [PubMed] |
| 9. | Ljungdahl M, Eriksson LG, Nyman R, Gustavsson S. Arterial embolisation in management of massive bleeding from gastric and duodenal ulcers. Eur J Surg. 2002;168:384-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Holme JB, Nielsen DT, Funch-Jensen P, Mortensen FV. Transcatheter arterial embolization in patients with bleeding duodenal ulcer: an alternative to surgery. Acta Radiol. 2006;47:244-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Laine L, Peterson WL. Bleeding peptic ulcer. N Engl J Med. 1994;331:717-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 439] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 12. | Langman MJ, Jensen DM, Watson DJ, Harper SE, Zhao PL, Quan H, Bolognese JA, Simon TJ. Adverse upper gastrointestinal effects of rofecoxib compared with NSAIDs. JAMA. 1999;282:1929-1933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 409] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 13. | Hunt RH, Harper S, Watson DJ, Yu C, Quan H, Lee M, Evans JK, Oxenius B. The gastrointestinal safety of the COX-2 selective inhibitor etoricoxib assessed by both endoscopy and analysis of upper gastrointestinal events. Am J Gastroenterol. 2003;98:1725-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Mamdani M, Juurlink DN, Kopp A, Naglie G, Austin PC, Laupacis A. Gastrointestinal bleeding after the introduction of COX 2 inhibitors: ecological study. BMJ. 2004;328:1415-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Llach J, Elizalde JI, Guevara MC, Pellisé M, Castellot A, Ginès A, Soria MT, Bordas JM, Piqué JM. Endoscopic injection therapy in bleeding Mallory-Weiss syndrome: a randomized controlled trial. Gastrointest Endosc. 2001;54:679-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Huang SP, Wang HP, Lee YC, Lin CC, Yang CS, Wu MS, Lin JT. Endoscopic hemoclip placement and epinephrine injection for Mallory-Weiss syndrome with active bleeding. Gastrointest Endosc. 2002;55:842-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Morales P, Baum AE. Therapeutic Alternatives for the Mallory-Weiss Tear. Curr Treat Options Gastroenterol. 2003;6:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Lee YT, Walmsley RS, Leong RW, Sung JJ. Dieulafoy's lesion. Gastrointest Endosc. 2003;58:236-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Savides TJ, Jensen DM, Cohen J, Randall GM, Kovacs TO, Pelayo E, Cheng S, Jensen ME, Hsieh HY. Severe upper gastrointestinal tumor bleeding: endoscopic findings, treatment, and outcome. Endoscopy. 1996;28:244-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 100] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Chapman WC, Abecassis M, Jarnagin W, Mulvihill S, Strasberg SM. Bile duct injuries 12 years after the introduction of laparoscopic cholecystectomy. J Gastrointest Surg. 2003;7:412-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Loffroy R, Guiu B, Cercueil JP, Lepage C, Cheynel N, Steinmetz E, Ricolfi F, Krausé D. Transcatheter arterial embolization of splenic artery aneurysms and pseudoaneurysms: short- and long-term results. Ann Vasc Surg. 2008;22:618-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Cappell MS, Abdullah M. Management of gastrointestinal bleeding induced by gastrointestinal endoscopy. Gastroenterol Clin North Am. 2000;29:125-67, vi-vii. [PubMed] |
| 23. | Frisoli JK, Sze DY, Kee S. Transcatheter embolization for the treatment of upper gastrointestinal bleeding. Tech Vasc Interv Radiol. 2004;7:136-142. [PubMed] |
| 24. | Kadir S, Lundell C, Saeed M. Celiac, superior, and inferior mesenteric arteries. Atlas of normal and variant angiography anatomy. Philadelphia: WB Saunders 1991; 297-308. |
| 25. | Loffroy R, Guiu B, D'Athis P, Mezzetta L, Gagnaire A, Jouve JL, Ortega-Deballon P, Cheynel N, Cercueil JP, Krausé D. Arterial embolotherapy for endoscopically unmanageable acute gastroduodenal hemorrhage: predictors of early rebleeding. Clin Gastroenterol Hepatol. 2009;7:515-523. [PubMed] |
| 26. | Porter DH, Kim D. Angiographic intervention in upper gastrointestinal bleeding. Gastrointestinal emergencies. Baltimore: Williams & Wilkins 1997; 63-180. |
| 27. | Walsh RM, Anain P, Geisinger M, Vogt D, Mayes J, Grundfest-Broniatowski S, Henderson JM. Role of angiography and embolization for massive gastroduodenal hemorrhage. J Gastrointest Surg. 1999;3:61-5; discussion 66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Smith R, Copely DJ, Bolen FH. 99mTc RBC scintigraphy: correlation of gastrointestinal bleeding rates with scintigraphic findings. AJR Am J Roentgenol. 1987;148:869-874. [PubMed] |
| 29. | Gunderman R, Leef J, Ong K, Reba R, Metz C. Scintigraphic screening prior to visceral arteriography in acute lower gastrointestinal bleeding. J Nucl Med. 1998;39:1081-1083. [PubMed] |
| 30. | Lo SK. Capsule endoscopy in the diagnosis and management of inflammatory bowel disease. Gastrointest Endosc Clin N Am. 2004;14:179-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Kovacs TO. Small Bowel Bleeding. Curr Treat Options Gastroenterol. 2005;8:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Defreyne L, Vanlangenhove P, Decruyenaere J, Van Maele G, De Vos M, Troisi R, Pattyn P. Outcome of acute nonvariceal gastrointestinal haemorrhage after nontherapeutic arteriography compared with embolization. Eur Radiol. 2003;13:2604-2614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Whitaker SC, Gregson RH. The role of angiography in the investigation of acute or chronic gastrointestinal haemorrhage. Clin Radiol. 1993;47:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Miller M, Smith TP. Angiographic diagnosis and endovascular management of nonvariceal gastrointestinal hemorrhage. Gastroenterol Clin North Am. 2005;34:735-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Aina R, Oliva VL, Therasse E, Perreault P, Bui BT, Dufresne MP, Soulez G. Arterial embolotherapy for upper gastrointestinal hemorrhage: outcome assessment. J Vasc Interv Radiol. 2001;12:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 172] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 36. | Padia SA, Geisinger MA, Newman JS, Pierce G, Obuchowski NA, Sands MJ. Effectiveness of coil embolization in angiographically detectable versus non-detectable sources of upper gastrointestinal hemorrhage. J Vasc Interv Radiol. 2009;20:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Rösch J, Keller FS, Wawrukiewicz AS, Krippaehne WW, Dotter CT. Pharmacoangiography in the diagnosis of recurrent massive lower gastrointestinal bleeding. Radiology. 1982;145:615-619. [PubMed] |
| 38. | Koval G, Benner KG, Rösch J, Kozak BE. Aggressive angiographic diagnosis in acute lower gastrointestinal hemorrhage. Dig Dis Sci. 1987;32:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 71] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Malden ES, Hicks ME, Royal HD, Aliperti G, Allen BT, Picus D. Recurrent gastrointestinal bleeding: use of thrombolysis with anticoagulation in diagnosis. Radiology. 1998;207:147-151. [PubMed] |
| 40. | Bloomfeld RS, Smith TP, Schneider AM, Rockey DC. Provocative angiography in patients with gastrointestinal hemorrhage of obscure origin. Am J Gastroenterol. 2000;95:2807-2812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Ryan JM, Key SM, Dumbleton SA, Smith TP. Nonlocalized lower gastrointestinal bleeding: provocative bleeding studies with intraarterial tPA, heparin, and tolazoline. J Vasc Interv Radiol. 2001;12:1273-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Widlus DM, Salis AI. Reteplase provocative visceral arteriography. J Clin Gastroenterol. 2007;41:830-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Johnston C, Tuite D, Pritchard R, Reynolds J, McEniff N, Ryan JM. Use of provocative angiography to localize site in recurrent gastrointestinal bleeding. Cardiovasc Intervent Radiol. 2007;30:1042-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Shetzline MA, Suhocki P, Dash R, Rockey DC. Provocative angiography in obscure gastrointestinal bleeding. South Med J. 2000;93:1205-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 45. | Kim CY, Suhocki PV, Miller MJ, Khan M, Janus G, Smith TP. Provocative mesenteric angiography for lower gastrointestinal hemorrhage: results from a single-institution study. J Vasc Interv Radiol. 2010;21:477-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 46. | Burke SJ, Golzarian J, Weldon D, Sun S. Nonvariceal upper gastrointestinal bleeding. Eur Radiol. 2007;17:1714-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Barr JW, Lakin RC, Rösch J. Vasopressin and hepatic artery. Effect of selective celiac infusion of vasopressin on the hepatic artery flow. Invest Radiol. 1975;10:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 48. | Sherman LM, Shenoy SS, Cerra FB. Selective intra-arterial vasopressin: clinical efficacy and complications. Ann Surg. 1979;189:298-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 49. | Clark RA, Colley DP, Eggers FM. Acute arterial gastrointestinal hemorrhage: efficacy of transcatheter control. AJR Am J Roentgenol. 1981;136:1185-1189. [PubMed] |
| 50. | Eckstein MR, Kelemouridis V, Athanasoulis CA, Waltman AC, Feldman L, van Breda A. Gastric bleeding: therapy with intraarterial vasopressin and transcatheter embolization. Radiology. 1984;152:643-646. [PubMed] |
| 51. | Stump DL, Hardin TC. The use of vasopressin in the treatment of upper gastrointestinal haemorrhage. Drugs. 1990;39:38-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Loffroy R, Rao P, Ota S, De Lin M, Kwak BK, Geschwind JF. Embolization of acute nonvariceal upper gastrointestinal hemorrhage resistant to endoscopic treatment: results and predictors of recurrent bleeding. Cardiovasc Intervent Radiol. 2010;33:1088-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 53. | Loffroy R, Guiu B, Mezzetta L, Minello A, Michiels C, Jouve JL, Cheynel N, Rat P, Cercueil JP, Krausé D. Short- and long-term results of transcatheter embolization for massive arterial hemorrhage from gastroduodenal ulcers not controlled by endoscopic hemostasis. Can J Gastroenterol. 2009;23:115-120. [PubMed] |
| 54. | Lenhart M, Paetzel C, Sackmann M, Schneider H, Jung EM, Schreyer AG, Feuerbach S, Zorger N. Superselective arterial embolisation with a liquid polyvinyl alcohol copolymer in patients with acute gastrointestinal haemorrhage. Eur Radiol. 2010;20:1994-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 55. | Loffroy R, Guiu B, Cercueil JP, Lepage C, Latournerie M, Hillon P, Rat P, Ricolfi F, Krausé D. Refractory bleeding from gastroduodenal ulcers: arterial embolization in high-operative-risk patients. J Clin Gastroenterol. 2008;42:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 56. | Eriksson LG, Sundbom M, Gustavsson S, Nyman R. Endoscopic marking with a metallic clip facilitates transcatheter arterial embolization in upper peptic ulcer bleeding. J Vasc Interv Radiol. 2006;17:959-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 57. | Toyoda H, Nakano S, Takeda I, Kumada T, Sugiyama K, Osada T, Kiriyama S, Suga T. Transcatheter arterial embolization for massive bleeding from duodenal ulcers not controlled by endoscopic hemostasis. Endoscopy. 1995;27:304-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 58. | De Wispelaere JF, De Ronde T, Trigaux JP, de Cannière L, De Geeter T. Duodenal ulcer hemorrhage treated by embolization: results in 28 patients. Acta Gastroenterol Belg. 2002;65:6-11. [PubMed] |
| 59. | van Vugt R, Bosscha K, van Munster IP, de Jager CP, Rutten MJ. Embolization as treatment of choice for bleeding peptic ulcers in high-risk patients. Dig Surg. 2009;26:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Lang EK. Transcatheter embolization in management of hemorrhage from duodenal ulcer: long-term results and complications. Radiology. 1992;182:703-707. [PubMed] |
| 61. | Park JH, Kim HC, Chung JW, Jae HJ, Park JH. Transcatheter arterial embolization of arterial esophageal bleeding with the use of N-butyl cyanoacrylate. Korean J Radiol. 2009;10:361-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 62. | Lee CW, Liu KL, Wang HP, Chen SJ, Tsang YM, Liu HM. Transcatheter arterial embolization of acute upper gastrointestinal tract bleeding with N-butyl-2-cyanoacrylate. J Vasc Interv Radiol. 2007;18:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 63. | Toyoda H, Nakano S, Kumada T, Takeda I, Sugiyama K, Osada T, Kiriyama S. Estimation of usefulness of N-butyl-2-cyanoacrylate-lipiodol mixture in transcatheter arterial embolization for urgent control of life-threatening massive bleeding from gastric or duodenal ulcer. J Gastroenterol Hepatol. 1996;11:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 64. | Panagiotopoulos V, Gizewski E, Asgari S, Regel J, Forsting M, Wanke I. Embolization of intracranial arteriovenous malformations with ethylene-vinyl alcohol copolymer (Onyx). AJNR Am J Neuroradiol. 2009;30:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 154] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 65. | Loffroy R, Guiu B, Cercueil JP, Krausé D. Endovascular therapeutic embolisation: an overview of occluding agents and their effects on embolised tissues. Curr Vasc Pharmacol. 2009;7:250-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 66. | Lau KY, Wong TP, Wong WW, Chan JK, Kan WK, Chan YF, Lee AS. Transcatheter embolisation of visceral pseudoaneurysms--technical difficulties and modification of embolisation technique. Eur J Vasc Endovasc Surg. 2005;30:133-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 67. | Tokuda T, Tanigawa N, Shomura Y, Kariya S, Kojima H, Komemushi A, Shiraishi T, Sawada S. Transcatheter embolization for peripheral pseudoaneurysms with n-butyl cyanoacrylate. Minim Invasive Ther Allied Technol. 2009;18:361-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 68. | Lubicz B, Leclerc X, Gauvrit JY, Lejeune JP, Pruvo JP. Three-dimensional packing with complex orbit coils for the endovascular treatment of intracranial aneurysms. AJNR Am J Neuroradiol. 2005;26:1342-1348. [PubMed] |
| 69. | Moret J, Cognard C, Weill A, Castaings L, Rey A. [Reconstruction technic in the treatment of wide-neck intracranial aneurysms. Long-term angiographic and clinical results. Apropos of 56 cases]. J Neuroradiol. 1997;24:30-44. [PubMed] |
| 70. | Vanninen RL, Manninen I. Onyx, a new liquid embolic material for peripheral interventions: preliminary experience in aneurysm, pseudoaneurysm, and pulmonary arteriovenous malformation embolization. Cardiovasc Intervent Radiol. 2007;30:196-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |