Published online Dec 27, 2011. doi: 10.4240/wjgs.v3.i12.201
Revised: October 31, 2011
Accepted: November 8, 2011
Published online: December 27, 2011
Solid-pseudopapillary tumors of the pancreas (SPTs) are comparatively rare and have low malignancy, with a predilection for young women. Diagnosis is difficult when a SPT develops in a boundary region with other organs. Here, we report a 42-year old woman with a SPT of the pancreas mimicking a submucosal tumor of the stomach on imaging. She was admitted to our hospital complaining of abdominal pain. We suspected a submucosal tumor of the stomach from the findings of endoscopy, endoscopic ultrasonography and abdominal computed tomography. However, angiography showed that some of the tumor vessels arose from the pancreas. Intraoperative findings revealed the tumor originated from the pancreas. Therefore, distal pancreatectomy was performed. The pathological diagnosis was SPT of the pancreas.
- Citation: Furuhashi S, Takamori H, Abe S, Nakahara O, Tanaka H, Horino K, Beppu T, Iyama KI, Baba H. Solid-pseudopapillary pancreatic tumor, mimicking submucosal tumor of the stomach: A case report. World J Gastrointest Surg 2011; 3(12): 201-203
- URL: https://www.wjgnet.com/1948-9366/full/v3/i12/201.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v3.i12.201
Solid-pseudopapillary tumors (SPTs), including solid-pseudopapillary neoplasms (SPNs), are comparatively rare, accounting for less than 1% of pancreatic neoplasms[1]. Frantz described SPN as an entity among pancreatic tumors with solid and cystic components, which had been previously misdiagnosed as nonfunctioning islet cell tumors[2]. SPN usually presents as a large mass containing a cystic component induced by internal hemorrhage and partial necrosis[3]. On the other hand, gastrointestinal stromal tumors (GISTs) are a type of submucosal tumor (SMT) of the stomach. Macroscopic growth patterns are classified into endoluminal, intraluminal, extraluminal and mixed types[4]. The extraluminal type has been reported to represent 67%-70% of all GISTs[5,6]. Here, we present a case of extramural compression of the stomach wall caused by a SPT of the pancreatic tail, mimicking a SMT of the stomach.
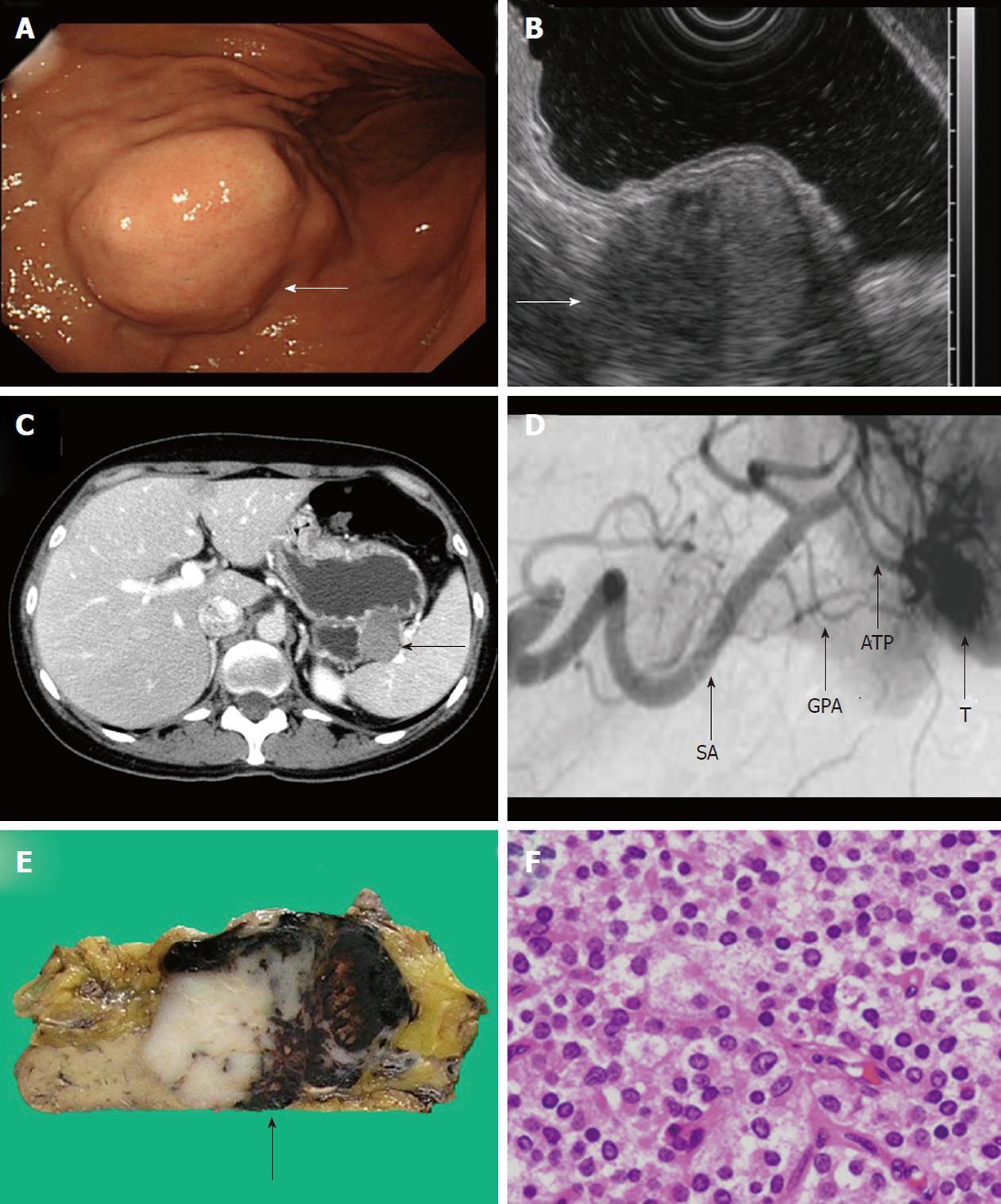
A 42-year old woman was admitted to our hospital complaining of abdominal pain in the upper left quadrant. All laboratory data were within normal limits, including tumor markers and hormones. Gastric endoscopy showed a smoothly elevated tumor in the posterior wall of the upper gastric body without mucosal abnormality. A working diagnosis of SMT of the stomach was made (Figure 1A). Endoscopic ultrasonography (EUS) revealed that the tumor had heterogeneous internal echoes and continuity of the muscularis propria. From these findings, we suspected GIST of the stomach (Figure 1B). Abdominal CT revealed that the tumor included an extragastric extension, close to the splenic hilum, 4.4 cm in diameter (Figure 1C). Angiography showed that tumor vessels diverged from the greater and dorsal pancreatic arteries (Figure 1D). Laparoscopic partial gastrectomy was planned based on the preoperative diagnosis of GIST of the stomach.
Intraoperative findings revealed that the tumor originated from the pancreas. The tumor was well encapsulated. Therefore, distal pancreatectomy was performed. The tumor was solid with focal necrosis and hemorrhage, macroscopically (Figure 1E). Histological examination revealed that the tumor cells had round to oval nuclei (Figure 1F), although a pseudopapillary pattern was not apparent. Immunohistochemical staining showed that the tumor cells were positive for CD10, CD56, α1-antitrypsin and α1-antichymotrypsin and negative for endocrine markers, including chromogranin A, somatostatin, vasoactive intestinal peptide, insulin, glucagon, gastrin and somatostatin receptor (SSTR)-2a, SSTR-3 and SSTR-5. These histological findings were used to make a final diagnosis of SPT of the pancreas.
The presenting features of SPT are known to be nonspecific. Abdominal pain is the most common symptom, followed by a slowly enlarging, non-tender, upper abdominal mass at the epigastrium or the left or right hypochondrium[7]. Some patients are completely asymptomatic and the lesions are detected either on routine examination or after injury[7]. The most common localization of SPT is in the tail of the pancreas, although extra-pancreatic localization occurs in 1.0% of cases[8]. In our case, the SPT was in the tail of the pancreas, enlarged beyond the boundary of the organ and compressing the stomach, which mimicked SMT of the stomach on endoscopy, EUS and CT. Only abdominal angiography indicated that the tumor derived from the pancreas because some tumor vessels diverged from the pancreatic arteries. Precisely identifying the origin of tumor vessels is useful in determining tumor origin.
Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is a useful modality for making the histological diagnosis of gastrointestinal neoplasms, with a diagnostic accuracy of 90%[9]. The usefulness of EUS-FNA to diagnose SPN is also reported[9], although it was not performed in this case. If a quantitative diagnosis had been carried out using EUS-FNA, a less invasive laparoscopic surgery might have been indicated. It is recommended that EUS-FNA be used to confirm the histological diagnosis preoperatively in such cases.
Some authors have reported similar cases to the present case[10,11], so this clinical situation may not be unique. Chen et al[12] reported that in 55 patients with extragastric compression, the stomach was compressed by normal extragastric organs (spleen in 10 cases, splenic vessels in 6, gallbladder in 9, liver in 3, pancreas in 3 and intestine in 1) (58%), benign pathological lesions (liver cyst in 7, liver hemangioma in 2, splenic cyst in 1, pancreatic cyst in 1 and pancreatic cystadenoma in 1) (22%), malignant tumors (hepatoma in 1, liver metastasis from colon cancer in 2, pancreatic cystadenocarcinoma in 1 and lymphoma of the spleen in 1) (9%) and in the remaining six patients, the external compression was considered transient (11%).
EUS is commonly agreed to be the best imaging method for diagnosing and differentiating between submucosal lesions and extragastric compression[13]. However, the accuracy of EUS in the differentiation of an extragastric compression from a submucosal tumor is not perfect. Therefore, it is important to evaluate such cases thoroughly using all the above mentioned examination findings.
Peer reviewer: Sonshin Takao, MD, PhD, Professor, Division of Advanced Medicine, Kagoshima University, Frontier Science Research Center, 8-35-1 Sakuragaoka, Kagoshima 890-8544, Japan
S- Editor Wang JL L- Editor Roemmele A E- Editor Zhang DN
| 1. | Klimstra DS, Wenig BM, Heffess CS. Solid-pseudopapillary tumor of the pancreas: a typically cystic carcinoma of low malignant potential. Semin Diagn Pathol. 2000;17:66-80. [PubMed] |
| 2. | Frantz VK. Tumor of the pancreas. Washington, DC, USA: Armed Forces Institute of Pathology 1959; 32-33 (fascicles 27 and 28). |
| 3. | Klöppel G, Kosmahl M. Cystic lesions and neoplasms of the pancreas. The features are becoming clearer. Pancreatology. 2001;1:648-655. [PubMed] |
| 4. | Skandalakis JE, Gray SW, Shepard D. Smooth muscle tumors of the stomach. Int Abstr Surg. 1960;110:209-226. [PubMed] |
| 5. | Ghanem N, Altehoefer C, Furtwängler A, Winterer J, Schäfer O, Springer O, Kotter E, Langer M. Computed tomography in gastrointestinal stromal tumors. Eur Radiol. 2003;13:1669-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Lee CM, Chen HC, Leung TK, Chen YY. Gastrointestinal stromal tumor: Computed tomographic features. World J Gastroenterol. 2004;10:2417-2418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 2005;200:965-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 536] [Article Influence: 26.8] [Reference Citation Analysis (1)] |
| 8. | Vander Noot MR, Eloubeidi MA, Chen VK, Eltoum I, Jhala D, Jhala N, Syed S, Chhieng DC. Diagnosis of gastrointestinal tract lesions by endoscopic ultrasound-guided fine-needle aspiration biopsy. Cancer. 2004;102:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 140] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Jhala N, Siegal GP, Jhala D. Large, clear cytoplasmic vacuolation: an under-recognized cytologic clue to distinguish solid pseudopapillary neoplasms of the pancreas from pancreatic endocrine neoplasms on fine-needle aspiration. Cancer. 2008;114:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Chen JH, Wang HP, Wu MS, Chou AL, Lin CC, Shun CT, Lee PH, Lin JT. Gastric leiomyosarcoma mimicking a cystic tumor at the pancreatic tail--one case report. Hepatogastroenterology. 1998;45:2468-2470. [PubMed] |
| 11. | Higuchi N, Akahoshi K, Honda K, Matsui N, Kubokawa M, Motomura Y, Nakamura K, Takayanagi R. Diagnosis of a small splenic artery aneurysm mimicking a gastric submucosal tumor on endoscopic ultrasound. Endoscopy. 2010;42 Suppl 2:E107-E108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (2)] |
| 12. | Chen TK, Wu CH, Lee CL, Lai YC, Yang SS, Tu TC. Endoscopic ultrasonography to study the causes of extragastric compression mimicking gastric submucosal tumor. J Formos Med Assoc. 2001;100:758-761. [PubMed] |
| 13. | Motoo Y, Okai T, Ohta H, Satomura Y, Watanabe H, Yamakawa O, Yamaguchi Y, Mouri I, Sawabu N. Endoscopic ultrasonography in the diagnosis of extraluminal compressions mimicking gastric submucosal tumors. Endoscopy. 1994;26:239-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 41] [Article Influence: 1.3] [Reference Citation Analysis (0)] |