INTRODUCTION
Gastric carcinoids are thought to be relatively rare tumors[1]. However, recently the prevalence of gastric carcinoids has risen, reported as 8.7% of all gastrointestinal carcinoid tumors in a large database[2]. In 1993, Rindi et al[3] advocated a classification of three subtypes of gastric carcinoid tumors, helpful for the prediction of malignant potential and commonly used. Among the three subtypes, type III, sporadic carcinoid, is known to possess a more aggressive behavior pattern than other subtypes with a higher malignant potential. Therefore, the recommended treatment is aggressive surgical management in the same manner as gastric cancer[4-7].
In this report, we describe a case of sporadic gastric carcinoid tumor with the appearance of a submucosal tumor. It was successfully treated by two-stage less invasive surgery which involved laparoscopic wedge resection and laparoscopy-assisted distal gastrectomy (LADG). We also discuss the strategy of surgical management for gastric carcinoids.
CASE REPORT
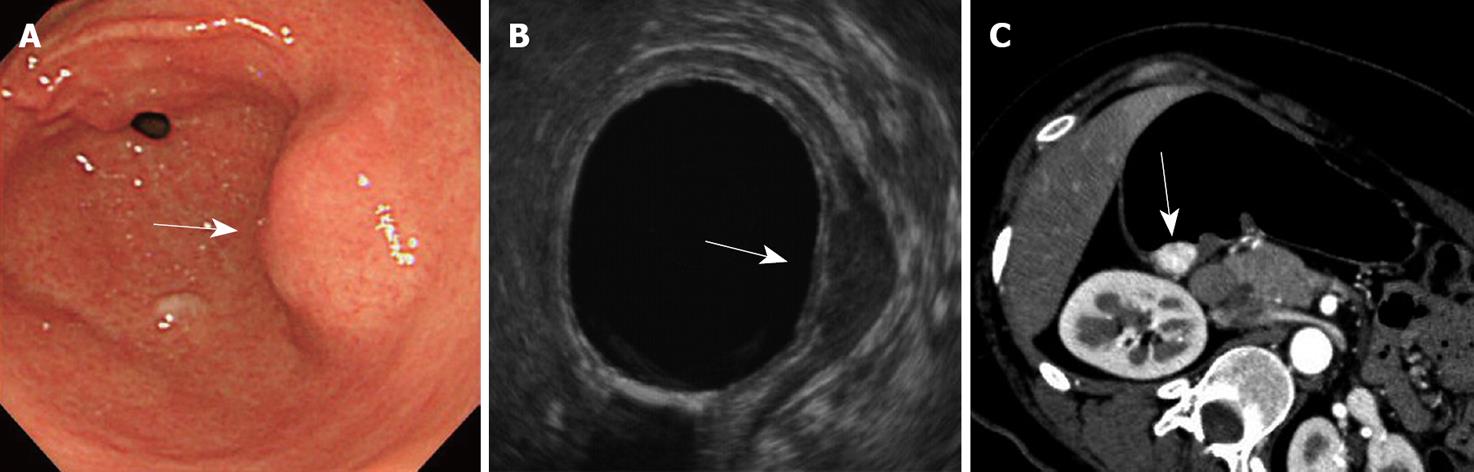
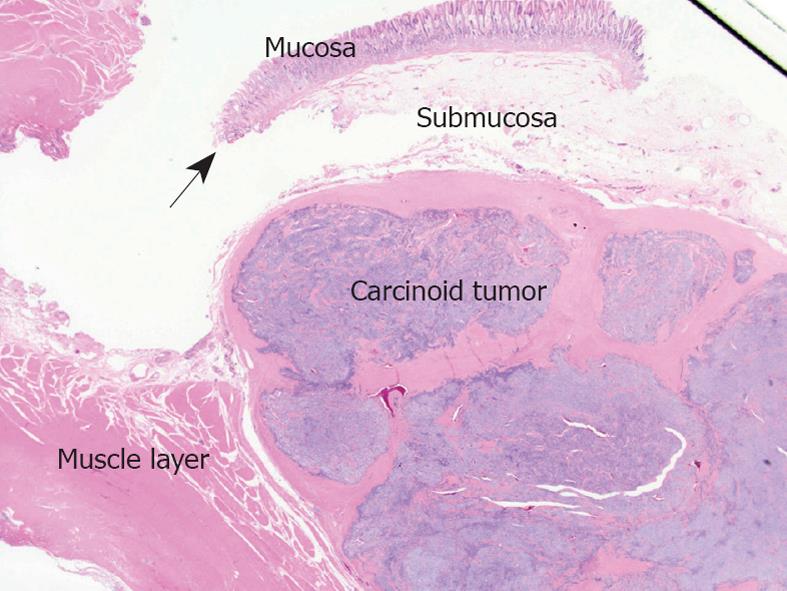
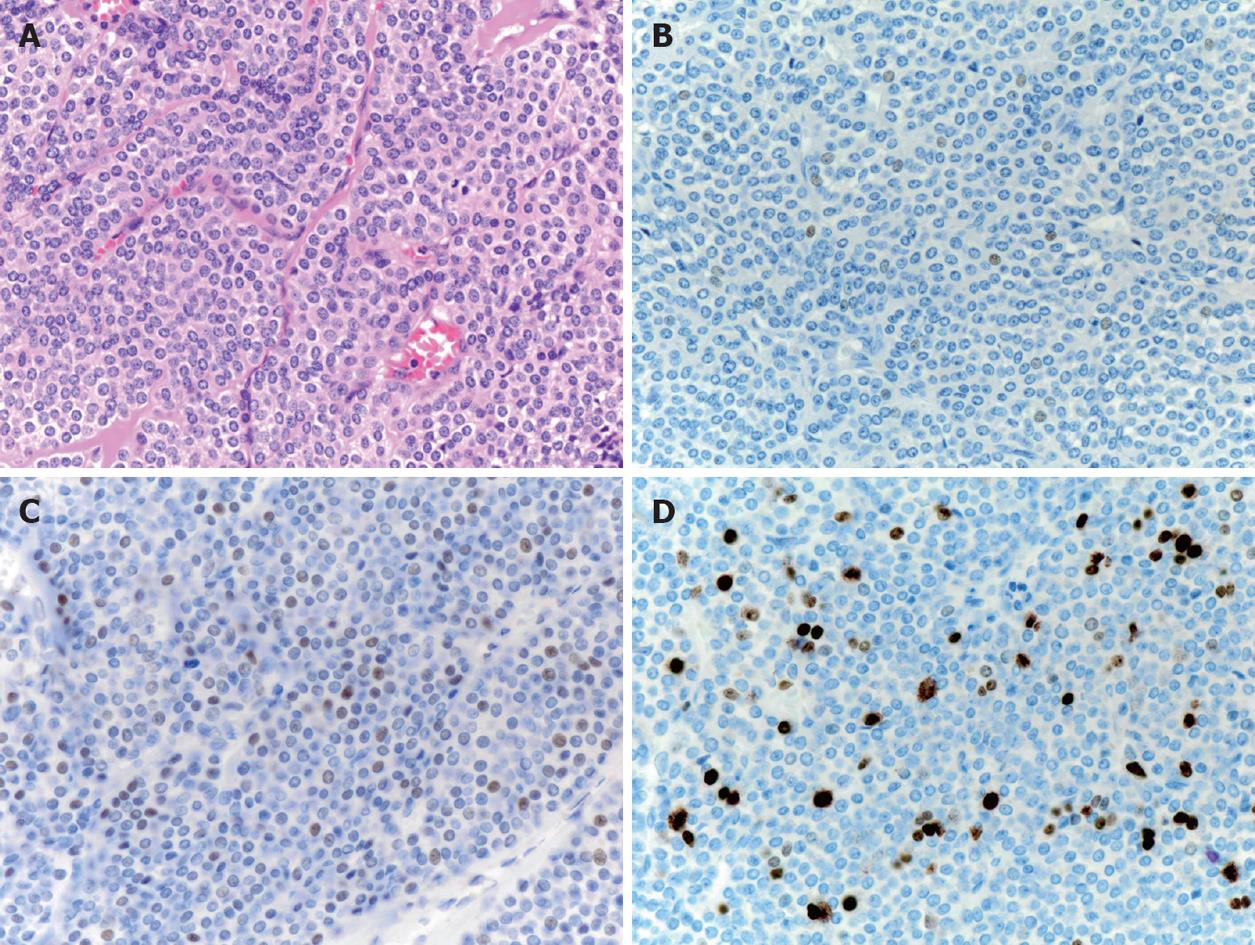
A 38-year old asymptomatic woman was referred to our hospital for evaluation of a submucosal tumor of the stomach. Gastroendoscopy showed a solitary submucosal tumor without ulceration or central depression on the posterior wall of the antrum (Figure 1A) with no atrophic gastritis. The surface of the tumor was covered completely by intact normal mucosa and biopsy specimens were not able to identify the tumor cells. Endoscopic ultrasound revealed the tumor, nearly 2 cm in diameter, arising from the muscle layer (Figure 1B). A computed tomography scan showed the tumor enhanced in the arterial phase (Figure 1C) and no tumors in other organs such as the liver or lung. Diagnosis could not be confirmed but a gastrointestinal stromal tumor was highly suspected according to these findings. For definitive diagnosis, laparoscopic wedge resection was performed with the assistance of peroral endoscopy as described by Hiki et al[8]. The tumor was excised manually using ultrasonic scissors and electrocautery and sutured manually. The operation time was 217 min and blood loss was 5 g. The postoperative course of the initial surgery was uneventful. Pathologically, the tumor was shown to be gastric carcinoid 13 mm × 12 mm in size and with a negative margin. The tumor was capsulated and localized mainly in the submucosal layer and had infiltrated the muscle layer. The mucosa and muscularis mucosa were intact (Figure 2). Microscopically, the tumor was uniform in shape and arranged in cribriform nests (Figure 3A). Immunological staining showed that it was positive for chromogranin A (Figure 3B) and synaptophysin, slightly positive for P53 (Figure 3C) and the Ki-67 labeling index was 10% (Figure 3D). Lymphovascular invasion was not seen. Laboratory tests showed that serum gastrin levels were within the normal range (74 pg/mL). The patient had no other tumors associated with multiple endocrine neoplasia. The patient was diagnosed with a sporadic gastric carcinoid tumor infiltrating the muscle layer, indicating the possibility of lymph node metastasis. For clearance of the regional lymph nodes and radical treatment, LADG was performed with Billroth-I reconstruction with D1+beta lymphadenectomy according to the classification of the Japanese Classification of Gastric Cancer (second English edition)[9] 3 wk after the initial surgery. The required incisions were a 50 mm mini-laparotomy in the epigastrium and another five 5-12 mm for trocar insertion. The operation time was 231 min and blood loss was 40 g. The postoperative course did not have any major complications but there was infection of the mini-laparotomy wound. Finally, pathological examination revealed no metastasis in 18 harvested lymph nodes and no residual tumor. The patient was followed up for 8 mo without findings indicative of recurrence or distant metastasis.
Figure 1 Endoscopy, endoscopic ultrasound and computed tomography findings of the patient.
A: Endoscopy revealed a submucosal tumor (arrow) without central depression on the posterior of the antrum; B: Endoscopic ultrasound revealed a tumor (arrow) arising from the muscle layer; C: Computed tomography scan showed the tumor (arrow) stained in the early phase.
Figure 2 Resected specimen viewed using low magnification.
The carcinoid tumors were located in the submucosal layer infiltrating the muscle layer. The muscularis mucosa (arrow) was intact.
Figure 3 Pathological findings of the resected carcinoid (× 40).
A: Hematoxylin and eosin; B: Chromogranin A staining; C: P53 staining; D: Ki-67 staining.
DISCUSSION
Gastric carcinoids arise from proliferating enterochromaffin-like cells of the fundus[10]. Rindi et al [3] classified gastric carcinoids into the following three subtypes based on their clinicopathological features as follows: (1) those that arise in a background of type A gastritis; (2) those associated with Zollinger-Ellison syndrome, usually combined with multiple endocrine neoplasia type 1; and (3) those that occur sporadically without hypergastrinemia. Among them, type III which are sporadic carcinoids, tend to be solitary, larger, invasive and more often metastatic.
The proportional rates of types I, II and III have been reported as 68%-83%, 0%-8% and 11%-23%[1-6] respectively. The rates of lymph node metastasis of each subtype have been reported to be 0%-7%, 0%-12% and 17%-58%[1-6] respectively which demonstrates a close relationship between the classification of subtypes and malignant potential. Soga[11] reported a statistical evaluation of 1094 cases of gastric carcinoids worldwide and noted that the behavior of gastric carcinoids is correlated with its size and infiltrating depth. In this previous study, the rates of metastasis by size in diameter were 8.2% (< 10 mm), 13.2% (11-20 mm) and 44.8% (> 20 mm)[11]. However, several case reports have shown that type III carcinoids of less than 10mm cause lymph node metastasis[12-14] which suggest that some types of type III gastric carcinoids possess considerable malignant potential, regardless of their size. The rates of metastasis by infiltrating depth have been reported to be 7.5% (mucosa), 13.2% (submucosa) and 44.8% (muscle layer)[11]. Therefore, management of gastric carcinoids should be determined taking into consideration these previous findings. In the present case, laparoscopic wedge resection revealed the tumor as a type III carcinoid that infiltrated the muscle layer which suggested a high possibility of lymph node metastasis. Pathological examination showed no atypical histology but the Ki-67 labeling index was 10% and p53 was slightly positive which suggested a moderate potential of tumor proliferation. Considering these results, we decided that radical surgery was necessary for lymph node clearance. Although there was no lymph node metastasis, we believe that this management was appropriate.
Definitive preoperative diagnosis of gastric submucosal tumors is frequently difficult, such as in the present case. Studies on experimental animals have demonstrated that carcinoids originate in the lower portion of the gastric glands and invade through the muscularis mucosae down to the submucosal layer, then forming a nodule larger than the original portion of the mucosa[15]. In most gastric carcinoids, the tumor simultaneously invades upward to the mucosal layer intraluminally, resulting in central depression. Interestingly, in the present case, not only the mucosa but also the muscularis mucosa was intact and there was a distance between the muscularis mucosa and the tumor capsule (Figure 2) which is unusual with gastric carcinoids. For this reason, the tumor appeared as the usual “submucosal tumor”. It is difficult to explain this phenomenon. One possibility is that, in the present case, the tumor may have originated from enterochromaffin-like cells in the heterotopic submucosal gastric gland[16]; however, it is difficult to determine this. Indeed, there were no diffuse heterotopic submucosal cysts in resected specimens of the second operation.
Laparoscopic wedge resection for the diagnosis and treatment of gastric submucosal tumors has been previously employed and its efficacy has been established[17-19]. En-bloc excision of the tumor with intact surrounding tissue allows precise pathological examination, resulting in an accurate assessment for the necessity of additional intervention. Our strategy is that gastric submucosal tumors larger than 2 cm should be laparoscopically removed, considering the possibility of gastrointestinal stromal tumors, and tumors less than 2 cm can be observed if there is no growing tendency or ulceration. Furthermore, in the present case, LADG with lymph node dissection was performed as a completion surgery. LADG is being increasingly performed in Eastern countries[20] where there are high incidences of early gastric cancer. Its feasibility, acceptable oncological outcomes and contribution to the patient’s quality of life have been previously reported. In our department, LADG has been mainly performed in the treatment of early-stage gastric cancer located in the middle or lower portion of the stomach; we have currently experienced over 250 cases. The laparoscopic approach generally provides fewer postoperative adhesions. In the present case, the second operation was able to be performed safely under laparoscopy because postoperative adhesions were minimal and only slight adhesions between the stomach and the pancreas were recognized.
In summary, we describe a case of a sporadic gastric carcinoid tumor, corresponding to Rindi’s type III, treated by two-stage laparoscopic surgery. Such management can be applied to patients in whom definitive diagnosis is difficult preoperatively. These less invasive surgeries may contribute to the quality of life of patients with gastric cancer and endocrine neoplasms.