Published online Jan 27, 2011. doi: 10.4240/wjgs.v3.i1.16
Revised: September 19, 2010
Accepted: September 26, 2010
Published online: January 27, 2011
A peritoneovenous shunt has become one of the most efficient procedures for intractable ascites due to liver cirrhosis. A case of intractable ascites due to hepatic lymphorrhea after hepatectomy for hepatocellular carcinoma that was successfully treated by the placement of a peritoneovenous shunt is presented. A 72-year-old Japanese man underwent partial resection of the liver for hepatocellular carcinoma associated with hepatitis C viral infection. After hepatectomy, a considerable amount of ascites ranging from 800-4600 mL per day persisted despite conservative therapy, including numerous infusions of albumin and plasma protein fraction and administration of diuretics. Since the patient’s general condition deteriorated, based on the diagnosis of intractable hepatic lymphorrhea, a subcutaneous peritoneovenous shunt was inserted. The patient’s postoperative course was uneventful and the ascites decreased rapidly, with serum total protein and albumin levels and hepatic function improving accordingly. For intractable ascites due to hepatic lymphorrhea after hepatectomy, we recommend the placement of a peritoneovenous shunt as a procedure that can provide immediate effectiveness without increased surgical risk.
- Citation: Inoue Y, Hayashi M, Hirokawa F, Takeshita A, Tanigawa N. Peritoneovenous shunt for intractable ascites due to hepatic lymphorrhea after hepatectomy. World J Gastrointest Surg 2011; 3(1): 16-20
- URL: https://www.wjgnet.com/1948-9366/full/v3/i1/16.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v3.i1.16
In abdominal surgery, especially after extended lymphadenectomy for gastroenterological cancer, lymphatic vessel injury causes lymphorrhea[1-4]. The postoperative lymphorrhea usually disappears spontaneously within a short time. However, intractable ascites sometimes develops in patients with liver cirrhosis[5,6], heart failure or renal failure. When a copious lymphatic discharge occurs, it is often difficult to improve the patient’s general condition, serum protein and electrolyte stores.
In 1974, LeVeen was the first to describe the placement of a peritoneovenous shunt (PVS) for intractable ascites due to liver cirrhosis[7]. Later, with the development of the Denver shunt, placement of PVS became an effective procedure for such cases.
The case of a patient with intractable ascites due to hepatic lymphorrhea from the hepatoduodenal ligament after radical hepatectomy for hepatocellular carcinoma (HCC), successfully treated by PVS placement with excellent recovery from copious ascites, is presented.
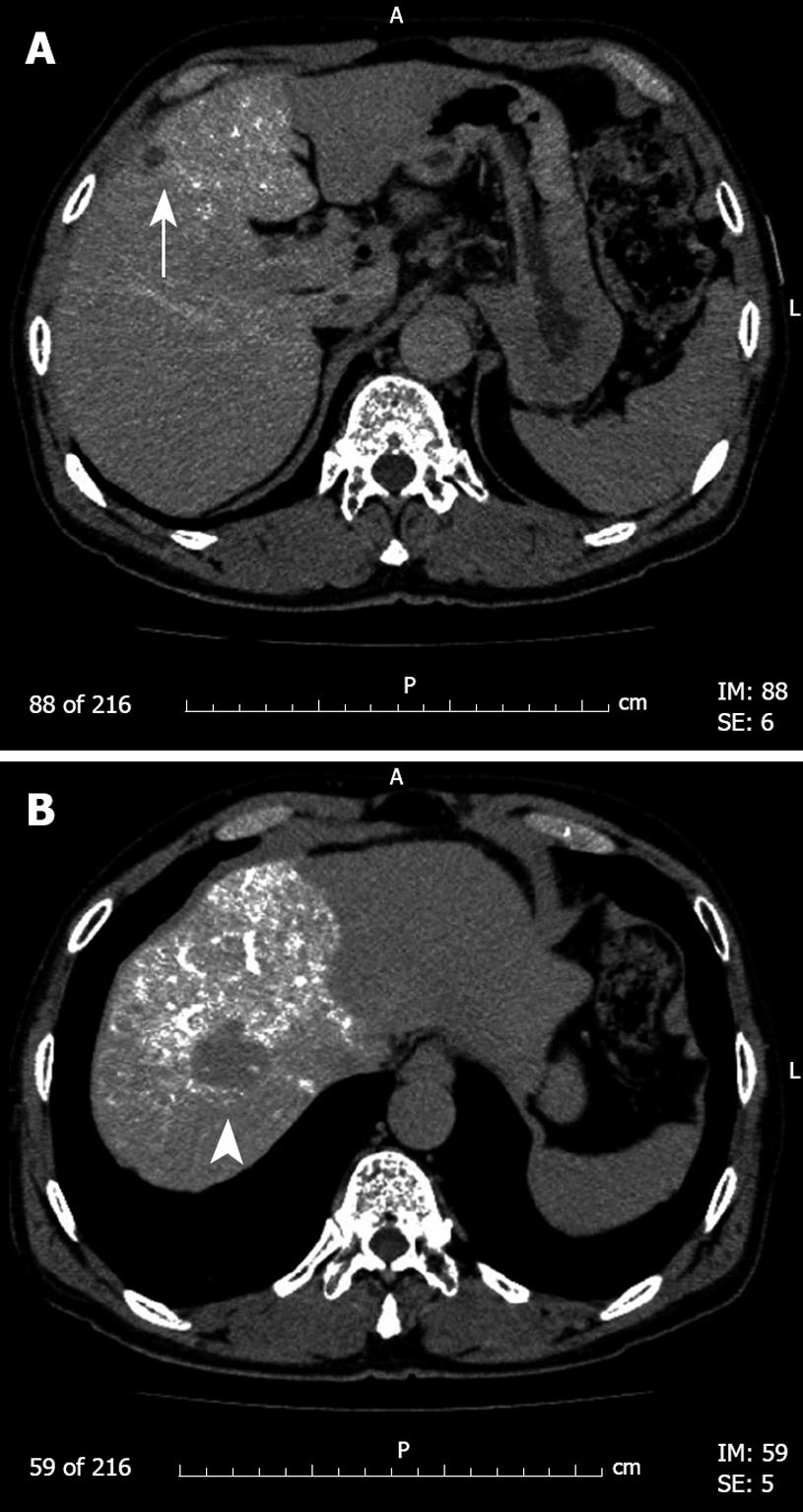
A 73-year old Japanese man was referred to our hospital for diagnostic work-up of two space-occupying lesions in the liver detected during follow-up abdominal ultrasonography for hepatitis C viral infection. The patient was asymptomatic and free from ascites. Physical examination revealed cool moist skin, pulse rate of 68 beats per min and blood pressure of 131/83 mmHg. He had a previous history of treatment including interferon therapy for hepatitis C infection aged 59 years. Laboratory findings were as follows: serological examination was positive for hepatitis C virus antibody and negative for hepatitis B surface antigen; hematocrit 37.1%, platelets 193 × 103 /μL (normal range, 162-329 × 103 /μL); serum aspartate aminotransferase 90 IU/L (normal range, 10-35 IU/L), alanine aminotransferase 154 IU/L (normal range, 5-35 IU/L), bilirubin 0.4 mg/dL (normal range, 0.1-1.0 mg/dL), total protein 8.8 g/dL (normal range, 6.3-8.0 g/dL), albumin 4.2 g/dL (normal range, 3.5-5.0 g/dL) and prothrombin time 82% (normal range, 80%-120%). The indocyanine green retention rate at 15 min after injection was 18.7% (normal range, < 10%). Serum alpha-fetoprotein was 29.5 ng/mL (normal range, < 15 ng/mL) and des-gamma carboxyprothrombin (PIVKA-II) was 29 mAU/mL (normal range, < 40 mAU/mL). Computed tomography during angiography showed two tumors, 3 and 1.3 cm in diameter, in liver segments S4 and S8, respectively (Figure 1). Abdominal ultrasound and magnetic resonance imaging (MRI) also showed similar findings. No abnormal findings were seen in other abdominal organs; there was no ascites.
With a preoperative diagnosis of HCC in S4 and S8 of the liver, partial resection of the liver was conducted in October 2007. At laparotomy, there was no ascites or peritoneal metastasis. The liver showed early stage cirrhotic change and tumors were located in S4 and S8. After cholecystectomy, a vessel loop was placed around the hepatoduodenal ligament for the Pringle maneuver. At that time, well-developed lymphatic ducts were noticed mainly in and around the hepatoduodenal ligament which were meticulously ligated and severed. During this procedure, a lymphatic oozing point was detected and ligated as well. Partial resection of the liver was performed via an anterior approach using a Cavitron ultrasonic surgical aspirator (SonoSurg system; Olympus Inc., Tokyo, Japan) and bipolar electrocautery with a saline irrigation system without the Pringle maneuver. The operation lasted 5 h and 55 min and blood loss was 850 mL.
The resected liver specimens weighed 60 and 15 g and the tumors measured 2.8 cm × 2.2 cm and 1.4 cm × 1.2 cm, in S8 and S4, respectively. The histological diagnoses of both tumors were moderately differentiated HCC (Edmondson grade II) without invasion into portal or hepatic venous systems. No positive surgical margin or metastases to regional lymph nodes were confirmed microscopically.
Starting from 3 d postoperatively, a considerable amount of ascites fluid ranging from 800-4600 mL per day drained from the abdominal drainage tube which was intractable despite albumin or plasma protein infusion and diuretic administration. The ascites was clear-colored and was diagnosed biochemically as non-chylous lymphorrhea. Cytological examination revealed no malignant cells and bacterial culture was also negative. At this time, hepatic lymphorrhea derived from surgical injury to the lymphatic vessels in and/or around the hepatoduodenal ligament was suspected since complete resolution of the lymphatic leak was not achieved intraoperatively. Thus, intractable ascites occurred although we had vigorously attempted to deal with leakage from the lymphatics. Based on the diagnosis of hepatic lymphorrhea without contamination of malignant cells, we decided to place a subcutaneous PVS (Denver shunt®, Denver PAK Single-Valved Ascites Shunt; Denver Biomedical, Golden, Co., USA) to avoid further deterioration of the patient’s nutritional status and progression of his immunocompromised condition.
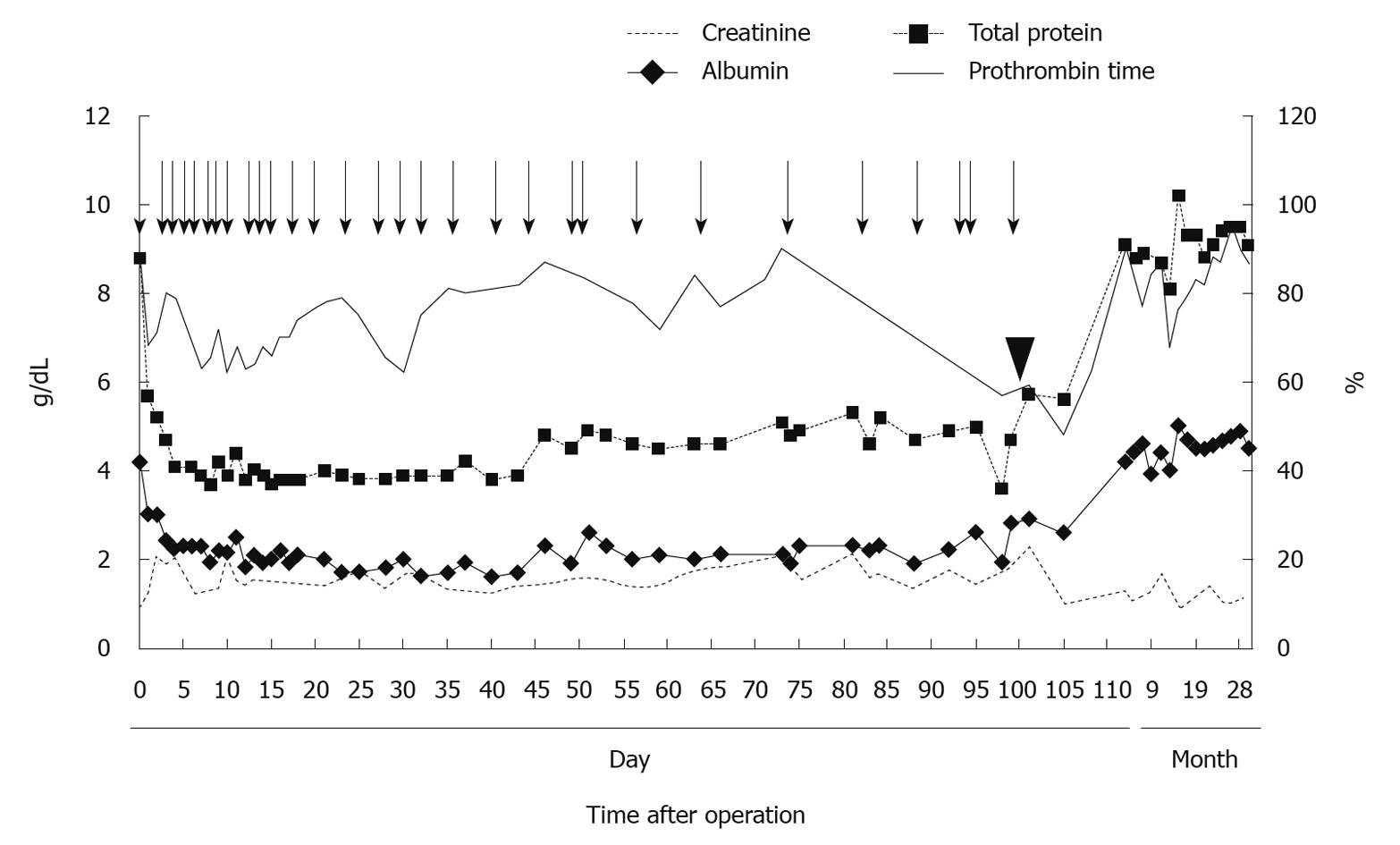
On the 98th postoperative day, a PVS was placed via the right subclavian vein under general anesthesia. The pump chamber site was created over the lower right rib cage to facilitate manual compression of the pump. The patient’s intra- and postoperative course was uneventful, his abdominal circumference decreased rapidly and his prothrombin time, serum creatinine, total protein and albumin levels improved accordingly (Figure 2). The patient was discharged on postoperative day 111 (12 d after PVS placement). Presently, he is doing well with no sign of HCC recurrence and on the last follow-up he had no ascites without using the PVS for which removal is now planned.
Intra abdominal lymph pathways are mainly classified into hepatic and intestinal lymph pathways. These two pathways both drain into the cisterna chylia round the first and second lumbar vertebra and subsequently into the circulatory system through the thoracic duct. The hepatic lymphatic system has two major pathways (i.e. ascending and descending) of the lymphatics. Via the ascending pathway, lymph from the surface of the upper part of the liver flows along the diaphragm into the cisterna chyli while lymph from the liver bed and in the liver flows along the hepatic veins. The descending pathway runs through the hepatoduodenal ligament including the portal vein, hepatic artery and bile duct. Intestinal lymph drains 50%-75% of intra abdominal lymph and contains many lipid droplets of long-chain fatty acids; thus its color is milky. On the other hand, hepatic lymph drains 25%-50% of intra abdominal lymph and the lymph is characterized as containing protein at a density as high as plasma without lipid droplets and so is clear-colored[1,2].
Although there are many reports describing the diagnosis, causes and treatment of chylous ascites from intestinal lymphorrhea[8], little is known regarding hepatic lymphorrhea following abdominal surgery[1-4]. Hepatic lymphorrhea is caused by injury of the lymphatic vessels during surgery, most of which occurs particularly within the hepatoduodenal ligament. In most instances, postoperative lymphatic leakage generally subsides spontaneously without special treatment. However, it becomes intractable in cases of substantial injury to major lymphatic vessels around the cisterna chylia and thoracic duct.
In the present case, the diameter and flow volume of the lymphatic vessels in and around the hepatoduodenal ligament were significantly notable due to underlying chronic hepatitis. Moreover, lymphatic vessel injury could not have been repaired completely during the surgery, subsequently causing persistent hepatic lymphorrhea.
In intractable ascites due to postoperative lymphorrhea, abundant lymphatic outflow from the drain usually leads to the loss of circulating proteins, depletion of electrolyte stores and a reduction in circulating blood volume, all of which results in further deterioration of the patient’s clinical condition. The conventional and conservative treatments for lymphorrhea consist of supplementary infusion of albumin or plasma protein fraction, diuretic therapy, total parenteral nutrition (TPN) and intravenous re-infusion of condensed ascitic fluid. Surgical interventions include ligation of the leaking point of the lymphatic vessels and placement of a PVS. A literature search in the English and Japanese medical literature yielded a further 14 reports of hepatic lymphorrhea following abdominal surgery. Clinical and operative details of these cases and the present case are given in Table 1.
| Case No. | Author | Age/sex | Operation | Treatment | Time to complete resolution (d) |
| 1 | Miyagawa, 1983 | 65/M | TG | Surgical ligation | 13 |
| 2 | Nakashima, 1985 | 58/M | DG | Surgical ligation + antibiotics + sclerotherapy | 30 |
| 3 | Nakano, 1987 | 49/M | TG | Surgical ligation | 14 |
| 4 | Kawata, 1989 | 52/M | DG | Surgical ligation + fibrin glue + sclerotherapy | 37 |
| 5 | Umehara, 1989 | 59/M | TG | Surgical ligation | 28 |
| 6 | Kaneko, 1991 | 44/M | DG | Surgical ligation + PVS | 30 |
| 7 | Imai, 1992 | 34/M | TG | Reoperation + antibiotics + sclerotherapy | 7 |
| 8 | Shimizu, 1992 | 62/M | DG | Surgical ligation | 30 |
| 9 | Ota, 1993[1] | 70/M | DG | Surgical ligation + fibrin glue | 50 |
| 10 | Mitsuno, 1993 | 42/M | DG | PVS | ND |
| 11 | Kawahira, 1994[2] | 58/M | DG | Surgical ligation + fibrin glue + OK-432 sclerotherapy | 10 |
| 12 | Matsumoto, 1995 | 44/M | DG | Re-re-surgical ligation + fibrin glue | 14 |
| 13 | Tanaka, 1998 | 49/M | DG | Surgical ligation + fibrin glue + OK-432 sclerotherapy | 12 |
| 14 | Tanaka, 2004[4] | 66/M | TG | Surgical ligation + fibrin glue + OK-432 sclerotherapy | 67 |
| 15 | Present report | 73/M | Partial resection of the liver | PVS | 12 |
Ligation of the lymphatic leaking point using a pigment was reported to be extremely useful[1,4]. The placement of a PVS is mainly used for intractable ascites due to decompensated liver cirrhosis and it is a simple and cost-effective procedure. The present case was resistant to diuretic therapy, TPN and numerous plasma protein products; the patient’s activities of daily life (ADL) gradually worsening due to disturbance of mobility, impairment of oral intake and compromised respiratory function. Therefore, we finally placed a PVS before the patient’s condition became irreversible. PVS was preferred, mainly because of the expected technical difficulty in detecting the leakage point based on our impression during the previous surgery, in addition to predictable intra-abdominal adhesions.
In 1974, LeVeen was the first to describe the placement of a PVS for intractable ascites due to liver cirrhosis[7]. Later, with the subsequent development of the Denver shunt, surgical placement of a PVS for malignant ascites, chylous ascites and lymphorrhea was reported[9]. To the best of our knowledge, there have been no reports of PVS use for hepatic lymphorrhea following hepatectomy. The main characteristic of PVS is its immediate effectiveness by rapid reduction of the ascites whereby patients become able to take enough orally and to resume ADL. Moreover, ascites from hepatic lymphorrhea would eventually cease while using a PVS as time passes, as seen in the present case. Major complications that have been described include disseminated intravascular coagulation, occlusion and shunt infection[5,8,9]. Moreover, it cannot be denied that PVS for hepatic lymphorrhea after hepatectomy for malignant tumor may prompt hematogenous dissemination of malignant cells; this needs further observation although HCC is generally not closely associated with lymphatic metastasis. In summary, if the pathological diagnosis for HCC can rule out residual malignant cells such as vascular and lymphatic invasion by the tumor and positive surgical margin of hepatectomy, PVS is a good option as an alternative to ligation on re-laparotomy which can provide a modality that is quite safe, simple and effective.
In conclusion, for intractable ascites due to hepatic lymphorrhea after hepatectomy, we recommend the placement of a PVS as an option that can provide immediate effectiveness without the increased surgical risk associated with re-operation.
Peer reviewer: Chen-Guo Ker, MD, PhD, Professor, Department of Surgery, Kaohsiung Medical University, No. 100, Tz-You 1st Rd, Kaohsiung, Taiwan, China
S- Editor Wang JL L- Editor Roemmele A E- Editor Lin YP
| 1. | Ota H, Miyazawa T, Hiizu I, Ueda N, Maeura Y, Matsunaga S, Tomita K. A case report of intractable ascites due to hepatic lymphorrhea from hepatoduodenal ligament after radical gastrectomy for gastric cancer (In Japanese with English abstract). Jpn J Gastroenterol Surg. 1993;26:1115-1119. |
| 2. | Kawahira Y, Nakao K, Nakahara M, Hamaji M, Ogino N, Miyazaki S. A case of intractable hepatic lymphorrhea after gastrectomy for gastric cancer (In Japanese with English abstract). Jpn J Gastroenterol Surg. 1994;27:117-120. |
| 3. | Endo M, Maruyama K, Kinoshita T, Sasako M. Chylous ascites after extended lymphnode dissection for gastric cancer (In Japanese with English abstract). Jpn J Gastroenterol Surg. 1994;27:917-921. |
| 4. | Tanaka K, Ohmori Y, Mohri Y, Tonouchi H, Suematsu M, Taguchi Y, Adachi Y, Kusunoki M. Successful treatment of refractory hepatic lymphorrhea after gastrectomy for early gastric cancer, using surgical ligation and subsequent OK-432 (Picibanil) sclerotherapy. Gastric Cancer. 2004;7:117-121. |
| 5. | Miyamoto K, Kusumoto C, Kawabata Y. The effectiveness of Denver peritoneovenous shunt for the treatment of refractory ascites (In Japanese with English abstract). Jpn J Gastroenterol Surg. 2006;39:422-427. |
| 6. | Lasheen AE, Elzeftawy A, Ibrahim S, Attia M, Emam M. Implantation of a skin graft tube to create a saphenoperitoneal shunt for refractory ascites. Surg Today. 2007;37:622-625. |
| 7. | Le Veen HH, Christoudias G, Moon IP, Luft R, Falk G, Grosberg S. Peritoneovenous shunting for ascites. Ann Surg. 1974;180:580-591. |
| 8. | Makino Y, Shimanuki Y, Fujiwara N, Morio Y, Sato K, Yoshimoto J, Gunji Y, Suzuki T, Sasaki S, Iwase A. Peritoneovenous shunting for intractable chylous ascites complicated with lymphangioleiomyomatosis. Intern Med. 2008;47:281-285. |
| 9. | Mamada Y, Yoshida H, Taniai N, Bandou K, Shimizu T, Kakinuma D, Mizuguchi Y, Ishikawa Y, Akimaru K, Tajiri T. Peritoneovenous shunts for palliation of malignant ascites. J Nippon Med Sch. 2007;74:355-358. |