Published online Oct 27, 2010. doi: 10.4240/wjgs.v2.i10.319
Revised: September 28, 2010
Accepted: October 5, 2010
Published online: October 27, 2010
Asymptomatic pancreatic lesions (APL) are a commonly encountered problem in today’s pancreatic surgical practices. Current literature regarding etiologies and incidence of APLs, particularly intraductal papillary mucinous neoplasm (IPMN), is presented. APLs constitute a wide spectrum of pathology (solid/cystic, benign/premalignant/malignant) but, overall, IPMN is now the most common diagnosis. The Sendai Guidelines and their function as a basis for risk stratification in branch duct IPMN are presented. The importance of traditionally analyzed cyst characteristics including size, presence of mucin or nodules and cyst fluid aspirate as indicators of malignancy is emphasized, noting also the potential correlation of main duct dilatation, thickened septae and elevated cyst fluid CEA with increased risk of malignancy. Current complication rates after resection of APLs are reviewed and found to be generally equivalent to those for symptomatic resections. A potential multidisciplinary treatment strategy is offered considering the costs of surgery versus repeated imaging or follow up endoscopy for these lesions. The decision for intervention is ultimately based on the Sendai Guidelines in the context of the individual patient.
- Citation: Kent TS, Jr CMV, Callery MP. Intraductal papillary mucinous neoplasm and the pancreatic incidentaloma. World J Gastrointest Surg 2010; 2(10): 319-323
- URL: https://www.wjgnet.com/1948-9366/full/v2/i10/319.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v2.i10.319
Asymptomatic pancreatic lesions (APL), first described in 2001 as “incidentalomas”[1], are now known to comprise between 6% and 23% of pancreatic resections for any cause[2-4]. Largely attributed to increasing numbers of radiological studies obtained, the prevalence of cystic APLs on axial imaging is now reported to be between 1.2% and 2.6%[5,6]. Additional lesions can be identified from abnormal blood work or endoscopy evaluations[2,3,7]. APLs are noted most commonly during the evaluation of genitourinary complaints, chest pain or screening/cancer surveillance tests[3,4].
Up to half of such lesions are solid[3] and the vast majority of these are either malignant or at least premalignant. Traditional pancreatic resection remains the mainstay of treatment as it does for similar lesions which are symptomatic. On the other hand, determining the best management strategy for cystic APLs has been complicated because not all lesions have malignant potential and accurate preoperative determination of that threat remains problematic. Given the imperfect diagnostic information available, surgeons must therefore weigh up the risks and benefits of performing a potentially morbid operation for a perhaps benign condition.
Incidentally-identified cystic lesions of the pancreas are most commonly intraductal papillary mucinous neoplasms (IPMN), mucinous cystic neoplasms (MCN), serous cystadenomas (SCA), true cysts or pseudocysts as well as a variety of other rare etiologies. In about a tenth of incidental lesions noted radiographically or endoscopically, no definitive pathological diagnosis is obtained[3,8]. Of 212 consecutive pancreatic cystic lesions in one series, 37% were incidental[8]. Pseudocysts, not surprisingly, comprised only 4% of the asymptomatic group. MCN (28%) and IPMN (27%) were the most common diagnoses of resected APLs. However, a substantial number (17%) were ultimately found to be a benign SCA, drawing attention to the limitations in our preoperative evaluation of these patients.
IPMN, which develops along a spectrum of epithelial dysplasia from non-invasive to frankly malignant, is a common diagnosis in this scenario. Two primary varieties occur: Main-Duct (MD; dilatation of the main pancreatic duct) or Branch-Duct (Br; dilatation of side branches in the absence of main-duct dilatation). A third type called mixed-variant is less frequently seen and involves elements of both MD and Br histology[9]. In a series from Johns Hopkins which includes both solid and cystic lesions[2], IPMN (mostly non-invasive) constituted 35.6% of incidental pancreatic head lesions. A full 1/3 of their IPMN cases had a malignant diagnosis (high-grade dysplasia or invasive adenocarcinoma) though, of note, no distinction was made between MD IPMN and Br-IPMN. However, incidental cases had a disproportionally lower stage compared to their symptomatic counterparts, equating to an improved survival by 10 mo. The authors acknowledge the different proportion of favorable pathology and the effect of lead-time bias on the incidental group[2]. Lahat et al[5] expanded this idea to examine APLs situated throughout the gland. Of 465 pancreatic resections in their series, 13.5% were for incidental lesions. The percentage of malignant diagnoses in this group (34.3%) was about half that for the symptomatic cohort. IPMN was again the most common diagnosis in the incidental group (23.4%) whereas it constituted only 9% of the symptomatic cases where adenocarcinoma (PDAC) was by far the most common diagnosis[5]. Eighty seven percent of their incidental IPMNs were classified as adenomas or borderline lesions vs 59.4% in the symptomatic group (Table 1). From our own practice over a recent 5-year period, resected APLs were most commonly IPMN (17%), SCA (14%) and neuroendocrine tumors (13%). Overall, including both solid and cystic APLs, 71% were malignant or pre-malignant tumors. Of cystic APLs, a full one-third were IPMN, 26% SCA and 12% MCN. The rate of invasive malignancy among these lesions was 1.7% but, including lesions with high-grade dysplasia, the total malignancy rate for cystic APLs was 10.5%[3]. Symptomatic patients resected during the same time-period were more likely to have pancreatitis, pseudocysts and benign strictures.
| Winter et al[2] | Spinelli et al[6] | Sachs et al[3] | Fernández-del Castillo et al[8] | Bruzoni et al[4] | |
| Diagnosis | |||||
| IPMN | 35.6 | 24.5 | 17 | 27 | 9 |
| MCN | 17 | 32.6 | 28 | 7 | |
| SCN | b | 20.4 | 14 | 16.6 | 12 |
| Pseudocyst | 0 | - | 3.8 | - | |
| Adenocarcinoma | 18.6 | 6.1 | 2.5 | 30 | |
| Neuroendocrine | 9.3 | 8.2 | 13 | - | 19 |
| Other | 19.8 | 8.2 | 10.2 | 14 | |
| No diagnosis | N/A | N/A | 11.5 | 9 | |
| > 1 diagnosis | - | - | 6.4 | ||
| Operation | |||||
| Whipple | 100%c | 41 | 29.1 | 32 | 26.4 |
| Distal panc | 31 | 38.2 | 23 | 22.8 | |
| Central panc | 0 | 6.4 | 11.5 | 5.3 | |
| Total panc | 0 | 2.7 | 6.4 | 3.5 | |
| Enucleation | 22 | 4.5 | 2.5 | 0 | |
| Pseudocyst dr. | 0 | 0 | 0 | 0 | |
| Exp laparotomy/other | 6 | 19.1 | 2.5 | 0 | |
| No surgery | N/A | N/A | 21.8 | 42 |
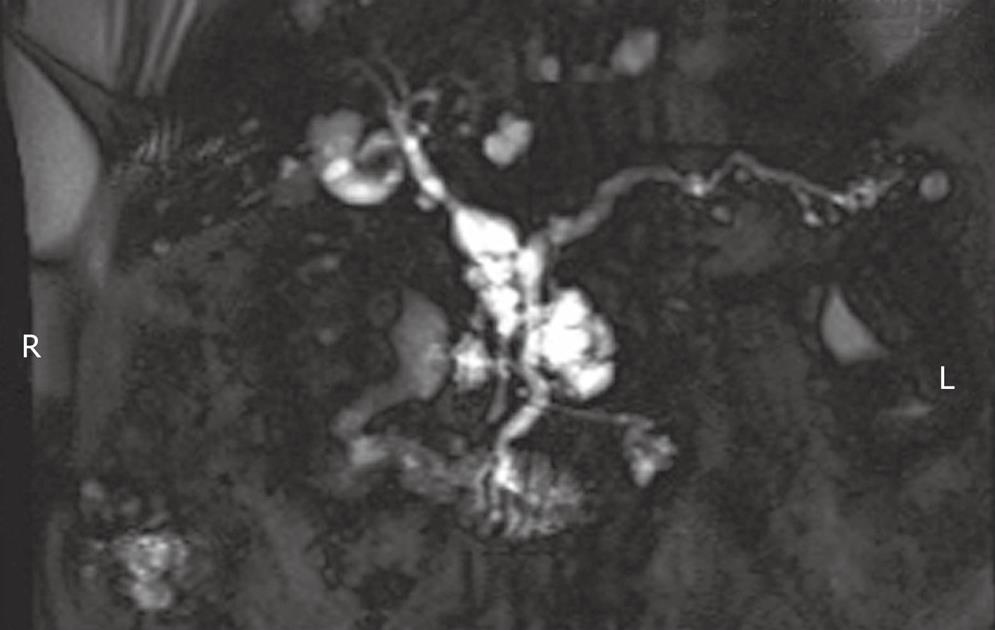
As emphasized elsewhere in this collection, the distinction between MD-IPMN and Br-IPMN is a crucial one given their different rates of progression to malignancy (63% vs 15% respectively)[10]. Both forms are frequently found incidentally (Figure 1). Of 145 patients with resected Br-IPMN, 40% were identified incidentally and there was no difference in malignancy between symptomatic and incidental lesions[11]. This review, representing the combined efforts of the Massachusetts General Hospital and the University of Verona, provided important justification of the Sendai consensus guidelines (below). Five years survival data among this large sample of resected Br-IPMN differed significantly for non-invasive (100%) compared to invasive cases (63%)[11], underscoring the fundamental issue with Br-IPMN; that is, they are significantly less likely to become malignant compared to MD-IPMN. Yet, waiting to operate until the Br-IPMN has become malignant significantly diminishes survival. Thus, it is critical to identify which Br-IPMN lesions are more likely to progress.
From the accrued literature, we can conclude that incidental lesions may be either solid or cystic. Solid tumors generally require resection just as if they were symptomatic. The majority of incidental cystic lesions are mucinous with both IPMN and MCN occurring frequently. However, SCAs still comprised a relevant proportion of resections. Overall, the proportion of malignant cases in this group was low but premalignant lesions were quite common. It is critical to distinguish between MD-IPMN and Br-IPMN because of their variable aggressiveness.
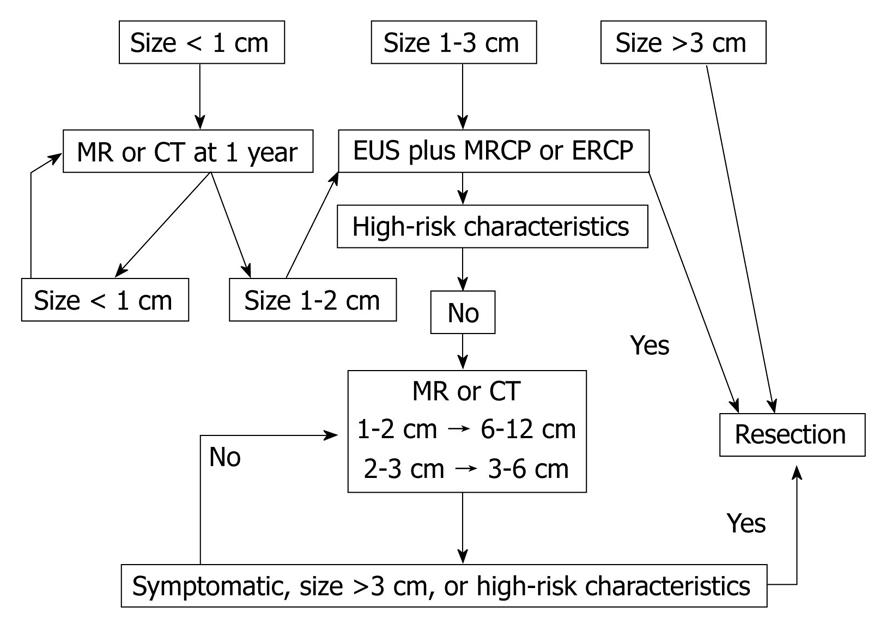
Since the initial reports of APLs, considerable effort has been devoted to the study of pancreatic cystic lesions and their management, culminating in 2006 with the publication of the Sendai Consensus Guidelines for the management of mucinous neoplasms of the pancreas[12]. This important position paper addressed the distinction between branch-duct and main-duct IPMN and further highlighted the need for appropriate preoperative classification. These guidelines recommend traditional resection including lymph node dissection for all MD-IPMN and MCN in reasonable surgical candidates. Resection is also recommended for Br-IPMN that is symptomatic, > 3 cm, have mural nodules or demonstrate cyst-aspirate cytology which is positive for malignancy[12]. Algorithms for follow-up of unresected IPMN were also provided, calling for computed tomography (CT), MRCP and/or EUS at intervals depending on size (< 1 cm, yearly; 1-2 cm, 6-12 mo; > 2 cm, 3-6 mo). Development of symptoms, nodules, cyst size > 3 cm or main duct dilatation > 6 mm during observation would then prompt consideration for resection. In the absence of change over a 2-year period, the interval between reevaluation may be lengthened. Those patients whose resected lesions are benign MCNs do not warrant follow-up but those with IPMN (particularly malignant) do have a risk of recurrence and should be reimaged yearly[12]. See Figure 2 for summary of guideline recommendations.
Despite the presence of these guidelines, medical and surgical pancreatologists continue to struggle with questions of what is really the best strategy for IPMN; specifically, which cystic lesions require resection and what follow-up is required for those patients undergoing resection as well as those who are observed. Subsequent work has augmented the body of knowledge on cystic lesions since Sendai. For instance, Tanno et al[13] have found that patients with a main pancreatic duct > 6 mm are more likely to demonstrate increasing cyst size or nodule development during follow-up and propose that main-duct diameter may help us predict which lesions will ultimately progress[13]. Among patients with solid or cystic APLs, elevated LFTs have also been found to correlate with the presence of malignancy[3]. Application of the Sendai guidelines has a negative predictive value of approximately 85%, indicating that several malignancies would be missed without further risk stratification[14].
While resection is generally recommended for premalignant lesions, the risks of the intervention must be weighed against the chance of progression to malignancy. Strict adherence to guidelines is not practically possible and reason dictates that a more flexible approach should be tailored to the individual patient’s circumstances. For example, an elderly patient with multiple comorbidities and an asymptomatic cystic lesion, even if MD-IPMN, may not be best served by a pancreatectomy. The difficulty is that we still do not know precisely the rate at which progression to malignancy will occur - either for the overall population or for any given patient who harbors an IPMN[15].
To help evaluate the benefit of resection for APLs, we must consider the necessary operations and their associated outcomes. Of studies incorporating lesions throughout the gland, APLs have accounted for 9%-31% of all pancreaticoduodenectomies and 22%-38% of distal resections[3,5,8] in focused pancreatic surgical practices.
Most groups report equivalent rates of overall morbidity (roughly 50%)[2,5,11] in patients with symptomatic and asymptomatic lesions. We had a 28% overall morbidity rate in our APL resections without any mortality[3]. Pancreatic fistula rates have varied by study depending in part on the inclusion or exclusion of distal resections and specific definitions employed. Rodriguez et al[11] reported a 17% fistula rate equivalent with their symptomatic patients and, in our series at BIDMC, clinically relevant (ISGPF grade B and C) fistulas occurred in 9%[3]. Winter et al[2] and Lahat et al[5] demonstrated higher fistula rates for their asymptomatic patients (25% and 18.4% vs 10.5% and 8.5% respectively). Important information is also available on the rarely reported outcomes of development of exocrine insufficiency (22%) and new or worsened diabetes (28%) in patients undergoing resection for benign Br-IPMN[11].
Comparison of survival among symptomatic and asymptomatic patients must be cautiously interpreted. A difference in survival can be attributed to a different breakdown of diagnoses (i.e. higher proportion of PDAC) in each group, as is seen and acknowledged by the Hopkins group[2]. Lahat et al[5] were able to compare survival specifically for their mucinous tumors; although median survival was not yet reached, there was a trend toward improved survival in the incidentally-identified IPMN and/or MCN (94%) compared to the symptomatic lesions (68%)[5].
In today’s practice environment, cost-effectiveness must also be considered as an outcome. Costs of long-term surveillance must be considered against the immediate costs of a high-acuity operation. This issue is not yet well delineated. In our recently published paper, patients with APLs were submitted to a median of 3 radiological tests prior to proceeding to surgery[3]. EUS and associated biopsy/FNA/cyst fluid analysis adds considerable additional cost. In IPMN cases, patients require follow-up even if they have a resection initially to identify possible recurrence. Das et al[16] conducted a decision analysis comparing surgery for all patients to follow-up for all to a cohort of intervention guided by EUS/cyst fluid analysis and subsequent risk stratification. Risk stratification-based treatment demonstrated the highest quality added life years and cost-effectiveness. Lastly, it is difficult to measure the true cost of a high-acuity operation with potential additive costs for complications for what turns out to be benign disease (i.e. an unnecessary resection) or, alternatively, the psychological burden and cost for a patient submitted to repeated scans and lengthy follow-up for a potentially pre-malignant tumor.
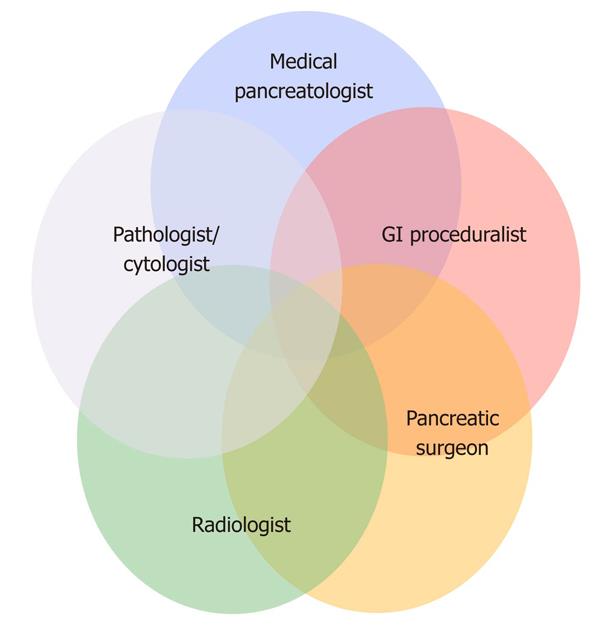
At our institution, we approach these lesions in a multidisciplinary fashion so that each patient is initially evaluated by a surgeon, a medical pancreatologist and a gastroenterologic proceduralist (Figure 3). Imaging exams are interpreted with dedicated pancreatic radiologists and efforts are made to accrue and evaluate antecedent scans in order to determine the natural history (growth or change of lesion morphology) of the lesion in question. In recent years, we have seen a stark increase in referrals for the evaluation of APLs to the point where they now comprise half of all referrals to our pancreatic surgical practice and nearly a quarter of all our resections[3].
Initial management typically includes treatment of the presenting problem if present. CT angiography of the pancreas is preferred for solid lesions whereas MRI is the primary modality used for follow-up of cystic lesions to best delineate the cyst and its relationship to the ductal system. With previous reports of only moderate sensitivity (69%) and specificity (90%) for EUS/ FNA[8] and unclear utility of cyst fluid analysis, we used EUS infrequently in the past. However, recent work has found EUS to be useful for predicting mucinous lesions by virtue of elevated cyst CEA (> 200 ng/mL)[3,17]. The value of additional biochemical cyst fluid analysis is debatable and we have found that it provides additive value to CEA analysis[18]. Furthermore, in our experience, atypical cytology on FNA was always associated with an ultimately premalignant tumor[3]. Thus, we now utilize EUS more frequently in the evaluation of both solid and cystic lesions. ERCP is rarely required to further evaluate side branch communication with the main duct or perhaps extent of involvement of MD-IPMN.
As solid lesions are much more likely to be malignant, most of these patients will undergo resection assuming they are reasonable surgical candidates. Care should be taken to rule out the rare occurrence of an aberrant splenule within the pancreas. For cystic lesions, we generally ascribe to the Sendai Consensus guidelines, as already mentioned above, and have seen a reversal of the ratio of resection to observation since their adoption. However, each patient’s particular circumstances contribute to decision making. Given the higher risk of malignant transformation, MCNs and MD-IPMN will generally undergo resection with a traditional pancreatectomy including regional lymphadenectomy in suitable surgical candidates. Of note, for multifocal Br-IPMN, we will typically resect the dominant disease if technically possible rather than proceed to total pancreatectomy in order to preserve function. Otherwise, cystic lesions that are < 3 cm, lack mural nodules, thickened septae, ductal obstruction or atypical/malignant FNA may qualify for observation on a case-by-case basis. Subsequent evaluation with MRI and/or EUS is then warranted as described above.
Development of the concerning features delineated above prompts reconsideration for resection. Furthermore, we consider anxiety in some cases to be a significant burden for many patients and have had many so anxious at the prospect of continued observation that they ultimately requested resection instead. This mandates a thorough and balanced discussion of risks and benefits with these patients. Postoperative follow-up is also regularly employed which entails additional imaging and clinical examination for those patients at risk for recurrence (malignant MCN, malignant IPMN, IPMN with retained dysplastic margins or multifocal disease which was not resected as well as other solid neoplasms).
APLs are a commonly encountered problem in today’s pancreatic surgical practices. IPMN is a frequent cause of the asymptomatic presentation, whether main- or branch-duct. Mucinous lesions generally should be resected due to the risk of malignancy. The Sendai Guidelines are a solid foundation on which to begin risk stratification for Br-IPMN. Aside from cyst size > 3 cm, presence of nodules and cyst fluid aspirate positive for malignancy, others have found main duct dilatation, thickened septae and elevated cyst fluid CEA to correlate with increased risk of malignancy. Complication rates after resection of APLs are generally equivalent to those for symptomatic resections, although some groups report a higher fistula rate. Exocrine and endocrine insufficiency will occur in approximately one-quarter of such resections. Although high-acuity surgery as required for resection of these lesions is costly, so, too, is repeated imaging or endoscopic intervention for follow-up. Ultimately, the Sendai Guidelines should be considered in the context of the individual patient, weighing up their anxiety, comorbidities and cyst characteristics against the risks and benefits of a pancreatic resection.
Peer reviewer: Shinichi Yachida, MD, PhD, Department of Gastrointestinal Surgery, Kagawa University, 1750-1, Ikenobe, Miki-cho, Kita-gun, Kagawa 761-0793, Japan
S- Editor Wang JL L- Editor Roemmele A E- Editor Yang C
| 2. | Winter JM, Cameron JL, Lillemoe KD, Campbell KA, Chang D, Riall TS, Coleman J, Sauter PK, Canto M, Hruban RH. Periampullary and pancreatic incidentaloma: a single institution's experience with an increasingly common diagnosis. Ann Surg. 2006;243:673-680; discussion 680-683. |
| 3. | Sachs T, Pratt WB, Callery MP, Vollmer CM Jr. The incidental asymptomatic pancreatic lesion: nuisance or threat? J Gastrointest Surg. 2009;13:405-415. |
| 4. | Bruzoni M, Johnston E, Sasson AR. Pancreatic incidentalomas: clinical and pathologic spectrum. Am J Surg. 2008;195:329-332; discussion 332. |
| 5. | Lahat G, Ben Haim M, Nachmany I, Sever R, Blachar A, Nakache R, Klausner JM. Pancreatic incidentalomas: high rate of potentially malignant tumors. J Am Coll Surg. 2009;209:313-319. |
| 6. | Spinelli KS, Fromwiller TE, Daniel RA, Kiely JM, Nakeeb A, Komorowski RA, Wilson SD, Pitt HA. Cystic pancreatic neoplasms: observe or operate. Ann Surg. 2004;239:651-657; discussion 657-659. |
| 7. | Laffan TA, Horton KM, Klein AP, Berlanstein B, Siegelman SS, Kawamoto S, Johnson PT, Fishman EK, Hruban RH. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802-807. |
| 8. | Fernández-del Castillo C, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138:427-433; discussion 433-444. |
| 9. | Singh M, Maitra A. Precursor lesions of pancreatic cancer: molecular pathology and clinical implications. Pancreatology. 2007;7:9-19. |
| 10. | Lévy P, Jouannaud V, O'Toole D, Couvelard A, Vullierme MP, Palazzo L, Aubert A, Ponsot P, Sauvanet A, Maire F. Natural history of intraductal papillary mucinous tumors of the pancreas: actuarial risk of malignancy. Clin Gastroenterol Hepatol. 2006;4:460-468. |
| 11. | Rodriguez JR, Salvia R, Crippa S, Warshaw AL, Bassi C, Falconi M, Thayer SP, Lauwers GY, Capelli P, Mino-Kenudson M. Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology. 2007;133:72-79; quiz 309-310. |
| 12. | Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17-32. |
| 13. | Tanno S, Nakano Y, Nishikawa T, Nakamura K, Sasajima J, Minoguchi M, Mizukami Y, Yanagawa N, Fujii T, Obara T. Natural history of branch duct intraductal papillary-mucinous neoplasms of the pancreas without mural nodules: long-term follow-up results. Gut. 2008;57:339-343. |
| 14. | Sawhney MS, Al-Bashir S, Cury MS, Brown A, Chuttani R, Pleskow DK, Callery MP, Vollmer CM. International consensus guidelines for surgical resection of mucinous neoplasms cannot be applied to all cystic lesions of the pancreas. Clin Gastroenterol Hepatol. 2009;7:1373-1376. |
| 15. | DiMagno EP. The pancreatic cyst incidentaloma: management consensus? Clin Gastroenterol Hepatol. 2007;5:797-798. |
| 16. | Das A, Ngamruengphong S, Nagendra S, Chak A. Asymptomatic pancreatic cystic neoplasm: a cost-effectiveness analysis of different strategies of management. Gastrointest Endosc. 2009;70:690-699.e6. |
| 17. | Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218-1226. |
| 18. | Sawhney MS, Devarajan S, O'Farrel P, Cury MS, Kundu R, Vollmer CM, Brown A, Chuttani R, Pleskow DK. Comparison of carcinoembryonic antigen and molecular analysis in pancreatic cyst fluid. Gastrointest Endosc. 2009;69:1106-1110. |