Published online May 27, 2010. doi: 10.4240/wjgs.v2.i5.157
Revised: February 6, 2010
Accepted: February 13, 2010
Published online: May 27, 2010
AIM: To develop a pure transvaginal access to the retroperitoneum, that is simple, reproducible and uses endoscopic material available on the market.
METHODS: From February 2008 to April 2009, 31 pigs were operated on, with 17 as an acute experiment and 14 with a survival protocol. The animals were placed in a supine position and a 12-mm double-channel endoscope (Karl Storz™, Tuttlingen) was used for vision and dissection. During the same time period, the access experiment was reproduced on 3 human cadavers using material similar to that used in the animal model.
RESULTS: In the animal model, 37 interventions were done on the kidney, adrenal gland and pancreas. The mean time to fashion the access was 10 min (range 5 to 20 min). No intraoperative death was observed. Two major (5%) intraoperative complications occurred: one hemorrhage on the aorta and one tearing of the right renal vein. Peritoneal laceration was encountered in 5 cases without impairing the planned task. In the survival group, good clinical outcome was observed at a mean follow-up of 3 wk (range 2 to 6 wk). In the 3 cadavers, access was performed correctly. The mean time to fashion the access was 52 min (range 40 to 60 min). All the anatomical landmarks described in the pig model were clearly identified in the same sequence.
CONCLUSION: A retroperitoneal natural orifice translumenal surgical transvaginal approach is feasible in both animal and human models and allows performance of a large panel of interventions.
- Citation: Allemann P, Perretta S, Asakuma M, Dallemagne B, Marescaux J. NOTES new frontier: Natural orifice approach to retroperitoneal disease. World J Gastrointest Surg 2010; 2(5): 157-164
- URL: https://www.wjgnet.com/1948-9366/full/v2/i5/157.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v2.i5.157
The retroperitoneal space contains many different organs that can be affected by a large variety of pathologies. For this reason, concern is shared by three surgical specialties: digestive surgery, urology and gynecology. Because these three disciplines evolved independently and relatively isolated, they have developed many different approaches to this space, based on their specific working habits. Basically 3 different accesses have been described: anterior (trans-abdominal), posterior and trans-vaginal, each carrying specific complications and limitations.
During the 1980s, the onset of video assisted surgery has dramatically changed the surgical field. The development of new approaches has also been applied to retroperitoneal surgery in the early 1990s[1-4], decreasing the invasiveness of interventions and improving the clinical outcome of patients[5-22]. As in open surgery, laparoscopy was developed in a dichotomist fashion, where either the rigid endoscope was inserted through the abdominal cavity (conventional laparoscopy, LS in the following text)[1,2,7-13] or through a posterior approach, a technique called retroperitoneoscopy (RPS)[3,4,14-22], which was described many years previous as a diagnostic tool[23]. If the advantage upon open surgery was obvious[24-26], no differences were observed between these two approaches until now.
Specific complications during access were observed in large series[21,27-29]. Because the two concepts are very different, they do not share the same problems, even if the rates of these difficulties are similar[27-29]. Trocar site complications (hematoma, infections, cell seeding and hernia) are shared by both approaches, as are general complications of every endoscopic surgery, such as hemorrhage, gas loss and gas embolism. The laparoscopic approach is limited due to the risk of visceral damages (enhanced with previous open surgery) because of the trans-abdominal approach[29], whereas pneumothorax and lesion of the 12th intercostals nerve are well-known complications of RPS[27,28].
Another drawback proposed for LS (and for all anterior approaches) was opening of the peritoneum to reach an extra-peritoneal organ. Even if this concept is still debated, some authors advocated an immune role of the peritoneal barrier[30-34], which could be misbalanced in the case of surgical trauma, which is a point particularly important in oncological surgery[30,32]. Even though this concept seems interesting, there is still lack of clear evidence to scientifically support these assumptions.
After 20 years of experience, almost everything has been attempted using minimally invasive approaches in the retroperitoneum. However, in the literature, some specific situations are still considered limited for these technologies. One example is the highly technically demanding intervention, duodenopancreatectomy[35-39], which is still strongly debated after 10 years of application. Another illustration, which may evolve in a few years, is the treatment of large adrenal tumors or primary malignant mass of the adrenal gland[40-42]. Even though these two interventions were demonstrated as being feasible using laparoscopy/RPS, these works remain highly debated and the open approach is still considered as the gold standard in these two situations.
Another aspect that tends to disappear with time is the longer learning curve of RPS compared to LS. Described as a limitation of this technique in the first trials, this point seems not to be a real limitation, as experience is growing worldwide[14].
After three decades, an alternative approach to laparoscopy has been proposed: the natural orifices transluminal endoscopic surgery® (NOTES). This emerging concept is at its dawn, but clinical experience is growing worldwide, offering to pass another step in minimally invasive concepts[43-46]. This technique has been applied to retroperitoneal surgery in both animal models and human applications, mainly centered on renal and pancreatic interventions[47-57]. All these attempts used trans-abdominal approaches through the stomach or the vagina. As we have seen, many complications can occur with such approaches[43-46] and, because it is thought that the peritoneum should not be touched to access extra-peritoneal organs, we decided to develop a reproducible extra-peritoneal access using the potential that NOTES approaches could offer[58-64].
The aim of the current experiment was to build a transvaginal retroperitoneal access to various organs. This approach was to be simple, reproducible and should use endoscopic material available on the market.
Our research institute is officially authorized to conduct animal experimentation (No. B-67-482-16). Our animal models were managed according to the Directive of the European Community Council (86/609/EEC).
For all interventions, a dual channel 12 mm flexible endoscope (Karl Story™) was used for access, dissection and vision. A laparoscopic gas insufflator using CO2 was employed to maintain constant gas pressure. Various endoscopic instruments (Karl Storz™, Olympus™ and Boston Scientific™) were used to dissect, cut, coagulate and clip the vessels.
The interventions were accomplished under general anesthesia in 25-30 kg female pigs. Anesthesia was induced with propofol 10 mL/kg + 2 mL pancuronium. Endotracheal intubation was performed and sleep was maintained with isofluorane 2%.
On 17 pigs, the experiment was done based on an acute protocol. A lethal dose of propofol and potassium chloride were successively administrated at the end of the intervention.
The remaining animals were awake at the end of the procedure and kept alive for various periods of time (from 2 to 6 wk), depending on the outcome measured. Their social comportment, feeding patterns and weight gain were used as markers for a good clinical course.
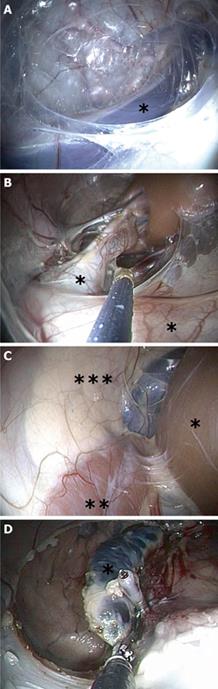
From February 2008 to April 2009, 31 pigs were operated on. With the pig placed in a supine position, a 10 mm latero-posterior colpotomy was performed at mid length of the vagina. Blunt dissection with the finger was used to create a 3 cm-long postero-lateral tunnel into which the flexible endoscope was inserted through the vagina. A retropneumoperitoneum of CO2 was insufflated at a pressure of 12 mmHg via one channel. Dissection progressed cranially and posteriorly, using only the tip of the endoscope and the pressure of the carbon dioxide. No extra instruments were employed. A complete and reproducible sequence of anatomical landmarks were visualized in the following sequence: the internal obturator muscle on the lateral side, the common iliac vessels (Figure 1A), the aorta or the IVC , depending on the side, the Gerota’s fascia (pre-renal fascia), the psoas muscle, the ureters (Figure 1B), the kidney (Figure 1B and C), the adrenal gland (Figure 1C and D) and the tail of the pancreas on the left side (Figure 1C).
Various procedures were attempted using a large panel of commercially available endoscopic devices. They were defined as following: (1) Total nephrectomy: dissection of the vessels, clipping and cutting, dissection of the ureters, clipping and cutting, dissection of the whole kidney, no retrieval due to limitation of the size of the vagina in our pig model; (2) Partial nephrectomy: dissection of the vessels, temporary clamping of one arterial branch, division of the parenchyma at the border of the ischemic tissue, hemostasis control, relies of the clamp, extraction of the specimen transvaginally; (3) Adrenalectomy: dissection of the lateral attachments, selective control of the vascular pedicles, complete dissection of the gland, extraction of the specimen transvaginally; and (4) Distal pancreatectomy: opening of the Gerota’s fascia, dissection of the anterior aspect of the pancreas up to the body, dissection of the posterior side with separation of the splenic vein (spleen sparing technique), control of the parenchyma with non-absorbable endoloop and cutting of the specimen with an endoscopic monopolar snare, extraction of the specimen transvaginally.
Experiments were conducted on frozen human cadavers, warmed at ambient temperature for 12 h. From December 2008 to April 2009, the same access principles were applied on 3 human cadavers, using material similar to that used in the animal model.
The colpotomy was performed on the posterior wall of the vagina, approximately 3 cm proximal from the posterior fornix. A posterior and lateral tunnel (left side) was then created under direct vision, using standard and laparoscopic instruments. Once the para-rectal space was entered, a 12-mm dual channel endoscope was introduced and insufflation using 15 mmHg of CO2 was applied through one of the channels.
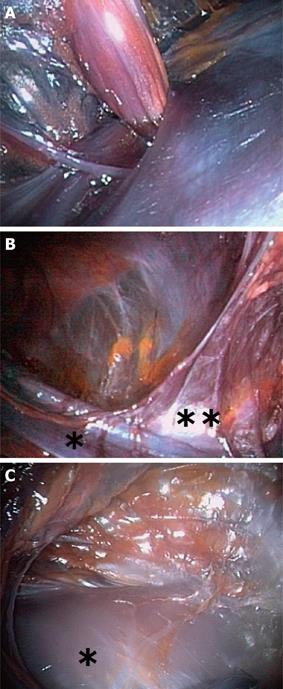
The successive anatomical landmarks identified were: the internal obturator nerve and artery entering Alcock’s canal (Figure 2A), the sacral nerves (running on the sacrum), the median rectal artery, emerging from the pelvic ring, the left external iliac vessel (Figure 2B) and the left inferior epigastric artery (Figure 2B). Progressing cranially, the lower pole of the kidney (Figure 2C) was dissected on its anterior aspect. The dissection was then prevented because of frozen tissues.
Various interventions were performed using the endoscope with a totally NOTES technique, without any percutaneous instruments. Surgical principles driving these interventions in standard surgery were preserved in all cases, but adapted with the endoscopic devices.
Thirty-seven interventions were performed on the kidney, the adrenal gland and the pancreas: 23 with an acute model and 14 with a survival model. A more detailed description of our lab experience is presented in Table 1.
| Intervention | Model (Number of intervention) |
| Lymphadenectomy | Acute (n = 3) Survival (n = 6) |
| Nephrectomy | Acute [n = 5 (left) +7 (right) +2 (partial)] Survival [n = 1 (right) +6 (partial)] |
| Adrenalectomy | Acute [n = 2 (left)+1 (right)] |
| Distal pancreatectomy | Acute (n = 2) Survival (n = 1) |
All operative steps described previously for each intervention were successfully conducted. The mean time to fashion the access was 10 min (range 5 to 20 min).
No intraoperative death was observed. Two major (5%) intraoperative complications occurred: one hemorrhage on the aorta and one tearing of the right renal vein. These two complications were successfully managed with endoscopic clips. Peritoneal laceration was encountered in 5 cases, without impairing the task planned. They were all managed with the placement of an intraperitoneal Veress needle.
In the survival group, a satisfying clinical outcome was observed in all animals, with a mean follow-up of 3 wk (range 2 to 6 wk). Three occult postoperative complications were discovered at necropsy: one pancreatic fistula after distal pancreatectomy and two collections containing urine after partial nephrectomies. No clinical signs were present in the animals concerned. Concerning the colpotomy, all accesses were found to be closed at 3 wk, without local complications such as abscesses or infection. The retroperitoneal space was found to be collapsed in all cases, without any objective infection.
The access was performed correctly in the 3 cadavers up to the iliac vessels. In the first case, frozen tissues prevented complete dissection up to the kidney. In the 2 remaining, the lower pole of the kidney was clearly visualized. The mean time to fashion the access was 52 min (range 40 to 60 min).
All the anatomical landmarks described in the pig model were clearly identified in the same sequence. Moreover, the sacral nerves and the middle rectal artery were identified in 2 of 3 cases.
We developed a model of transvaginal extra-peritoneal access to the retroperitoneum in both animal and human cadaver models[58-64]. Using this access and simple endoscopic instruments, various procedures were performed[59-64]. The mean time to fashion the access decreased dramatically with experience showing a quick learning curve and was strongly correlated with the introduction of a standardized anatomical landmarks-based dissection. As described previously for RPS[14,21,22], the orientation is more difficult in the retroperitoneum due to the lack of a real space (compared to the abdominal cavity). In order to overcome this limitation, we developed a highly standardized technique in which every anatomical landmark observed allowed the operator to progress in the direction of the next structure[58]. This is critical to guarantee reproducibility for other operators. This fact was clearly observed at the NOTES hands-on information session given at our institute during which our access was easily reproduced by endoscopic-naïve operators (data not published).
A complete mapping and extraction of all lymphatic stations of the pig was possible (up to renal pedicle lymph nodes) on both sides using the same vaginal incision[60]. As the first structures encountered during our dissection are the pelvic lymph nodes, this seems to represent a more practical intervention to be performed with such an approach and could be interesting for gynecology during the mapping of uterine cancer.
NOTES approaches have been widely used in urology for a few years with success[47-55], but only through transabdominal accesses. Our approach seems to be a valuable option for such interventions, as the kidney was always dissected freely in all of our interventions, including in the human cadavers[64]. Despite this easy access, important technical limitations have to be ruled-out (vascular control, cutting, and hemostasis) to allow more safe and practical interventions. In order to push the limits of our relatively simple instrumentation, we developed a survival model of partial nephrectomies (data not published). These interventions were possible, using advanced tactical tricks to perform temporary vascular control, but the need of suturing material was found to be a major limitation during the interventions. Attempts to close opened pyelocaliceal structures with endoscopic clips resulted in urine leak in two animals.
Interventions on adrenal glands were found to be feasible in the pig model[63], however, we encountered anatomical difficulties due to the firm attachments of the glands to vascular structures on both sides (the inferior vena cava and the aorta). This topographic distinction was responsible for two major intraoperative complications. No clinical repercussions were encountered as these two complications were managed quickly with compression and endoscopic control using clips. As in laparoscopy, the working space is closed and gas pressure greatly contributed to contain the hemorrhage, but measures to avoid massive gas embolism must also be quickly taken. In the two cases, the adrenal glands were situated deep into the wall of the vascular structure. In this context, we decided to limit our experiment on this model. These limits should not be extended to the human model, as the glands are well separated from these two major structures.
Even if laparoscopy remains a debated approach, it seemed interesting to try this approach for the distal part of the pancreas, due to its close proximity during the others interventions. Using simple and basic material, it was possible to perform resection of the distal part of this organ without touching the splenic vessels and the peritoneum[62]. Due to the shape of the pelvis and the size of the vagina in the pig, it was not possible to insert a stapler for the transaction and this was done using endoscopic endoloop. This is probably the reason for the pancreatic stump leakage observed in one animal. Despite this technical drawback, this approach allows pushing our model to the limits. Posterior access to treat pancreatic pathologies has already been proposed for a long time, in open or endoscopic surgery, and has shown many interesting benefits as a dissection of the pancreas without opening of the peritoneum[16-20]. This was particularly important to prevent peritoneal seeding of aggressive pancreatic juice during acute pancreatitis.
Many advantages were discovered during this experiment. One of the leading was the use of the endoscope by itself. This provides an “all-in-one” flexible platform for vision, insufflation and access to deliver a large variety of endoscopic instruments without the need to retrieve the platform to clean the lens or change the instruments. Conceptually, the endoscope could be considered as a flexible long single port and allows us to save time and movements.
As this transvaginal approach could not be considered as a pure anterior or posterior access, it allows us to avoid all of the complications related to both LS and RPS. The risk of pneumothorax, intercostal nerve injury and abdominal viscera is per se almost impossible to occur. Moreover, through the same incision, it was possible to gain access to both sides from the pelvis to the diaphragm[58]. This bilateral and full exploration of the retroperitoneum through one access is not possible in both RPS and LS (due to the interposition of abdominal organs).
This retroperitoneum-based access allows us to progress up to the targeted organ without opening the peritoneum in the vast majority of cases. In the few animals where this barrier was opened, it was limited to a small tear and a Veress needle was used to take the pneumoperitoneum out, which is a technique routinely used during extraperitoneal hernia repair (TEP)[65]. Another advantage in not opening the peritoneum is that the space is perfectly dry, allowing the CO2 to dissolve into the tissues and to enhance pneumo dissection. This effect, shared with RPS, is present during LS but to a lesser extent. Working in a closed space under pressure carries other advantages, such as a natural retraction coming from the areolar tissue surrounding the organs created by a selective and comprehensive dissection during the approach.
The orientation of the instruments and vision was found to be completely different compared to LS and RPS. Of particular interest was the direct access to the renal pedicle, allowing simple control of all the vessels, which is sometimes difficult during RPS nephrectomy for a large kidney.
As already pointed out, in all transvaginal access for NOTES, these techniques remain limited to women. If this point seems impossible to overcome, some other possibilities could be considered, such as transrectal access to the retroperitoneum, but close control of the infectious problems have to be studied first.
Another point concerning the access is the outcome of the colpotomy in terms of pain, fertility and local infections. If a transvaginal procedure is used many times in gynecology for intra-abdominal interventions (e.g. hysterectomy and fertility assessment), it may be an important issue for all transvaginal NOTES interventions, either transperitoneal or retroperitoneal.
Although complex interventions were feasible using simple endoscopic instruments, a revolution in terms of material is mandatory to transpose such technique to clinical applications. If actual endoscopic clips are sufficient to control a 3 mm artery, such devices were not designed for larger structures. Moreover, if flexible stapling devices are available on the market, their miniaturization and handling should be improved.
One of the limitations of our pig model was the size of the vagina. This prevents retrieving the kidney in one piece after complete dissection or to insert another instrument alongside the endoscope. We believe that this limitation will not be encountered in a human model, as transvaginal retrieval of kidney was already performed and described[47]. However, there could be a clear limit in the case of large tumors. Another detail concerning the extraction is the prevention of cell seeding. This point could be ruled out using plastic protectors, as in LS/RPS.
If this technique is going to be applied to regular practice, more research is needed to develop the same stepwise approach in a cadaver model. Indeed, this approach was designed to avoid the complications of existing techniques. This objective will only be reached with complete knowledge of surgical anatomy encountered during the endoscopic dissection.
In conclusion, the retroperitoneal NOTES transvaginal approach is feasible in both animal and human models and allows performing a large panel of interventions, even using basic instrumentation. This technique may contribute to a decrease in surgical trauma and the complications associated with currents approaches.
Surgery of the organs situated in the retroperitoneum is shared by different operative specialties: general surgery, visceral surgery, urology and gynecology. This multidisciplinary approach comes from the different organs targeted. Technical evolutions have been applied in this field, particularly the arrival of video-assisted endoscopic surgery. This new approach has dramatically decreased the trauma induced by the surgical intervention and has been proved to have clear benefits for the patient’s recovery. Basically two approaches have been developed: an anterior approach, through the abdominal cavity (laparoscopy) and a direct posterior approach (retroperitoneoscopy).
Nowadays, new fields of research tend to lower again and again the invasiveness of this approach. Transluminal endoscopic surgery performed through natural orifices (NOTES) is one of these promising targets. Both laparoscopy and retroperitoneoscopy have some limitations and potential complications. NOTES approaches could eventually overcome some of the morbidity arising from the skin incisions. NOTES has been applied on a large panel of interventions in the retroperitoneum, in both animal and human models.
In the past experiments on retroperitoneal organs with NOTES, the access was always done through an anterior approach,viathe peritoneal cavity, despite the targeted organs are situated behind the peritoneum. The main risk of such access is to damage intraperitoneal organs (bowel, liver, blood vessels). For this reason, the authors tried the purpose of this research was to study the potentialities of NOTES approach through a posterior approach, avoiding touching the abdominal cavity.
The results of this experiment demonstrate that a posterior approach of the retroperitoneum is feasible with NOTES technique in an animal model. Anatomical landmarks were essentials to provide a large reproducibility. Application to Human seems promising, but will require more advanced experiments.
Retroperitoneum: anatomical space situated behind the peritoneal cavity. It contains important organs such as the kidneys, the pancreas, the adrenal glands, the aorta,etc. NOTES: Endoscopic surgery performed through the natural orifices (the mouth, the vagina, the anus). Using these orifices avoids the need of the small incisions of the conventional endoscopic surgery, allowing surgery without visible scar.
The author described retroperitoneal approach of NOTES technique for animal and human cadaver model. This experimental report may contribute for the surgeons who are going to perform NOTES in the retroperitoneum.
Peer reviewers: Takeyama Hiromitsu, MD, PhD, Professor, Department of Gastroenterological Surgery, Nagoya City University, Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya 467-8601, Japan; Simone Ferrero, MD, San Martino Hospital and University of Genoa, Largo Rosanna Benzi1, Genova 16132, Italy
S- Editor Li LF L- Editor Lutze M E- Editor Yang C
| 1. | Higashihara E, Tanaka Y, Horie S, Aruga S, Nutahara K, Homma Y, Minowada S, Aso Y. [A case report of laparoscopic adrenalectomy]. Nippon Hinyokika Gakkai Zasshi. 1992;83:1130-1133. |
| 2. | Gagner M, Lacroix A, Bolté E. Laparoscopic adrenalectomy in Cushing's syndrome and pheochromocytoma. N Engl J Med. 1992;327:1033. |
| 3. | Mercan S, Seven R, Ozarmagan S, Tezelman S. Endoscopic retroperitoneal adrenalectomy. Surgery. 1995;118:1071-1075; discussion 1075-1076. |
| 4. | Walz MK, Peitgen K, Hoermann R, Giebler RM, Mann K, Eigler FW. Posterior retroperitoneoscopy as a new minimally invasive approach for adrenalectomy: results of 30 adrenalectomies in 27 patients. World J Surg. 1996;20:769-774. |
| 5. | Brunt LM. Minimal access adrenal surgery. Surg Endosc. 2006;20:351-361. |
| 6. | Cadeddu MO, Mamazza J, Schlachta CM, Seshadri PA, Poulin EC. Laparoscopic excision of retroperitoneal tumors: technique and review of the laparoscopic experience. Surg Laparosc Endosc Percutan Tech. 2001;11:144-147. |
| 7. | Liao JC, Breda A, Schulam PG. Laparoscopic renal surgery for benign disease. Curr Urol Rep. 2007;8:12-18. |
| 8. | Tseng D, Sheppard BC, Hunter JG. New approaches to the minimally invasive treatment of pancreatic cancer. Cancer J. 2005;11:43-51. |
| 9. | Madeb R, Koniaris LG, Patel HR, Dana JF 2nd, Nativ O, Moskovitz B, Erturk E, Joseph JV. Complications of laparoscopic urologic surgery. J Laparoendosc Adv Surg Tech A. 2004;14:287-301. |
| 10. | Melman L, Matthews BD. Current trends in laparoscopic solid organ surgery: spleen, adrenal, pancreas, and liver. Surg Clin North Am. 2008;88:1033-1046, vii. |
| 11. | Turna B, Aron M, Gill IS. Expanding indications for laparoscopic partial nephrectomy. Urology. 2008;72:481-487. |
| 12. | Ariyan C, Strong VE. The current status of laparoscopic adrenalectomy. Adv Surg. 2007;41:133-153. |
| 13. | Micali S, Peluso G, De Stefani S, Celia A, Sighinolfi MC, Grande M, Bianchi G. Laparoscopic adrenal surgery: new frontiers. J Endourol. 2005;19:272-278. |
| 14. | Barczyński M, Konturek A, Gołkowski F, Cichoń S, Huszno B, Peitgen K, Walz MK. Posterior retroperitoneoscopic adrenalectomy: a comparison between the initial experience in the invention phase and introductory phase of the new surgical technique. World J Surg. 2007;31:65-71. |
| 15. | Molina WR, Desai MM, Ng CS, Spaliviero M, Gill IS. Retroperitoneoscopic radical nephrectomy with concomitant distal pancreatectomy: case report. J Endourol. 2004;18:665-667. |
| 16. | Takada M, Ichihara T, Toyama H, Suzuki Y, Kuroda Y. Retroperitoneoscopic laparoscopic distal pancreatectomy with spleen salvage. Hepatogastroenterology. 2004;51:925-927. |
| 17. | Connor S, Ghaneh P, Raraty M, Sutton R, Rosso E, Garvey CJ, Hughes ML, Evans JC, Rowlands P, Neoptolemos JP. Minimally invasive retroperitoneal pancreatic necrosectomy. Dig Surg. 2003;20:270-277. |
| 18. | Nakasaki H, Tajima T, Fujii K, Makuuchi H. A surgical treatment of infected pancreatic necrosis: retroperitoneal laparotomy. Dig Surg. 1999;16:506-511. |
| 19. | Fagniez PL, Rotman N, Kracht M. Direct retroperitoneal approach to necrosis in severe acute pancreatitis. Br J Surg. 1989;76:264-267. |
| 20. | Nagakawa T, Kurachi M, Konishi K, Miyazaki I. Translateral retroperitoneal approach in radical surgery for pancreatic carcinoma. Jpn J Surg. 1982;12:229-233. |
| 21. | Walz MK, Alesina PF, Wenger FA, Deligiannis A, Szuczik E, Petersenn S, Ommer A, Groeben H, Peitgen K, Janssen OE. Posterior retroperitoneoscopic adrenalectomy--results of 560 procedures in 520 patients. Surgery. 2006;140:943-948; discussion 948-950. |
| 22. | Zhang X, Fu B, Lang B, Zhang J, Xu K, Li HZ, Ma X, Zheng T. Technique of anatomical retroperitoneoscopic adrenalectomy with report of 800 cases. J Urol. 2007;177:1254-1257. |
| 23. | Bartel M. [Retroperitoneoscopy. An endoscopic method for inspection and bioptic examination of the retroperitoneal space]. Zentralbl Chir. 1969;94:377-383. |
| 24. | Lee J, El-Tamer M, Schifftner T, Turrentine FE, Henderson WG, Khuri S, Hanks JB, Inabnet WB 3rd. Open and laparoscopic adrenalectomy: analysis of the National Surgical Quality Improvement Program. J Am Coll Surg. 2008;206:953-959; discussion 959-961. |
| 25. | Lang B, Fu B, OuYang JZ, Wang BJ, Zhang GX, Xu K, Zhang J, Wang C, Shi TP, Zhou HX. Retrospective comparison of retroperitoneoscopic versus open adrenalectomy for pheochromocytoma. J Urol. 2008;179:57-60; discussion 60. |
| 26. | Hemal AK, Kumar R, Misra MC, Gupta NP, Chumber S. Retroperitoneoscopic adrenalectomy for pheochromocytoma: comparison with open surgery. JSLS. 2003;7:341-345. |
| 27. | Hanssen WE, Kuhry E, Casseres YA, de Herder WW, Steyerberg EW, Bonjer HJ. Safety and efficacy of endoscopic retroperitoneal adrenalectomy. Br J Surg. 2006;93:715-719. |
| 28. | Siperstein AE, Berber E, Engle KL, Duh QY, Clark OH. Laparoscopic posterior adrenalectomy: technical considerations. Arch Surg. 2000;135:967-971. |
| 29. | Strebel RT, Müntener M, Sulser T. Intraoperative complications of laparoscopic adrenalectomy. World J Urol. 2008;26:555-560. |
| 30. | Jung IK, Kim MC, Kim KH, Kwak JY, Jung GJ, Kim HH. Cellular and peritoneal immune response after radical laparoscopy-assisted and open gastrectomy for gastric cancer. J Surg Oncol. 2008;98:54-59. |
| 31. | Luk JM, Tung PH, Wong KF, Chan KL, Law S, Wong J. Laparoscopic surgery induced interleukin-6 levels in serum and gut mucosa: implications of peritoneum integrity and gas factors. Surg Endosc. 2009;23:370-376. |
| 32. | Hegarty N, Dasgupta P. Immunological aspects of minimally invasive oncologic surgery. Curr Opin Urol. 2008;18:129-133. |
| 33. | Jesch NK, Kuebler JF, Nguyen H, Nave H, Bottlaender M, Teichmann B, Braun A, Vieten G, Ure BM. Laparoscopy vs minilaparotomy and full laparotomy preserves circulatory but not peritoneal and pulmonary immune responses. J Pediatr Surg. 2006;41:1085-1092. |
| 34. | Ost MC, Tan BJ, Lee BR. Urological laparoscopy: basic physiological considerations and immunological consequences. J Urol. 2005;174:1183-1188. |
| 35. | Merchant NB, Parikh AA, Kooby DA. Should all distal pancreatectomies be performed laparoscopically? Adv Surg. 2009;43:283-300. |
| 36. | Palanivelu C, Rajan PS, Rangarajan M, Vaithiswaran V, Senthilnathan P, Parthasarathi R, Praveen Raj P. Evolution in techniques of laparoscopic pancreaticoduodenectomy: a decade long experience from a tertiary center. J Hepatobiliary Pancreat Surg. 2009;16:731-740. |
| 37. | Cho A, Yamamoto H, Nagata M, Takiguchi N, Shimada H, Kainuma O, Souda H, Gunji H, Miyazaki A, Ikeda A. Laparoscopic major hepato-biliary-pancreatic surgery: formidable challenge to standardization. J Hepatobiliary Pancreat Surg. 2009;16:705-710. |
| 38. | Warner EA, Ben-David K, Cendan JC, Behrns KE. Laparoscopic pancreatic surgery: what now and what next? Curr Gastroenterol Rep. 2009;11:128-133. |
| 39. | Briggs CD, Mann CD, Irving GR, Neal CP, Peterson M, Cameron IC, Berry DP. Systematic review of minimally invasive pancreatic resection. J Gastrointest Surg. 2009;13:1129-1137. |
| 40. | Parnaby CN, Chong PS, Chisholm L, Farrow J, Connell JM, O'Dwyer PJ. The role of laparoscopic adrenalectomy for adrenal tumours of 6 cm or greater. Surg Endosc. 2008;22:617-621. |
| 41. | Walz MK, Petersenn S, Koch JA, Mann K, Neumann HP, Schmid KW. Endoscopic treatment of large primary adrenal tumours. Br J Surg. 2005;92:719-723. |
| 42. | Haleblian GE, Wilson C, Haddad D, Albala DM. Adrenocortical carcinoma: role of laparoscopic surgery in treatment. Expert Rev Anticancer Ther. 2007;7:1295-1300. |
| 43. | Yan SL, Thompson-Fawcett M. NOTES: new dimension of minimally invasive surgery. ANZ J Surg. 2009;79:337-343. |
| 44. | Sumiyama K, Gostout CJ, Gettman MT. Status of access and closure techniques for NOTES. J Endourol. 2009;23:765-771. |
| 45. | Sodergren MH, Clark J, Athanasiou T, Teare J, Yang GZ, Darzi A. Natural orifice translumenal endoscopic surgery: critical appraisal of applications in clinical practice. Surg Endosc. 2009;23:680-687. |
| 46. | Mintz Y, Horgan S, Cullen J, Stuart D, Falor E, Talamini MA. NOTES: a review of the technical problems encountered and their solutions. J Laparoendosc Adv Surg Tech A. 2008;18:583-587. |
| 47. | Branco AW, Branco Filho AJ, Kondo W, Noda RW, Kawahara N, Camargo AA, Stunitz LC, Valente J, Rangel M. Hybrid transvaginal nephrectomy. Eur Urol. 2008;53:1290-1294. |
| 48. | Alcaraz A, Peri L, Molina A, Goicoechea I, García E, Izquierdo L, Ribal MJ. Feasibility of transvaginal NOTES-assisted laparoscopic nephrectomy. Eur Urol. 2010;57:233-237. |
| 49. | Castillo OA, Vidal-Mora I, Campos R, Fonerón A, Feria-Flores M, Gómez R, Sepúlveda F. [Laparoscopic simple nephrectomy with transvaginal notes assistance and the use of standard laparoscopic instruments]. Actas Urol Esp. 2009;33:767-770. |
| 50. | Boylu U, Oommen M, Joshi V, Thomas R, Lee BR. Natural orifice translumenal endoscopic surgery (NOTES) partial nephrectomy in a porcine model. Surg Endosc. 2010;24:485-489. |
| 51. | Kaouk JH, White WM, Goel RK, Brethauer S, Crouzet S, Rackley RR, Moore C, Ingber MS, Haber GP. NOTES transvaginal nephrectomy: first human experience. Urology. 2009;74:5-8. |
| 52. | Ribal Caparrós MJ, Peri Cusí L, Molina Cabeza A, García Larrosa A, Carmona F, Alcaraz Asensio A. [First report on hybrid transvaginal nephrectomy for renal cancer]. Actas Urol Esp. 2009;33:280-283. |
| 53. | Aron M, Berger AK, Stein RJ, Kamoi K, Brandina R, Canes D, Sotelo R, Desai MM, Gill IS. Transvaginal nephrectomy with a multichannel laparoscopic port: a cadaver study. BJU Int. 2009;103:1537-1541. |
| 54. | Haber GP, Brethauer S, Crouzet S, Berger A, Gatmaitan P, Kamoi K, Gill I. Pure 'natural orifice transluminal endoscopic surgery' for transvaginal nephrectomy in the porcine model. BJU Int. 2009;104:1260-1264. |
| 55. | Sotelo R, de Andrade R, Fernández G, Ramirez D, Di Grazia E, Carmona O, Moreira O, Berger A, Aron M, Desai MM. NOTES hybrid transvaginal radical nephrectomy for tumor: stepwise progression toward a first successful clinical case. Eur Urol. 2010;57:138-144. |
| 56. | Matthes K, Yusuf TE, Willingham FF, Mino-Kenudson M, Rattner DW, Brugge WR. Feasibility of endoscopic transgastric distal pancreatectomy in a porcine animal model. Gastrointest Endosc. 2007;66:762-766. |
| 57. | Ryou M, Fong DG, Pai RD, Tavakkolizadeh A, Rattner DW, Thompson CC. Dual-port distal pancreatectomy using a prototype endoscope and endoscopic stapler: a natural orifice transluminal endoscopic surgery (NOTES) survival study in a porcine model. Endoscopy. 2007;39:881-887. |
| 58. | Zacharopoulou C, Nassif J, Allemann P, Dallemagne B, Perretta S, Marescaux J, Wattiez A. Exploration of the retroperitoneum using the transvaginal natural orifice transluminal endoscopic surgery technique. J Minim Invasive Gynecol. 2009;16:198-203. |
| 59. | Perretta S, Allemann P, Asakuma M, Cahill R, Dallemagne B, Marescaux J. Feasibility of right and left transvaginal retroperitoneal nephrectomy: from the porcine to the cadaver model. J Endourol. 2009;23:1887-1892. |
| 60. | Nassif J, Zacharopoulou C, Marescaux J, Wattiez A. Transvaginal extraperitoneal lymphadenectomy by Natural Orifices Transluminal Endoscopic Surgery (NOTES) technique in porcine model: feasibility and survival study. Gynecol Oncol. 2009;112:405-408. |
| 61. | Allemann P, Perretta S, Marescaux J. Surgical access to the adrenal gland: the quest for a "no visible scar" approach. Surg Oncol. 2009;18:131-137. |
| 62. | Allemann P, Perretta S, Asakuma M, Dallemagne B, Mutter D, Marescaux J. Multimedia manuscript. NOTES retroperitoneal transvaginal distal pancreatectomy. Surg Endosc. 2009;23:882-883. |
| 63. | Perretta S, Allemann P, Asakuma M, Dallemagne B, Marescaux J. Adrenalectomy using natural orifice translumenal endoscopic surgery (NOTES): a transvaginal retroperitoneal approach. Surg Endosc. 2009;23:1390. |
| 64. | Singh-Ranger D, Taneja T, Sroden P, Peters J. A rare complication following laparoscopic TEP repair: case report and discussion of the literature. Hernia. 2007;11:453-456. |
| 65. | Lau H, Patil NG, Yuen WK, Lee F. Management of peritoneal tear during endoscopic extraperitoneal inguinal hernioplasty. Surg Endosc. 2002;16:1474-1477. |