Published online Nov 27, 2010. doi: 10.4240/wjgs.v2.i11.373
Revised: September 5, 2010
Accepted: September 12, 2010
Published online: November 27, 2010
The purpose of this article is to review pertinent literature assessing the evidence regarding adjuvant chemoradiotherapy for adenocarcinoma of the pancreas following curative resection. This review looks at randomized controlled studies with the emphasis on adjuvant chemoradiotherapy. In assessing the evidence from the studies reviewed in this article, the trials have been grouped according to the positive or negative results for or against adjuvant treatment. In addition, data from two large, single-institution studies affirming the role for adjuvant chemoradiotherapy has been included. Understanding the evidence from all of the randomized studies is important in shaping current practice recommendations for adjuvant therapy of surgically resected pancreas cancer. Adjuvant chemoradiotherapy following surgery is the current approach at many cancer treatment centers in the United States. In Europe, chemotherapy alone is the preferred adjuvant therapy. However, the type of adjuvant treatment recommended remains controversial due to conflicting study results. The debate will likely continue. Current practice should be based on the weight of evidence available at this time, which is in favor of adjuvant chemotherapy with chemoradiotherapy.
- Citation: Iott M, Neben-Wittich M, Quevedo JF, Miller RC. Adjuvant chemoradiotherapy for resected pancreas cancer. World J Gastrointest Surg 2010; 2(11): 373-380
- URL: https://www.wjgnet.com/1948-9366/full/v2/i11/373.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v2.i11.373
The most recent cancer statistics show that even though pancreatic cancer accounts for only 3% of all cancer cases, it remains the fourth most prevalent cause of cancer death among men and women (6% of cancer deaths for both sexes) in the United States. The outlook continues to be bleak considering that only 5% of patients diagnosed with pancreatic cancer will survive to 5 years. This survival rate has persisted, essentially unchanged, over the past few decades. The American Cancer Society’s most recent projected estimates indicate there will be 42 470 new pancreas cancer diagnoses per year and 35 420 subsequent deaths. Surgery continues to be vitally important in achieving a potential cure for these patients. However, only a minority of pancreatic cancer patients have resectable disease at the time of diagnosis[1,2].
To date, surgery has been considered the mainstay for optimal treatment of pancreatic cancer and this situation is likely to continue for the foreseeable future. Unfortunately, due to the aggressive nature of this cancer, even surgical resections with histologically negative margins (R0) do not affect a cure for the majority of patients. Therefore, adjuvant therapy must be considered for improvement in survival rates of patients who have undergone a potentially curative resection.
Prospective, randomized trials are the gold standard in evaluating treatment outcomes for adjuvant treatment of pancreatic cancer. However, randomized studies evaluating adjuvant chemoradiotherapy following curative resection for pancreatic cancer have produced inconsistent results. The conflicting findings from these studies make it difficult for clinicians to recommend optimal effective adjuvant treatment. Current recommendations by the National Comprehensive Cancer Network are for adjuvant therapy (chemotherapy and/or chemoradiotherapy) after surgery[3]. Therefore, the objective of this article is to assess and clarify current evidence and the underlying principles for recommending adjuvant chemoradiotherapy for patients with a resected pancreatic cancer.
Gastrointestinal Tumor Study Group 9173 trial: The first prospective, randomized, multi-institutional study for determining the effectiveness of adjuvant chemoradiotherapy for pancreas cancer was conducted by Kalser and Ellenberg[4]. In this seminal trial, 43 patients with histologically confirmed non-metastatic pancreatic adenocarcinoma with R0 surgical resections were randomly assigned to observation (22) or chemoradiotherapy (21). Radiotherapy was given in two 20 Gy courses separated by a two-week break. Concomitant, bolus 5-fluorouracil (5-FU) chemotherapy was administered on the first 3 d of each two-week course of radiotherapy. The study protocol also called for weekly maintenance 5-FU chemotherapy following completion of adjuvant chemoradiotherapy or until evidence of disease recurrence.
This study demonstrated a statistically significant prolonged survival rate for patients who received adjuvant chemoradiotherapy following surgery. A 20-mo median survival was achieved with adjuvant chemoradiotherapy as compared to 11 mo for patients who had surgery alone. A nonrandomized, confirmatory study with 30 additional patients given adjuvant chemoradiotherapy as dictated by the study protocol [conducted by Gastrointestinal Tumor Study Group (GITSG)] provided additional evidence substantiating the results of the randomized trial.
The size and extent of tumor at surgery and the Eastern Cooperative Oncology Group performance status were strongly predictive of overall survival. The most obvious limitation of this study was the small number of patients studied. Other adverse aspects of the study included the prolonged 8-year accrual time of study participants, the lack of a central standardized quality assurance for radiotherapy, the delay in starting adjuvant treatment after surgery (protocol time limit was 4-10 wk), and the small number of patients (2) who received the protocol prescribed maintenance chemotherapy following adjuvant chemoradiotherapy[4,5]. The findings from GITSG provided the incentive for performing additional larger studies such as the trial conducted by the European Organisation for Research and Treatment of Cancer (EORTC).
European Organisation for Research and Treatment of Cancer: This study was the second randomized, multicenter trial performed to look at the value of adjuvant chemoradiotherapy following surgery. Twenty-nine institutions across Europe participated in this trial. This was an attempt to validate results noted in the GITSG randomized study. The treatment protocol was similar to the GITSG trial in that radiotherapy was given in two, 20 Gy courses separated by a two-week break using a 3-4 field technique. However, chemotherapy differed from the GITSG study because concomitant chemotherapy was not given in bolus doses; it was administered via continuous infusion during the first two-week course of radiotherapy and then for either 0, 3 and 5 d during the second course of radiotherapy as determined by prior toxicity/patient tolerance during the first course. Another variance from the previous study was that there was no maintenance chemotherapy given following adjuvant chemoradiotherapy. Participants in this study (as determined by central pathology review) had either a resected T1-2N0-1aM0 adenocarcinoma of the head of the pancreas (114) or T1-3N0-1aM0 periampullary adenocarcinoma (104). The trial required patients to start adjuvant treatment within two to eight weeks of surgery. Following surgery, patients were randomized to observation (108) and treatment (110). Following removal of ineligible patients (five patients in the observation arm and six patients in the treatment arm), there were 103 patients observed and 104 patients treated.
The reported findings indicated that adjuvant chemoradiotherapy did not demonstrate an advantage for disease progression-free survival or overall survival. The observation group and treatment group had a progression-free survival of 16 and 17.4 mo respectively. Median overall survival for the observation and treatment groups were 19 and 24.5 mo respectively, and 2-year survival rates of 41% and 51% in the respective groups (P = 0.208). When evaluating only head of pancreas cancer patients, the authors noted a greater divergence between the groups with a median survival of 12.6 mo in the observation group and 17.1 mo in the treatment group. Although there was a trend for improvement with adjuvant chemoradiotherapy, it did not reach statistical significance (P = 0.099) using a two-sided log-rank test[6].
The inclusion of patients with positive surgical margins (R1) and patients with periampullary cancer complicated the interpretation of the trial results. Periampullary cancer is known to have a better prognosis over pancreatic cancer. Other limitations of this study include the lack of a centralized quality assurance for radiotherapy and the absence of maintenance chemotherapy following chemoradiotherapy.
Critics have since argued that a more appropriate statistical test to assess for improvement or harm (implied by the GITSG trial) would be to use a one-sided log-rank test as opposed to a two-sided log-rank test. Reanalysis of the data using the one-sided log-rank test suggests a statistically significant improvement in overall survival at two years for pancreatic cancer patients (P = 0.049). On this basis, the findings from this trial would be considered a positive trial for adjuvant chemoradiotherapy[7,8]. Similar benefits of improved survival have been noted in more recent randomized-controlled cooperative trials directed by the Radiation Therapy Oncology Group (RTOG) 97-04 study and the Charité Onkologie Phase III trial.
RTOG 97-04 study: Regine et al[9] conducted the most contemporary, multi-institutional, randomized trial assessing the effectiveness of adding gemcitabine chemotherapy to adjuvant 5-FU-based chemoradiotherapy. This study was not intended to confirm the utility of adjuvant chemoradiotherapy with 5-FU since it was included in both regimens. This intergroup trial (from July 1998 through July 2002) evaluated resected pancreatic cancer patients and stratified them according to pathologic stage T1-4N0-1M0 and margin status (positive, negative or unknown). There were two treatment arms to which patients were randomized. One regimen called for 5-FU chemotherapy given via continuous venous infusion (CVI) for three weeks followed by chemoradiotherapy (using 5-FU). Then 3 to 5 wk later, this was followed by additional (CVI) 5-FU chemotherapy for 4 wk, 2 wk off and then repeated for another 4 wk. The second regimen involved once weekly gemcitabine chemotherapy for 3 wk followed by chemoradiotherapy (using 5-FU). Then 3 to 5 wk later, this was followed by further weekly gemcitabine chemotherapy for 3 wk then 1 wk off, repeated for a total of 3 cycles. There was no observation arm since RTOG considers chemoradiotherapy the current standard of care for resected pancreatic cancer. Radiotherapy (after central review) was given over a period of 5.5 wk (unlike GITSG and EORTC trials which had a split-course) to a dose of 50.4 Gy at 1.8 Gy/fraction per day along with concurrent continuous infusion 5-FU chemotherapy.
Of the 538 patients enrolled, 451 were eligible for the study. The analysis of patients with adenocarcinoma of the head of the pancreas (388) demonstrated that patients receiving the gemcitabine-based regimen had a 20.5-mo median survival and a 31% 3-year survival as opposed to the 5-FU-based regimen with 16.9 mo and 22% respectively, although this did not reach statistical significance (P = 0.09). On univariate analysis, the addition of gemcitabine to adjuvant, 5-FU based chemoradiotherapy was associated with a trend toward survival benefit but without a statistically significant improvement in overall or disease-free survival. However, with adjustments made for prognostic variables, multivariate analysis of head of pancreas tumors revealed that the treatment effect of the gemcitabine-regimen yielded a statistically significant effect for enhanced survival (P = 0.05 with a 0.80 hazard ratio). The study authors asserted that accounting for the prognostic variables with multivariate analysis is a more accurate assessment of treatment outcome. Therefore, this study was included as evidence for adjuvant chemoradiotherapy. However, the primary endpoint of this trial to detect a statistically significant improvement in overall and disease-free survival for resected pancreatic cancer patients receiving adjuvant therapy with the addition of gemcitabine to chemoradiotherapy was not achieved. The trial only demonstrated that a trend toward improved survival. Confounding the results of this study is the fact, that many patients were given salvage chemotherapy at the time of disease recurrence with a majority of those receiving gemcitabine for salvage treatment. This salvage therapy probably lessened the capacity to discover a significant survival benefit[7,9,10].
An important finding from this study is that lymph node involvement was found to be a poor prognostic factor, demonstrating statistical significance with a P-value of 0.001. Tumor size (< 3 cm or ≥ 3cm) and surgical margin status (negative, positive or unknown) did not reach statistical significance as prognostic factors. Further analysis of postoperative CA 19-9 levels as a secondary endpoint of the study revealed that a postresection CA 19-9 value of ≤ 90 U/mL was also an independent predictor for survival. A significant increased risk of death was associated with CA 19-9 levels > 90 (P < 0.001)[7,9-12]. The study authors recommended the inclusion of gemcitabine-based therapy in future trials of adjuvant chemoradiotherapy. Even without the use of radiotherapy, the evidence for the positive effect of gemcitabine-based chemotherapy has been seen, as noted in a study performed by the German Charité Onkologie group.
Charité Onkologie Phase III trial: This collaborative German and Austrian, multi-institutional, randomized, controlled trial (July 1998 to December 2004) conducted by Oettle et al[13] sought to determine if resected pancreatic cancer patients would see a benefit in disease-free survival of six months or greater when given gemcitabine-based adjuvant chemotherapy. A total of 368 patients recruited were randomized to receive gemcitabine (n = 186) or observation (n = 182) following a macroscopic complete R0 or R1 resection. Presurgical staging required patients to have a T1-T4N0-N1M0 disease. Patients were stratified according to T-stage (T1-2 vs T3-4) and lymph node status (positive or negative). Of these participants, seven were excluded from each arm due to enrollment criteria violations. In the end, 179 patients received gemcitabine-based adjuvant chemotherapy and 175 patients were observed.
Chemotherapy for the gemcitabine group consisted of 6 cycles of gemcitabine (one weekly dose × 3 wk and one week off). Commencement of chemotherapy between postoperative day 10 and day 42 depending on wound status was recommended. There were 111 (62%) of patients received the complete 6 cycles of gemcitabine and 90% received a minimum of one dose. At least 87% were given one complete cycle of adjuvant gemcitabine chemotherapy.
Findings from this cooperative study demonstrated an improvement in the median disease-free survival that was statistically significant. The median disease-free survival for participants receiving adjuvant gemcitabine chemotherapy was 13.4 mo along with an estimated 3-year and 5-year disease-free survival of 23.5% and 16.5% as compared to 6.9 mo and 7.5% and 5.5% for the observation group (P < 0.001). However, overall survival only showed a trend toward improvement; it did not reach statistical significance. Follow up performed over a median of 53-mo demonstrated that participants receiving gemcitabine had a 22.1 mo median survival as compared to participants in the observation group with a 20.2 mo median survival (P = 0.06).
A limitation of this study was the lack of central review for validation of surgical resection and staging. In addition, participants in the observation group were given gemcitabine and other chemotherapy upon relapse of disease. This limits the study’s capacity to identify a benefit in overall survival. Although the change in overall survival did not show statistical significance, this trial does demonstrate that adjuvant gemcitabine chemotherapy has a positive affect on disease-free survival. This trial’s results combined with the results of RTOG 97-04 (chemoradiotherapy) adds weight to the evidence in favor of adjuvant therapy for resected pancreatic cancer[9,11,13]. However, there are other studies with negative results that should be considered when evaluating recommendations for adjuvant chemoradiotherapy.
European Study Group for Pancreatic Cancer trial: In this complex, multicenter, prospective, randomized study (February 1994 to June 2000), Neoptolemos et al[14] attempted to answer questions regarding the utility of adjuvant chemotherapy and adjuvant chemoradiotherapy vs no adjuvant therapy for potentially curatively resected pancreatic cancer patients. The updated analysis of the original study was done by way of comparison using a (2 × 2) factorial design with participants randomized to groups defined as (1) observation (no adjuvant therapy); (2) adjuvant chemoradiotherapy; (3) adjuvant chemotherapy; and (4) chemotherapy following adjuvant chemoradiotherapy. This design was used to detect the independent effect of each treatment and its interaction with other treatments in the study.
The regimen for chemoradiotherapy involved a similar course of radiotherapy as the GITSG trial which was given as two, 20 Gy courses separated by a two-week break. As well, the 5-FU chemotherapy was administered in bolus doses on the first three days of each two-week course of radiotherapy. The adjuvant chemotherapy regimen included a bolus dose of leucovorin followed by bolus 5-FU on five consecutive days of a 28-d cycle for a total of six cycles. The combined regimen (chemoradiotherapy + chemotherapy) involved the chemoradiotherapy regimen noted above followed by the previously described 28-d cycle chemotherapy regimen.
As patients (n = 289) were randomized within the 2 × 2 factorial design, the group breakdown was as follows: Observation (no adjuvant therapy) group (n = 69); chemoradiotherapy group (n = 73); chemotherapy group (n = 75) and chemoradiotherapy with additional chemotherapy group (n = 72). As the authors assessed group outcomes, the factorial design then allowed for combination of the randomized patient groups noted above into subsets as follows; patients assigned to receive chemoradiotherapy [chemoradiotherapy (73) + chemoradiotherapy and chemotherapy (72) = 145] vs assigned not to receive chemoradiotherapy [chemotherapy (75) + observation (69) = 144] and the patients assigned to chemotherapy [chemotherapy (75) + chemoradiotherapy and chemotherapy (72) = 147] vs assigned not to receive chemotherapy alone [observation (69) + chemoradiotherapy (73) = 142].
Interpretation of the results from this trial is complicated due to its complex structure. There was a median follow up of 47 mo. Assessment of a 2-year survival rate was its primary endpoint. Calculation of survival rate was determined by the date of surgery until death from disease or other cause. Measurement of secondary endpoints involved the prevalence of adverse treatment effects, recurrence of disease and quality of life.
Patients assigned to the chemoradiotherapy subset (n = 145) had a median survival of 15.9 mo as compared to a median survival of 17.9 mo for patients assigned not to receive chemoradiotherapy subset (n = 144) with a P-value of 0.05. The 2-year and 5-year survival estimates were 29% and 10% for the chemoradiotherapy subset (145) and 40% and 20% respectively in the no chemoradiotherapy subset (144). In the chemotherapy subset (n = 147), the median survival was 20.1 mo as compared to 15.5 mo in the patients who did not receive chemotherapy subset (n = 142) with a P-value of 0.009. The 2-year and 5-year survival estimates were 40% and 21% for the chemotherapy subset (147) and 30% and 8% in the no chemotherapy subset (142). Additional analyses of survival demonstrated that the observation (no adjuvant therapy) group (69) had a 16.9 mo median survival and a 5-year survival estimate of 11% as compared to a 13.9 mo median survival and a 7% 5-year survival estimate of the chemoradiotherapy group (73). A median survival of 21.6 mo and estimated 5-year survival rate of 29% was seen for patients receiving chemotherapy (75) as compared to 19.9 mo and a 13% 5-year survival estimate for the combination treatment (chemoradiotherapy + chemotherapy) group (72). Other outcome analysis found that increased tumor differentiation, tumor size > 2 cm and positive lymph nodes had a significant adverse affect on survival. There was no statistically significant difference in quality of life measures between the groups[14].
This trial was the largest randomized study to date attempting to examine the advantage of adjuvant treatment (chemoradiotherapy and maintenance chemotherapy) for resected pancreatic cancer. The positive aspect of this study was that a survival advantage was noted for resected pancreatic cancer patients who received adjuvant chemotherapy. However, this study is considered negative for chemoradiotherapy as it was found to adversely affect survival and even more so than no adjuvant therapy. Critics have raised concern that there were notable delays in treatment start time with a mean of 61 d for the chemoradiotherapy group as opposed to 48 d for the chemotherapy group. There is also concern about selection bias and whether the group of patients undergoing chemoradiotherapy had a poorer performance status than patients in the no adjuvant therapy group which had a better survival outcome. The question was raised whether or not toxicity from the first treatment in the consecutive treatment group (chemoradiotherapy followed by chemotherapy) affected compliance with the second portion of therapy. A limitation of this study was that there was no central standardized quality assurance for radiotherapy. Critics also note that the split-course of radiotherapy used in this trial is now considered outdated. Further confounding the findings of European Study Group for Pancreatic Cancer Trial, is the fact that physicians enrolling patients to the trial were also allowed to give other “background” chemotherapy or chemoradiotherapy, thus making interpretation of the trial results difficult[7,15-17]. The results of this trial in addition to the initial analysis of the EORTC trial effectively changed the practice in Europe. Chemotherapy alone is currently the preferred treatment for resected pancreatic cancer patients. However, in many centers in the United States, the recommendation for adjuvant therapy continues to be a combination of postoperative chemotherapy and chemoradiotherapy. Therefore, review of two large, single-institution studies of adjuvantly treated resected pancreatic cancer patients are included here. These studies reflect the general practice for adjuvant treatment usually given to resected pancreatic cancer patients in the United States.
The Johns Hopkins Hospital experience: A large study of prospectively collected data of resected pancreatic cancer patients (n = 616) at Johns Hopkins Hospital was conducted by Herman et al[18]. The study patients underwent a pancreaticoduodenectomy followed by adjuvant chemoradiotherapy or observation (from August 30, 1993 through February 28, 2005). Patients with T4 or M1 disease were excluded from analysis. Findings from this study demonstrated a significant improvement in overall survival for patients that underwent adjuvant chemoradiotherapy following a pancreaticoduodenectomy compared with those patients who had surgery alone. There was a 21.2 mo median survival for adjuvantly treated patients as compared to 14.4 mo for surgery-only patients (P < 0.001). The 2-year and 5-year survival rates for resected patients were 43.9% and 20.1% following adjuvant treatment as compared to 31.9% and 15.4% respectively without adjuvant chemoradiotherapy. The study authors also noted that adjuvant chemoradiotherapy provided a survival benefit even when high risk tumor features (high histologic grade, nodal involvement and positive surgical margins) were present.
The Mayo Clinic experience: A similar retrospective study was conducted by Corsini et al[19] at Mayo Clinic for stage I-II, T1-3N0-1M0 pancreas cancer patients (n = 454) who had undergone a R0 resection and postoperative adjuvant chemoradiotherapy (1975 to 2005). The retrospective analysis demonstrated that providing adjuvant chemoradiotherapy after surgery significantly improved overall survival. There was a 25.2 mo vs a 19.2 mo median survival for patients receiving adjuvant treatment (n = 274) vs no adjuvant treatment (n = 180) following surgery (P = 0.001). The authors noted a 2-year and 5-year survival of 50% and 28% for the adjuvant therapy group as opposed to 39% 2-year and 17% 5-year survival for surgery-alone group. They also found that positive lymph nodes and tumors with high histological grade adversely affected prognosis. In addition, they noted that patients receiving adjuvant treatment had a higher number of these adverse prognostic factors.
The Johns Hopkins Hospital & Mayo Clinic Collaborative study: Hsu et al[20] took a collaborative approach by combining the single-institution data noted above from Johns Hopkins Hospital and Mayo Clinic in order to assess predictive factors for survival following surgery, and to determine the benefit of adjuvant chemoradiotherapy stratified by risk groups. They also performed propensity score analysis as well as matched-pair analysis to correct for treatment selection bias associated with retrospective data. There were 1092 patients who underwent resection who were appropriate for review. The adjuvant chemoradiotherapy group (n = 583) and surgery-alone group (n = 509) survival did not vary significantly by institution. Patients who received adjuvant therapy were younger (median age 64.7 years) than the no adjuvant therapy group (median age 70.2 years) with a P-value < 0.001. The adjuvant therapy group had higher grade (3 or 4) tumors (59%) and greater positive surgical margins (35%) as compared to 51% and 31% respectively for the surgery alone group. However, adjuvant chemoradiotherapy after surgery was found to significantly improve survival in spite of the age of patient, and the status of the tumor, surgical margin or lymph nodes. Following propensity score analysis, overall survival improved by about 33% following adjuvant chemoradiotherapy (P < 0.001). Median survival, 2-year and 5-year survival were better with 21.1 mo, 44.7% and 22.3% as compared to 15.5 mo, 34.6% and 16.1% for those not receiving adjuvant therapy (P < 0.001).
Analysis of these large single-institution treatment data affirms the results of the randomized studies showing positive results. Adjuvant chemoradiotherapy for patients with a resected pancreatic cancer provides a survival benefit. Adjuvant therapy with chemotherapy alone or with chemoradiotherapy should remain an essential part of the treatment recommendations for resected pancreatic cancer.
Our current approach for adjuvant therapy for pancreas cancer includes a complete restaging following their resection. For patients with non-metastatic disease, a discussion is carried out regarding the potential benefits and side effects of either chemotherapy alone vs chemotherapy followed by chemoradiotherapy and then further chemotherapy. For patients with higher risk factors for local-regional recurrence such as local extension beyond the pancreas, an incomplete resection (R1/R2 resection), or positive lymph nodes, chemoradiotherapy may be considered following initial treatment with chemotherapy, similar to that of the RTOG 97-04 trial. Our previous analysis of patients undergoing resection at the Mayo Clinic for pancreas cancer showed that the risk of recurrence and death from tumor progression rises significantly for patients with these risk factors. Survival is proportionally greater following adjuvant therapy in cases with higher numbers of risk factors despite the overall higher risk of recurrence[21].
Patients receive two months of gemcitabine, followed by a second restaging procedure consisting of a repeat computed tomography scan of the abdomen and pelvis, chest X-ray, and laboratory studies including a CA 19-9 analysis. If patients have no evidence of metastatic progression, they then go on to receive 50.4 Gy of external beam radiotherapy in 1.8 per day fractions along with either radiosensitizing 5-FU chemotherapy given by continuous intravenous infusion or capecitabine. Following completion of this six week course of therapy, patients have a 3 to 4 wk recovery period before moving on to a further two months of gemcitabine chemotherapy. Radiotherapy is directed at the bed of resection, areas adjacent to the original tumor at risk for occult, direct local spread in the retroperitoneum and along vascular structures, and lymph node regions at risk. Details of this technique have been published elsewhere by Corsini et al[19].
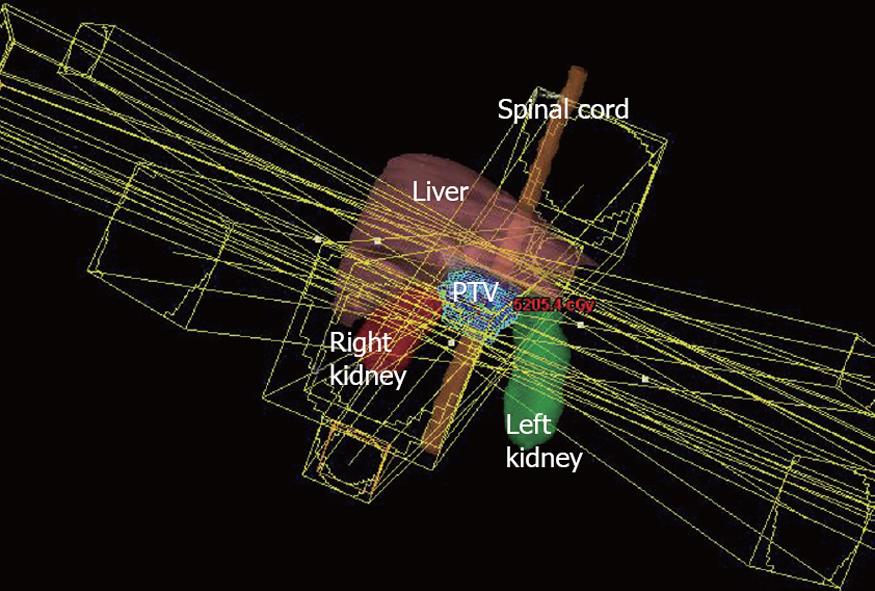
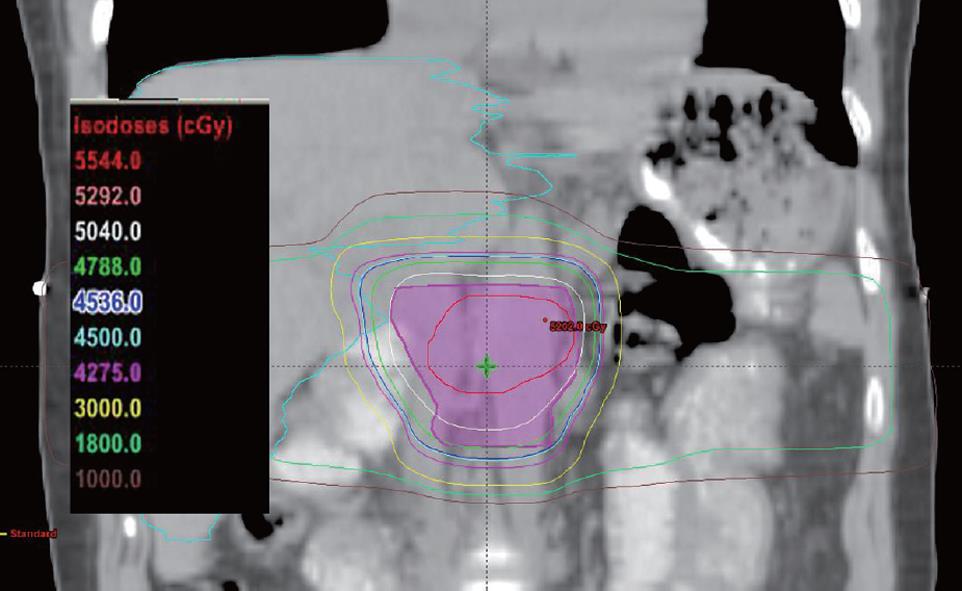
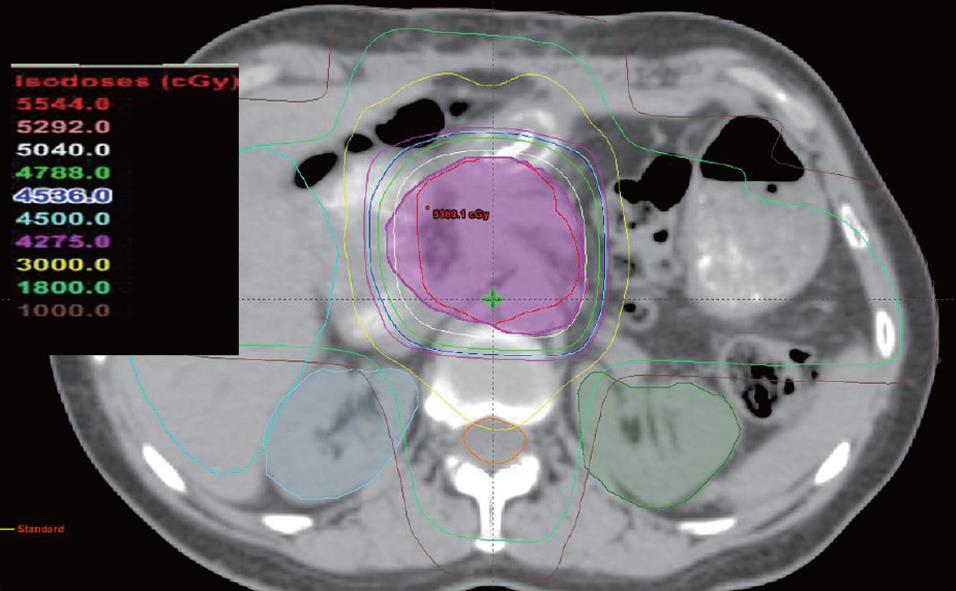
However, techniques for radiotherapy treatment delivery have changed over time. Currently, we favor a non-coplanar, six field approach for radiotherapy delivery that allows for enhanced sparing of normal tissues such as the liver, kidneys, and spinal cord. Anterior and posterior radiotherapy beams are angled inferiorly and superiorly to reduce the dose to the liver and kidneys, while the remaining radiotherapy dose is delivered through opposed lateral beams and two anterior oblique beams angled 45 degrees to either side of vertical. Figures 1, 2, and 3 illustrate a typical treatment geometry and isodose curves in the axial and coronal planes for a case receiving postoperative radiotherapy for resected pancreas cancer.
At this time, surgical resection will continue to be the most important first step in the treatment of pancreas cancer. The review of eminent, randomized-controlled studies (Table 1) is important in understanding the current practice for recommendations for adjuvant therapy of surgically resected pancreas cancer. The examination of the two single-institution studies completed by Johns Hopkins Hospital and Mayo Clinic (Table 1) adds weight to the evidence in favor of both adjuvant chemotherapy and chemoradiotherapy. The positive randomized-controlled trials and the large, single-institution studies reflect the current approach offered at many cancer treatment centers in the United States. However, the type of adjuvant treatment recommended remains controversial. In Europe, chemotherapy alone (5-FU/leucovorin or gemcitabine) is the preferred adjuvant therapy. The controversy regarding adjuvant therapy is likely to continue. However, this should not deter the search for the optimal treatment of this deadly disease. Current practice recommendations should be based on the weight of evidence in favor of adjuvant chemotherapy with chemoradiotherapy.
| Trial/authors, yr | Adjuvant therapy | Patients (n) | Median survival (mo) | Median disease free survival (mo) | Estimated 5-yr survival (%) | P-value |
| Evidence for adjuvant chemoradiotherapy in randomized trials | ||||||
| GITSG 9173/Kalser et al[4], 1985 | Chemoradiotherapy (5-FU) | 21 | 20 | 11 | 18 | 0.035 |
| No adjuvant therapy | 22 | 11 | 9 | 0 | ||
| EORTC Phase III/Klinkenbijl et al[6], 1999 | Chemoradiotherapy (5-FU) | 104 | 24.5a | 17.4a | 28a | 0.099a |
| 17.1b | ||||||
| No adjuvant therapy | 103 | 19a | 16a | 22a | 0.049c | |
| 12.6b | ||||||
| RTOG 97-04/Regine et al[9], 2008 | Chemoradiotherapy (5-FU) | 230 | 16.9 | 22d | 0.09 | |
| Chemoradiotherapy (gemcitabine) | 221 | 20.5 | 31d | |||
| CONKO Phase III/Oettle et al[13], 2007 | Chemotherapy (gemcitabine) | 186 | 22.1 | 13.4 | 18 | < 0.001e |
| No adjuvant therapy | 182 | 20.2 | 6.9 | 0 | 0.06f | |
| Evidence against adjuvant chemoradiotherapy in randomized trials | ||||||
| ESPAC-1/Neoptolemos et al[14], 2004 | Chemotherapy (CT) (5-FU) | 75 | 21.6 | 29 | 0.05 [(CT/RT), (CT/RT + CT) vs (CT), (no CT/RT)] | |
| Chemoradiotherapy (CT/RT) (5-FU) | 73 | 19.9 | 13 | |||
| Chemoradiotherapy + chemotherapy (CT/RT + CT) (5-FU) | 72 | 13.9 | 7 | 0.009 [(CT), (CT/RT + CT) vs (CT/RT), no (CT/RT)] | ||
| No adjuvant therapy | 69 | 16.9 | 11 | |||
| Evidence for adjuvant therapy in large single-institution studies | ||||||
| Johns Hopkins Hospital experience/Herman et al[18], 2008 | Chemoradiotherapy (5-FU) | 271 | 21.2 | 20.1 | < 0.001 | |
| No adjuvant therapy | 345 | 14.4 | 15.4 | |||
| The Mayo Clinic experience/Corsini et al[19], 2008 | Chemoradiotherapy (5-FU) | 274 | 25.2 | 28 | 0.001 | |
| No adjuvant therapy | 180 | 19.2 | 17 | |||
| The Johns Hopkins Hospital & Mayo Clinic Collaborative study/Hsu et al[20], 2010 | Chemoradiotherapy (5-FU) | 583 | 21.1 | 22.3 | < 0.001 | |
| No adjuvant therapy | 509 | 15.5 | 16.1 | |||
Peer reviewers: Sunil Krishnan, MD, Professor, Department of Radiation Oncology, MD Anderson Cancer Center, Unit 097, 1515 Holcombe Blvd, Houston, TX 77030, United States; Masashi Ishikawa, MD, PhD, Professor, Department of Surgery, Tokushima Red Cross Hospital, 103 Irinokuchi, Komatsushima city, Tokushima, Masa 1192, Japan
S- Editor Wang JL L- Editor Hughes D E- Editor Lin YP
| 1. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-249. |
| 2. | Ryan DP, Mamon H. Adjuvant and neoadjuvant therapy for pancreatic adenocarcinoma. Waltham, MA: UpToDate 2009; Accessed January 31, 2010. Available from: http://www.uptodate.com/online/content/topic.do?topicKey=gicancer/12842&selectedTitle=1%7E128&source=search_result. |
| 3. | National Comprehensive Cancer Network. Clinical Practice Guidelines in OncologyTM: Pancreatic Adenocarcinoma [v.1.2009]. 2009; Retrieved April 18, 2010. Available from: http://www.nccn.org/professionals/physician_gls/PDF/pancreatic.pdf. |
| 4. | Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899-903. |
| 5. | Gastrointestinal Tumor Study Group. Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Cancer. 1987;59:2006-2010. |
| 6. | Klinkenbijl JH, Jeekel J, Sahmoud T, van Pel R, Couvreur ML, Veenhof CH, Arnaud JP, Gonzalez DG, de Wit LT, Hennipman A. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230:776-782; discussion 782-784. |
| 7. | Garofalo MC, Nichols EM, Regine WF. Optimal adjuvant therapy for resected pancreatic cancer: chemotherapy or chemoradiotherapy? Gastrointest Cancer Res. 2007;1:182-187. |
| 8. | Garofalo MC, Regine WF, Tan MT. On statistical reanalysis, the EORTC trial is a positive trial for adjuvant chemoradiation in pancreatic cancer. Ann Surg. 2006;244:332-333; author reply 333. |
| 9. | Regine WF, Winter KA, Abrams RA, Safran H, Hoffman JP, Konski A, Benson AB, Macdonald JS, Kudrimoti MR, Fromm ML. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299:1019-1026. |
| 10. | Berlin J, Hoffman JP, Regine WF. Editorial: Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus conference. Ann Surg Oncol. 2009;16:1757-1759. |
| 11. | Abrams RA, Lowy AM, O'Reilly EM, Wolff RA, Picozzi VJ, Pisters PW. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1751-1756. |
| 12. | Berger AC, Garcia M Jr, Hoffman JP, Regine WF, Abrams RA, Safran H, Konski A, Benson AB 3rd, MacDonald J, Willett CG. Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: a prospective validation by RTOG 9704. J Clin Oncol. 2008;26:5918-5922. |
| 13. | Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267-277. |
| 14. | Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200-1210. |
| 15. | Bergenfeldt M, Albertsson M. Current state of adjuvant therapy in resected pancreatic adenocarcinoma. Acta Oncol. 2006;45:124-135. |
| 16. | Choti MA. Adjuvant therapy for pancreatic cancer--the debate continues. N Engl J Med. 2004;350:1249-1251. |
| 17. | Regine WF, Abrams RA. Adjuvant therapy for pancreatic cancer: current status, future directions. Semin Oncol. 2006;33:S10-S13. |
| 18. | Herman JM, Swartz MJ, Hsu CC, Winter J, Pawlik TM, Sugar E, Robinson R, Laheru DA, Jaffee E, Hruban RH. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol. 2008;26:3503-3510. |
| 19. | Corsini MM, Miller RC, Haddock MG, Donohue JH, Farnell MB, Nagorney DM, Jatoi A, McWilliams RR, Kim GP, Bhatia S. Adjuvant radiotherapy and chemotherapy for pancreatic carcinoma: the Mayo Clinic experience (1975-2005). J Clin Oncol. 2008;26:3511-3516. |
| 20. | Hsu CC, Herman JM, Corsini MM, Winter JM, Callister MD, Haddock MG, Cameron JL, Pawlik TM, Schulick RD, Wolfgang CL. Adjuvant chemoradiation for pancreatic adenocarcinoma: the Johns Hopkins Hospital-Mayo Clinic collaborative study. Ann Surg Oncol. 2010;17:981-990. |
| 21. | Miller RC, Iott MJ, Corsini MM. Review of adjuvant radiochemotherapy for resected pancreatic cancer and results from Mayo Clinic for the 5th JUCTS symposium. Int J Radiat Oncol Biol Phys. 2009;75:364-368. |