CASE REPORT
A 21-year old man came to the accident and emergency unit with a 4-d history of bilious vomiting (roughly 10 episodes per day), associated with progressive abdominal distention, asthenia and constipation, without fever. At physical examination on admission: the patient displayed cachexia; heart rate 103/min; blood pressure 90/60 mmHg; soft distended abdomen with dull percussion tones; mild pain on palpation but no sign of peritoneal irritation; digital rectal examination revealed pellet-like stools; generalized muscular atrophy; fingers flexed on both hands; and predominantly distal paresis. The patient walked with the aid of a prosthetic lower limb. Blood work on admission was as follows: leukocytes 20 000 cells/mcL; neutrophils 86.7%; Hb 15.6 g/dL; Hct 45.1%; platelets 291 000 cells/mcL; ActPT% 64.9%; INR 1.2; CRP 102 mg/L; glucose 179 mg/dL; urea 148 mg/dL; creatinine 1.18 mg/dL; potassium 2.8 mEq/L; sodium 132 mEq/L; chlorine 95 mEq/L; amylase 207; and total bilirubin 1.2 mg/dL. The patient had a history of Charcot Marie Tooth disease, which began at age 3 years with bilateral pes equinovarus and progressive mainly-distal paresis. He underwent club-foot repair surgery at the age of 6 years. The patient also displayed chronic protein-energy malnutrition and partial functional dependence.
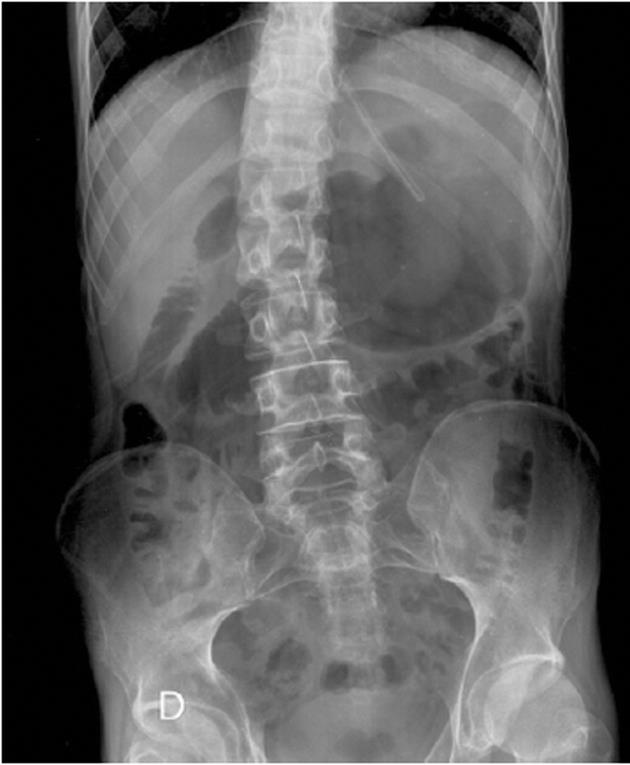
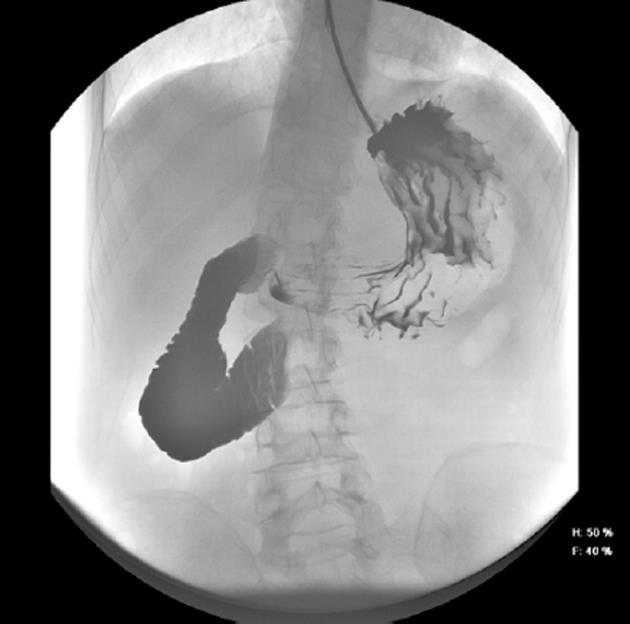
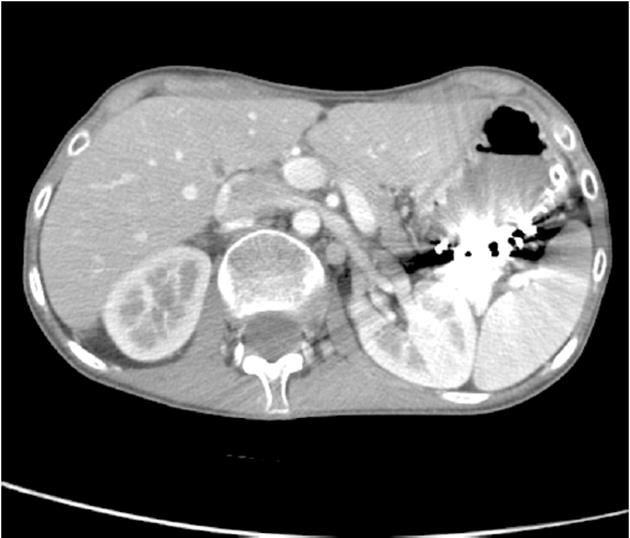
Following examination at the accident and emergency unit, the patient was admitted to the surgical unit with suspected partial bowel blockage. Abdominal X-ray showed gastric and duodenal dilatation (Figure 1). Barium-meal examination demonstrated duodenal dilatation, marked peristaltic reflex and an obstruction in the third part of the duodenum (Figure 2). The abdominal CT scan revealed marked distension of the stomach and duodenum as far as the third part; narrowing of the duodenal caliber was observed at the point where the duodenum passed between the aorta and the superior mesenteric artery (Figure 3). No free fluid or pneumoperitoneum were found. CT angiography revealed narrowing of the duodenum as it passed between the aorta and the superior mesenteric artery; the aorto-mesenteric angle was 16º and the aorto-mesenteric distance was 4 mm; no lymph-node enlargement was observed. The diagnosis was superior mesenteric artery syndrome.
Figure 1 Abdominal X-ray showing gastric and duodenal dilatation.
Figure 2 Upper gastrointestinal barium study showing blockage of the third part of the duodenum.
Figure 3 Reduced aorto-mesenteric distance evident in CT scan.
A nasogastric tube was inserted to maintain duodenal and gastric decompression. Attempted endoscopic introduction of a nasojejunal tube for enteral feeding failed due to complete blockage of the duodenum. A central venous catheter was placed in the left jugular vein. Following consultation with the nutrition unit, parenteral feeding was started. After three weeks of parenteral feeding support and, in view of the patient’s chronic malnutrition and consequently poor response to conservative management, surgical treatment was indicated.
A scheduled open duodenojejunostomy was performed. Midline supraumbilical laparatomy revealed dilatation of the duodenum from the third part onwards and compression by the superior mesenteric artery. Following a wide Kocher maneuver and division of the ligament of Treitz (Strong’s operation), a side-to-side transmesocolic duodenojejunostomy was performed and a feeding jejunostomy was created. A Penrose drain was placed near the anastomosis.
Initial postoperative progress was slow due to emesis. A double-contrast gastroduodenal study confirmed the permeability of the duodenojejunostomy and the absence of anastomotic leakage. When the feeding jejunostomy was removed, emesis ceased and oral tolerance gradually improved. Thereafter, progress was satisfactory and the patient was discharged.
DISCUSSION
This disorder was first described by Carl Von Rokitansky in 1842[3]. In 1878, Willett reported a case of death secondary to what he termed “fatal vomiting” following application of a body cast for scoliosis[4]. Autopsy revealed massive dilatation of the stomach and duodenum. This gave rise to the use of the term “cast syndrome”, although at this stage it was not clearly accepted that duodenal dilatation was due to extrinsic compression at the aorto-mesenteric angle. In the early twentieth century, the SMA syndrome was known as chronic duodenal ileus or gastromesenteric ileus. In 1927, Wilkie reported a series of 75 patients with duodenal ileus, 64 of who underwent duodenojejunostomy, which confirmed extrinsic mechanical compression of the third part of the duodenum by the superior mesenteric root. Wilkie argued that chronic duodenal ileus was thus a true clinical and pathological entity, which later became known as Wilkie’s syndrome[5].
The superior mesenteric artery arises from the right anterolateral surface of the abdominal aorta, anterior to vertebra L1. It immediately passes through the third part of the duodenum into the mesenteric root. The duodenojejunal flexure is attached to the posterior parietal peritoneum by the ligament of Treitz, whose band of muscle and connective tissue arises from the right crus of the diaphragm and spreads over the fourth portion of the duodenum and the jejunoduodenal angle, which are thus held in position at L2 level. According to an angiographic study by Mansberger et al[6], this specific anatomical configuration gives rise to a normal aorto-mesenteric angle ranging from 25º to 60º (abnormal 7º-22º) and a normal aorto-mesenteric distance, at duodenum level, of 10 to 28 mm (pathological 2 to 8 mm). SMAS results from the narrowing of this anatomical angle is due to a range of causes: loss of the fatty cushion around the superior mesenteric artery as a result of malnutrition due to diabetes, HIV, heart disease or neuropathy; alteration of the insertion or the length of the ligament of Treitz; inflammatory or tumoral infiltration of the mesenteric root; aortic aneurysm; increased lumbar lordosis; or the application of a body cast[7]. SMAS should be distinguished from a SMA-like syndrome. Duodenal dilatation may be due to a range of causes, including amyloidosis, dermatomyositis, lupus erythematosus, scleroderma, myxedema, or myotonic dystrophy, none of which lead to extrinsic vascular compression of the duodenum or to changes in the aorto-mesenteric angle or distance.
Clinical manifestations of SMAS may be chronic or acute; chronic symptoms include intermittent gastric pain, fullness and occasional episodes of postprandial vomiting, whilst acute symptoms include incoercible vomiting, oral intolerance, mainly epigastric abdominal distension and abdominal pain. Symptoms are generally relieved in the left lateral decubitus and knees-chest positions, and their intensity is directly proportional to the aorto-mesenteric distance. SMAS should be strongly suspected in patients with bilious vomiting and weight loss, and special attention should be paid to diabetic patients displaying weight loss and poor glycemic control, since symptoms might be mistakenly ascribed to gastroparesis[8,9]. Differential diagnosis should include pancreatitis, peptic ulcer disease, intestinal malrotation with volvulus, and secondary obstruction by adhesions or masses. SMAS may, in some cases, give rise to significant complications, such as massive gastric dilatation with intragastric pressures of over 30 cmH20 leading to parietal ischemia and necrosis, prompting gastric wall pneumatosis, portal venous gas and gastric rupture[10]. Moreover, persistent emesis may give rise to secondary Mallory-Weiss syndrome[11], whilst reflux and biliary stasis may trigger peptic ulcer disease.
The two routine diagnostic tests for SMAS are: barium studies of the upper gastointestinal tract, which reveal duodenal dilatation, retention of the contrast medium, marked retrograde peristalsis, positive Hayes’ maneuver (contrast flows into jejunum when patient is placed in left lateral decubitus position) and vertical indentation of the third part of the duodenum; and angiography, which enables measurement of the aorto-mesenteric angles. Where there is no evidence of duodenal dilatation, hypotonic duodenography may be used as a diagnostic alternative[12]. Upper endoscopy can be used to rule out stenosing masses in the duodenum. The aorto-mesenteric distance can be measured by abdominal ultrasonography, while abdominal radiography reveals the double-bubble sign of duodenal obstruction. Konen et al[13] report that CT angiography with 3D reconstructions allows more accurate measurements of the aorta-SMA angle and distance than angiography alone since the superior mesenteric artery is anterolateral to the aorta; as a result, this is currently considered the technique of choice, as it is both non-invasive and more precise.
Surgical treatment of SMAS is recommended only when initial conservative treatment has failed. Conservative treatment in the acute phase involves insertion of a nasogastric tube for decompression, together with aggressive fluid and electrolyte replacement therapy, associated with anti-emetic agents and posture therapy, e.g., placing the patient in the left lateral decubitus position in order to increase the aorto-mesenteric angle. Parenteral nutrition can then be instituted using a nasojejunal tube, starting with a slow infusion rate to avoid refeeding syndrome. Electrolyte balance and weight gain should be monitored regularly. The aim is to replenish mesenteric fat stores in order to decompress the third portion of the duodenum. Once on a stable regimen, the tube feeds can be given at home; after two or three weeks, oral food intake can be resumed[14]. Parenteral nutrition can be used where appropriate. However, surgical treatment should be the first choice if conservative management is deemed to have failed after 3 or 4 wk, if the patient displays long-standing chronic disease with progressive weight loss and duodenal dilatation with stasis, or if there is evidence of complicated peptic ulcer disease[15].
In 1908, Stavely recommended surgical treatment of gastromesenteric ileus, in the form of a duodenojejunostomy, with a view to bypassing the mechanical obstruction at superior mesenteric artery level[16]. Thereafter, this became the surgical treatment of choice for SMAS, with a reported success rate of 90%[17]. Nevertheless, in 1958, Strong, in view of the physiopathological mechanisms involved in SMAS, recommended wide division of the ligament of Treitz[18]. By 1997, with the advent of minimally-invasive surgery, Massoud[19] was the first to implement a laparoscopic version of Strong’s operation; one year later, Gersin and Heniford[20] performed the first laparoscopic duodenojejunostomy. Other surgical alternatives include anterior transposition of the third part of the duodenum, a technique described by Duvie[21], and gastrojejunostomy, which is useful in cases of underlying gastric dilatation or associated peptic ulcer disease and where it is difficult to mobilize the duodenum. Where secondary duodenal dysfunction is so marked that it leads to strong, continuous reversed peristalsis with opening of the pyloric duct and an atonic stomach, expansion of the duodenal cavity to 8 cm or more and intraluminal pressure of 15 to 17 cmH20, duodenojejunostomy does not cure the clinical symptoms. For such cases, Yang et al[22] designed a duodenal circular drainage method, comprising of triple duodenojejunal, gastrojejunal and jejunojejunal anastomosis, giving rise to a drainage circuit that compensates for reflux from the dysfunctional duodenum.
To conclude, this is the third report of SMAS in a patient with NHSM treated successfully with the surgical technique of choice using a conventional approach. In 1986, Daher et al[23] described the development of SMAS in a patient with CMT undergoing corrective surgery for scoliosis. In 2001, Lin et al[24] reported a patient with HMSN type II who developed SMAS and was treated in a conservative fashion. In this case, initial conservative management was ruled out due to the chronic nature of the symptoms and the lack of response to nutritional therapy.