Published online Aug 27, 2025. doi: 10.4240/wjgs.v17.i8.108600
Revised: May 22, 2025
Accepted: June 23, 2025
Published online: August 27, 2025
Processing time: 129 Days and 16.8 Hours
Morphomics, a computed tomography-based body composition assessment, helps predicting esophageal cancer outcomes, but its link to bioelectrical impedance analysis (BIA) and functional assessments such as hand grip strength (HGS) and cardiopulmonary exercise testing (CPET) remains unclear.
To investigate correlations between morphomics and BIA, HGS, CPET, and assess its ability to predict low cardiorespiratory fitness (CRF).
Fifty esophageal cancer patients underwent multi-level morphomics, BIA, HGS, and CPET. Correlations were analyzed using heatmaps and scatter plots, and logistic regression assessed morphomic predictive value for low CRF.
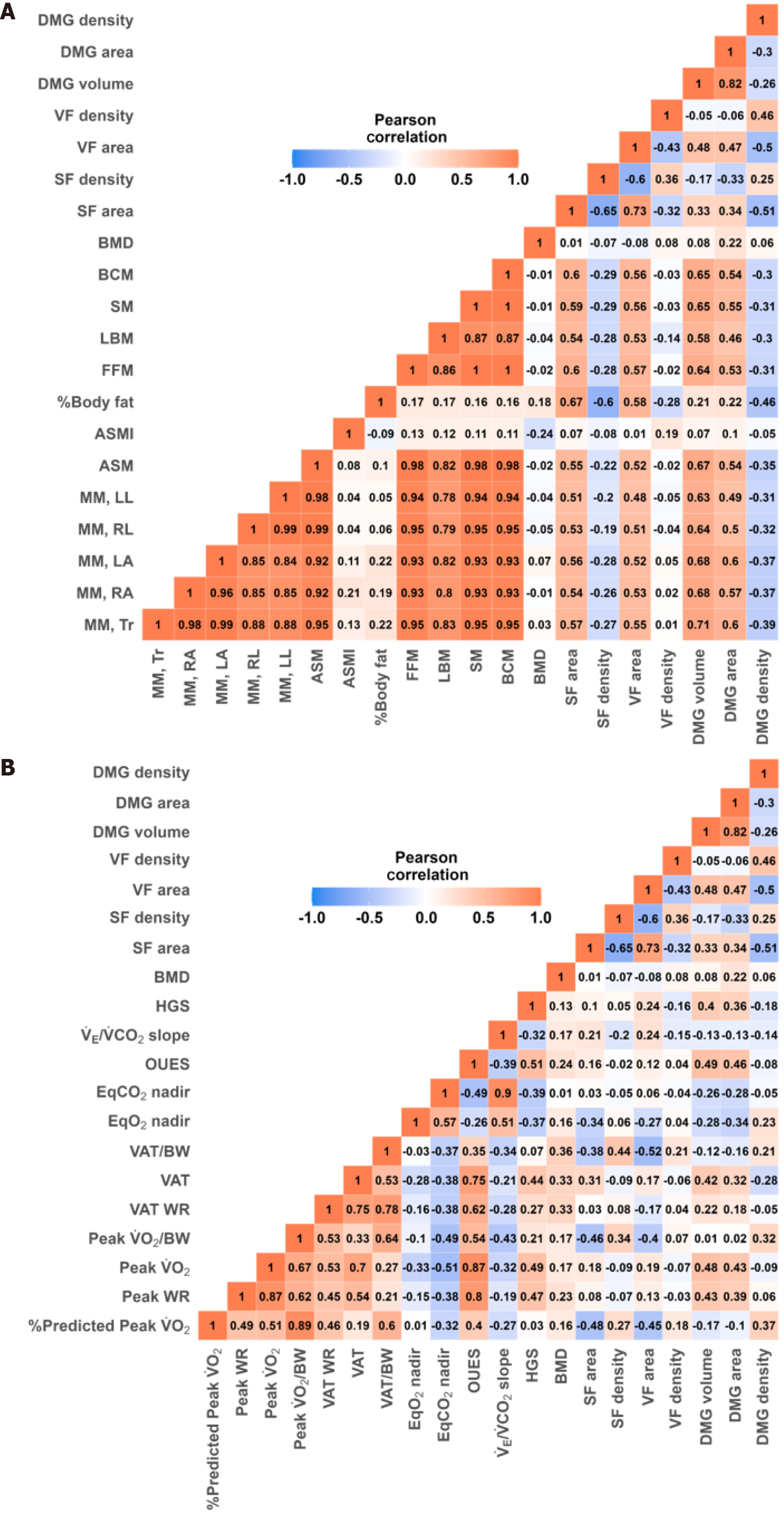
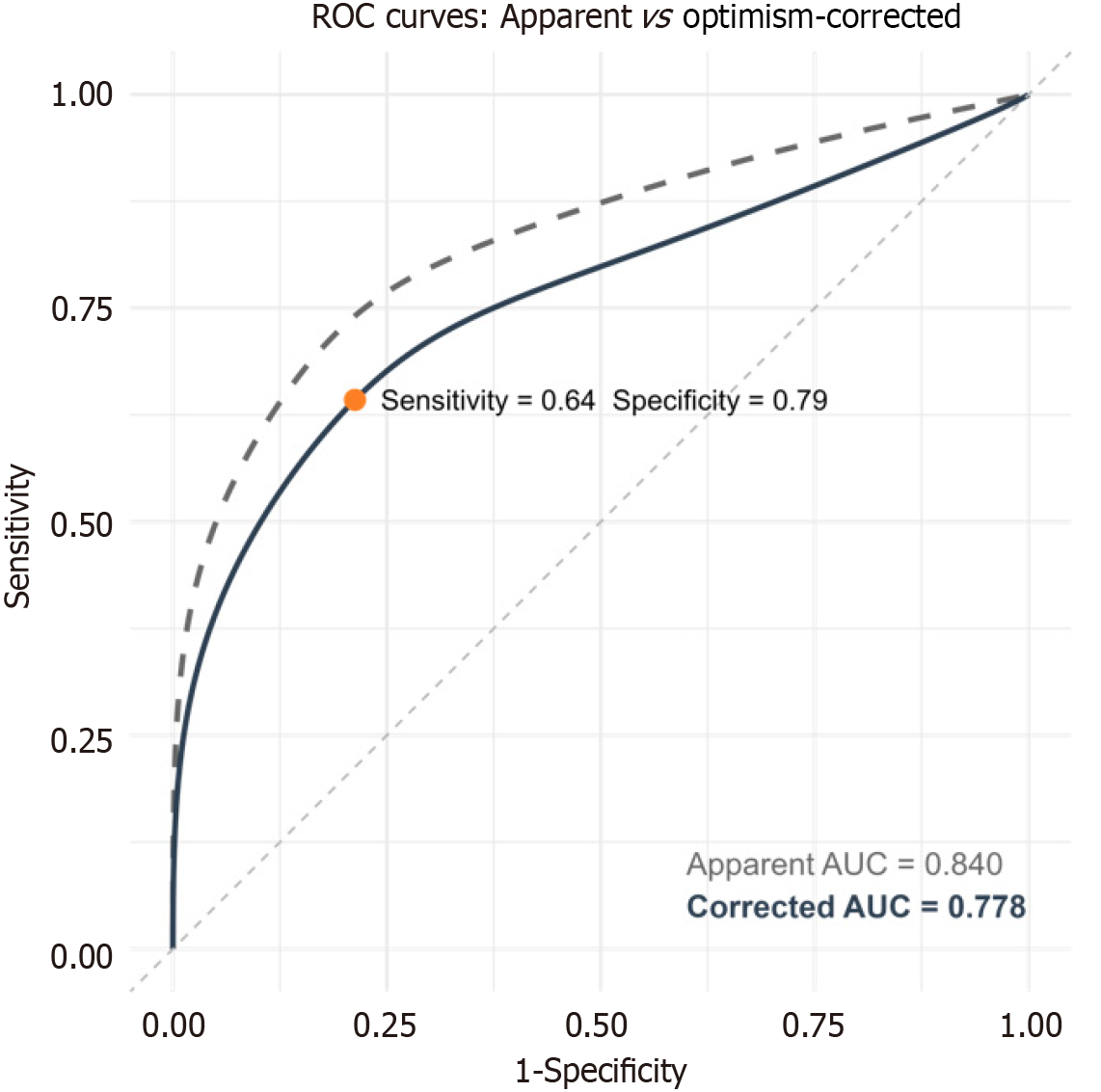
T11 is the only level with complete morphomic data, making it the most applicable. To ensure reliability, T11 and its adjacent levels, T10-12, were included in the subsequent analysis. Dorsal muscle group volume from T10-12 morphomics all correlated positively with BIA muscle components (r = 0.56-0.68, all P < 0.001), HGS (r = 0.4-0.48, all P < 0.001), and CPET variables (r = 0.43-0.51, all P < 0.001). Subcutaneous fat area and visceral fat area from morphomics correlated with body fat percentage (r = 0.58-0.67, all P < 0.001) and negatively with CPET parameters (r = -0.33 to -0.52, all P < 0.05). Morphomics also showed potential in identifying low CRF, with an area under the receiver operating characteristic curve of 0.778.
T11 morphomics shows strong correlation with BIA, HGS, and CPET, and may serve as a practical tool for preoperative risk assessment in esophageal cancer patients.
Core Tip: This study explored the relationship between computed tomography-based morphomics and physical assessments, including bioelectrical impedance analysis, hand grip strength, and cardiopulmonary exercise testing in esophageal cancer patients. Morphomics at the eleventh thoracic vertebra level had the most complete data and showed strong correlations with muscle mass, strength, and exercise capacity. Fat measurements from morphomics were inversely related to exercise performance. Morphomics also demonstrated potential to identify patients with low cardiorespiratory fitness, highlighting its clinical potential as a reliable tool for assessing physical fitness and guiding personalized care in esophageal cancer.
- Citation: Tseng WHS, Huang SC, Wang SC, Lin J, Zhang P, Liu YC, Chao YK, Chiu CH. Morphomics in esophageal cancer: Validation and association with muscular and cardiorespiratory fitness. World J Gastrointest Surg 2025; 17(8): 108600
- URL: https://www.wjgnet.com/1948-9366/full/v17/i8/108600.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i8.108600
Body composition is an essential part in the evaluation of physical health status, and studies have demonstrated its capacity to serve as a prognostic indicator[1]. Conventional approaches to body composition assessment, such as bioelectrical impedance analysis (BIA), have been widely adopted as one of the gold standards due to their accuracy and cost-effectiveness. In recent years, as computed tomography (CT) has become a standard procedure for patients with esophageal cancer, morphomics-a CT-derived body composition measurement-has garnered attention for its precision and its associations with surgical risks, outcome predictions, and long-term survival in patients with esophageal cancer[2-5]. However, most previous studies have focused on a single vertebral level for deriving morphomic parameters, comply L3[6-8]. Nevertheless, this level is not universally conducted in esophageal cancer cases. This study aimed to identify the spine level with the most comprehensive information for CT analysis as a feasible marker in esophageal cancer and to assess its validity by BIA using a novel multi-level approach.
Secondly, the prognostic predictive power of morphomics, is expected to align with muscular fitness and cardiorespiratory fitness (CRF)[9,10]. Hand grip strength (HGS) and cardiopulmonary exercise testing (CPET) are the most common tests for assessing these factors, both of which have well-established prognostic value[11-15]. However, no study to date has explored the association between morphomics and CRF in esophageal cancer patients[16]. This study aimed to strengthen this link.
Lastly, low CRF has been associated with increased risk of complications following major surgery[14,15,17]. In elective major intra-abdominal procedures, a ventilatory anaerobic threshold normalized by body weight (VAT/BW) below 10.9 mL/kg/min has been shown to predict all-cause hospital and 90-day mortality[15]. In the context of esophageal cancer surgery, a VAT/BW below 11 mL/kg/min has been linked to postoperative cardiopulmonary complications[14]. Accordingly, our study investigated whether morphomic parameters can effectively identify esophageal cancer patients with VAT/BW < 11 mL/kg/min.
To the best of our knowledge, this is the first investigation to establish morphomics using BIA as the reference standard in esophageal cancer patients examining the association with CRF. The present study explored the relationships between multi-level morphomics and BIA, HGS and CPET, as well as its potential predictive value.
The data were retrospectively collected from 50 newly diagnosed esophageal cancer patients at Chang Gung Memorial Hospital at Linkou (Taoyuan, Taiwan), between July 2019 and July 2022. All patients underwent CT of the chest and abdomen for esophageal cancer staging, along with assessments of muscular and CRF including body composition from morphomics and BIA, HGS and CPET. CT and CPET were performed within a 2-week interval, with HGS measured just prior to CPET. The study was conducted in accordance with the ethical standards of the institutional review board (IRB number: 202500080B0) at Chang Gung Memorial Hospital at Linkou.
Morphomics: Analytic morphomics processing was conducted following previously described methods[2,4,5]. All CT scans were processed using a fully automated, artificial intelligence-based algorithm implemented in MATLAB R2016b (The MathWorks, Inc, Natick, MA). Morphomic variables were analyzed at the cross-sectional level of the bottom of the specific vertebra, excluding bone mineral density (BMD). The specific analytic morphomics were as follows: (1) BMD: Average pixel intensity Hounsfield units (HU) within trabecular bone of vertebra at mid-vertebra level; (2) Subcutaneous fat (SF) area: Area of fat-intensity pixels (–205 to –51 HU) between the fascia and skin; (3) SF density: Median pixel intensity of fat-intensity pixels in SF area; (4) Visceral fat (VF) area: Area of fat-intensity pixels within the fascial envelope; (5) VF density: Median pixel intensity of fat-intensity pixels in VF area; (6) Dorsal muscle group (DMG) area: Area of muscle-intensity pixels (-29 to 150 HU) contained in the triangular region between the spinal canal and bilateral lateral seams; (7) DMG volume: Volume of DMG between this vertebra and its superior neighbor; and (8) DMG density: Median pixel intensity of muscle-intensity pixels in DMG area.
BIA: Whole-body composition was assessed using the InBody S10 device (Seoul, Korea), which measures electrical impedance at four frequencies (5, 50, 250, and 500 kHz)[18,19]. Participants remained seated upright on a non-conductive chair throughout the entire 10-minute assessment. Electrodes were positioned on each body segment according to the manufacturer’s guidelines to measure electrical impedance. Participants were instructed to fast for at least 2 hours prior to testing. The following parameters were recorded: Appendicular skeletal muscle index (ASMI), body cell mass (BCM), fat-free mass (FFM), lean body mass (LBM), and body fat percentage (BF).
HGS was measured using a dynamometer (Tsutsumi company, TTM, Tokyo). Participants stood naturally with palms facing their bodies. Grip distance was optimized for force. They squeezed the dynamometer with maximum force for about 3 seconds. Two trials were done for each hand, alternating sides. The average of the two highest values, one from each side, was calculated.
A symptom-limited incremental exercise test was conducted in an upright position using a calibrated cycle ergometer (Ergoselect 150P, Germany) to evaluate cardiopulmonary fitness. The CPET was performed 2-4 hours after a light meal. The protocol included 2 minutes of rest, a 1-minute warm-up at 10 watts, and a ramp increase of 10 watts per minute until the participant reached exhaustion. The participant was requested to keep the pedaling frequency at 60 ± 3 revolutions per minute.
Minute ventilation (Ve), oxygen consumption (VO2), and carbon dioxide production (VCO2) were measured breath by breath using a computerized system (MasterScreen CPX, Cardinal Health, Germany). Heart rate was monitored via R–R intervals on a 12-lead electrocardiogram, while arterial blood pressure was assessed with an automated system (Tango, SunTech Medical, United Kingdom). Arterial oxygen saturation was continuously monitored using finger pulse oximetry (Model 9500, Nonin Onyx, Plymouth, Minnesota). The exercise test was terminated under any of the following conditions: (1) The participant could no longer maintain a pedaling cadence of at least 50 rpm; (2) The participant experienced volitional fatigue and asked to stop; (3) Peak VO2 plateaued or declined despite continued effort; or (4) An adverse cardiovascular event occurred[20]. The data were averaged every 15 seconds.
The ventilatory anaerobic threshold (VAT) was primarily identified using the V-slope method and confirmed through the following ventilatory criteria: (1) A nonlinear increase in VCO2 relative to VO2; (2) An increase in the Ve/VO2 ratio without a corresponding rise in the Ve/VCO2 ratio; and (3) A rise in end-tidal oxygen pressure without a simultaneous drop in end-tidal carbon dioxide pressure. Ventilatory efficiency was evaluated using four parameters: (1) EqCO2 nadir: The lowest value of the ventilatory equivalent for CO2 (Ve/VCO2) during the incremental exercise test; (2) EqO2 nadir: The lowest value of the ventilatory equivalent for O2 (Ve/VO2) throughout The test; (3) Ve-VCO2 slope: Calculated using linear regression (Y = mX + b), where Y represents Ve, X represents VCO2, and m is the slope. Ve and VCO2 values were taken from the start of exercise to peak effort. A flatter slope indicates more efficient Ve; and (4) Oxygen uptake efficiency slope: Calculated from the slope of VO2 plotted against the logarithm of Ve [(L/min)/Log(L/min)], reflecting the efficiency of oxygen uptake during exercise. A higher slope indicates better oxygen utilization efficiency[21].
All statistical analyses were conducted using R (version 4.4.1) and RStudio (version 2014.12.0). Continuous variables were presented as mean ± SD, whereas categorical variables, such as gender, were reported as counts.
This study employed a novel multi-level approach, ultimately selecting levels with the least information loss to ensure feasibility. Pearson correlation coefficients were calculated to evaluate univariable relationships between BIA, CPET, HGS, and morphomics. Only those with correlation coefficients greater than 0.3 and reaching significance at all levels are considered reliable correlations.
Multivariable logistic regression was used to predict the likelihood of patients having an VAT/BW below 11 mL/kg/min, which has been identified as a clinically meaningful cutoff for predicting complications in major surgery[14,15]. Multicollinearity was examined with variance-inflation factors before model fitting. All eight morphomic variables were initially entered as covariates, including BMD, DMG area, DMG volume, DMG density, SF area, SF density, VF area and VF density. We then adopted a bidirectional stepwise variable-selection procedure and yielded a parsimonious model comprising BMD, VF area, and DMG volume. Logistic regression models were fitted with both the full and reduced predictor sets, and odds ratios (OR) with 95% confidence intervals (CI) were reported to quantify the contribution of each variable. To ensure validity, internal validation was done through 1000 bootstrap resamples. We reported OR of each variable in both full model and the parsimonious model to assess the importance of each variable. Receiver operating characteristic (ROC) analysis was then employed to assess the predictive performance of these variables in distinguishing individuals with VAT/BW ≤ 11 mL/kg/min. Significance of the area under the ROC curve (AUC) was tested using the Wilcoxon test, with statistical significance set at P value < 0.05. Both apparent ROC and optimism-corrected ROC were presented.
The majority of the cohort was male (98%), with a mean age of 57.3 years. The summary of variables of morphomics, BIA, HGS and CPET were recorded in Supplementary Table 1.
Morphomic variables were extracted from cross-sectional levels spanning the thoracic to lumbar vertebrae. To ensure data reliability, vertebral levels with more than 5% missing values were excluded from the analysis. Consequently, T10, T11, and T12 were selected, as their missing data rates were 0.5%, 0%, and 0.5%, respectively. In contrast, the missing data rates at L1, L2, and L3 were significantly higher (15.5%, 21.5%, and 34%, respectively), making them unsuitable for further analysis.
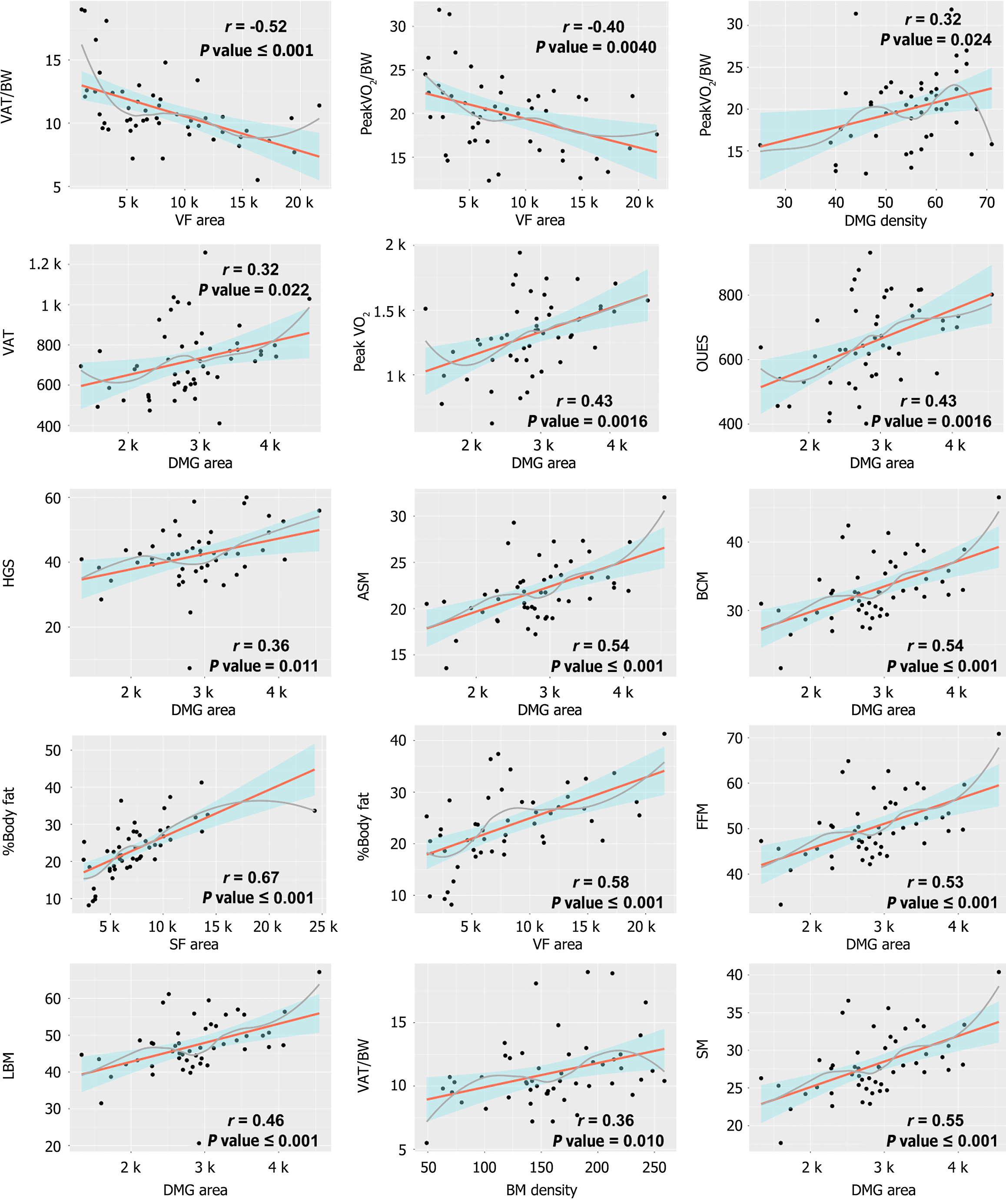
For muscle component, DMG volume from morphomics at T10-T12 Level had positive correlation with skeletal muscle mass (SMM) (r = 0.6-0.65), FFM (r = 0.6-0.64), LBM (r = 0.56-0.58), BCM (r = 0.6-0.68) and appendicular skeletal muscle (ASM) (r = 0.6-0.67) (all P < 0.001). A similar relationship was also observed between DMG area and SMM (r = 0.55-0.65), FFM (r = 0.53-0.64), LBM (r = 0.5-0.61), BCM (r = 0.54-0.66) and ASM (r = 0.54-0.65) (all P < 0.001) (Table 1; Figure 1A, Figure 2).
| Bioelectrical impedance analysis | Morphomics | Spine level | Correlation | P value |
| Body cell mass | DMG volume | T10 | 0.594 | < 0.001 |
| T11 | 0.647 | < 0.001 | ||
| T12 | 0.684 | < 0.001 | ||
| DMG area | T10 | 0.647 | < 0.001 | |
| T11 | 0.543 | < 0.001 | ||
| T12 | 0.664 | < 0.001 | ||
| Body fat percentage | SF area | T10 | 0.666 | < 0.001 |
| T11 | 0.672 | < 0.001 | ||
| T12 | 0.674 | < 0.001 | ||
| VF area | T10 | 0.576 | < 0.001 | |
| T11 | 0.578 | < 0.001 | ||
| T12 | 0.606 | < 0.001 | ||
| DMG density | T10 | -0.469 | < 0.001 | |
| T11 | -0.457 | < 0.001 | ||
| T12 | -0.513 | < 0.001 | ||
| Fat free mass | SF area | T10 | 0.597 | < 0.001 |
| T11 | 0.599 | < 0.001 | ||
| T12 | 0.590 | < 0.001 | ||
| SF area | T10 | -0.244 | 0.09 | |
| T11 | -0.281 | 0.048 | ||
| T12 | -0.420 | 0.002 | ||
| VF area | T10 | 0.491 | < 0.001 | |
| T11 | 0.568 | < 0.001 | ||
| T12 | 0.491 | < 0.001 | ||
| DMG volume | T10 | 0.589 | < 0.001 | |
| T11 | 0.642 | < 0.001 | ||
| T12 | 0.589 | < 0.001 | ||
| DMG area | T10 | 0.643 | < 0.001 | |
| T11 | 0.528 | < 0.001 | ||
| T12 | 0.643 | < 0.001 | ||
| Lean body mass | DMG volume | T10 | 0.556 | < 0.001 |
| T11 | 0.584 | < 0.001 | ||
| T12 | 0.556 | < 0.001 | ||
| DMG area | T10 | 0.613 | < 0.001 | |
| T11 | 0.459 | < 0.001 | ||
| T12 | 0.613 | < 0.001 | ||
| Skeletal muscle mass | DMG volume | T10 | 0.598 | < 0.001 |
| T11 | 0.650 | < 0.001 | ||
| T12 | 0.598 | < 0.001 | ||
| DMG area | T10 | 0.650 | < 0.001 | |
| T11 | 0.546 | < 0.001 | ||
| T12 | 0.650 | < 0.001 | ||
| Appendicular skeletal muscle | DMG volume | T10 | 0.603 | < 0.001 |
| T11 | 0.670 | < 0.001 | ||
| T12 | 0.671 | < 0.001 | ||
| DMG area | T10 | 0.638 | < 0.001 | |
| T11 | 0.542 | < 0.001 | ||
| T12 | 0.653 | < 0.001 | ||
| Muscle mass, left arm | DMG volume | T10 | 0.540 | < 0.001 |
| T11 | 0.679 | < 0.001 | ||
| T12 | 0.540 | < 0.001 | ||
| DMG area | T10 | 0.628 | < 0.001 | |
| T11 | 0.605 | < 0.001 | ||
| T12 | 0.628 | < 0.001 | ||
| Muscle mass, left leg | DMG volume | T10 | 0.594 | < 0.001 |
| T11 | 0.629 | < 0.001 | ||
| T12 | 0.594 | < 0.001 | ||
| DMG area | T10 | 0.601 | < 0.001 | |
| T11 | 0.494 | < 0.001 | ||
| T12 | 0.601 | < 0.001 | ||
| Muscle mass, right arm | DMG volume | T10 | 0.540 | < 0.001 |
| T11 | 0.677 | < 0.001 | ||
| T12 | 0.540 | < 0.001 | ||
| DMG area | T10 | 0.623 | < 0.001 | |
| T11 | 0.571 | < 0.001 | ||
| T12 | 0.623 | < 0.001 | ||
| Muscle mass, right leg | DMG volume | T10 | 0.605 | < 0.001 |
| T11 | 0.637 | < 0.001 | ||
| T12 | 0.605 | < 0.001 | ||
| DMG area | T10 | 0.621 | < 0.001 | |
| T11 | 0.497 | < 0.001 | ||
| T12 | 0.621 | < 0.001 | ||
| Muscle mass, trunk | DMG volume | T10 | 0.568 | < 0.001 |
| T11 | 0.705 | < 0.001 | ||
| T12 | 0.568 | < 0.001 | ||
| DMG area | T10 | 0.646 | < 0.001 | |
| T11 | 0.598 | < 0.001 | ||
| T12 | 0.646 | < 0.001 |
For fat component, BF showed moderate to strong positive relationship with SF area (r = 0.67) and VF area (r = 0.58-0.61) across the T10-T12 spinal levels (all P < 0.001). Conversely, a negative correlation was observed between BF and DMG density (r = -0.46 to -0.51, P < 0.001) (Table 1; Figure 1A, Figure 2). As for bone component, BMD exhibited little to no correlation with most BIA parameters, showing only a weak association with ASMI (r = -0.24, P = 0.099) (Figure 1A).
HGS demonstrated positive correlation with DMG volume at T10 (r = 0.48, P < 0.001), T11 (r = 0.40, P < 0.001) and T12 (r = 0.45, P < 0.001) and DMG area at T10 (r = 0.51, P < 0.001), T11 (r = 0.36, P = 0.011) and T12 (r = 0.35, P = 0.013) (Table 2; Figure 1B, Figure 2).
| Musclar/cardiorespiratory fitness | Morphomics | Spine level | Correlation | P value |
| Hand grip strength | DMG volume | T10 | 0.482 | < 0.001 |
| T11 | 0.396 | 0.004 | ||
| T12 | 0.455 | < 0.001 | ||
| DMG area | T10 | 0.514 | < 0.001 | |
| T11 | 0.358 | 0.01 | ||
| T12 | 0.350 | 0.01 | ||
| Peak VO2 | DMG volume | T10 | 0.504 | < 0.001 |
| T11 | 0.483 | < 0.001 | ||
| T12 | 0.466 | < 0.001 | ||
| DMG area | T10 | 0.460 | < 0.001 | |
| T11 | 0.435 | 0.002 | ||
| T12 | 0.424 | 0.002 | ||
| Peak WR | DMG volume | T10 | 0.435 | 0.002 |
| T11 | 0.430 | 0.002 | ||
| T12 | 0.436 | 0.002 | ||
| DMG area | T10 | 0.380 | 0.006 | |
| T11 | 0.394 | 0.005 | ||
| T12 | 0.338 | 0.016 | ||
| Peak VO2/BW | SF area | T10 | -0.469 | < 0.001 |
| T11 | -0.457 | < 0.001 | ||
| T12 | -0.461 | < 0.001 | ||
| VF area | T10 | -0.290 | 0.041 | |
| T11 | -0.400 | 0.004 | ||
| T12 | -0.436 | 0.002 | ||
| DMG density | T10 | 0.385 | 0.006 | |
| T11 | 0.319 | 0.024 | ||
| T12 | 0.408 | 0.003 | ||
| %predicted peak VO2 | SF area | T10 | -0.486 | < 0.001 |
| T11 | -0.478 | < 0.001 | ||
| T12 | -0.471 | < 0.001 | ||
| VF area | T10 | -0.335 | 0.017 | |
| T11 | -0.455 | < 0.001 | ||
| T12 | -0.464 | < 0.001 | ||
| DMG density | T10 | 0.341 | 0.015 | |
| T11 | 0.373 | 0.008 | ||
| T12 | 0.462 | < 0.001 | ||
| VAT | DMG area | T10 | 0.356 | 0.011 |
| T11 | 0.324 | 0.022 | ||
| T12 | 0.327 | < 0.001 | ||
| VAT WR | BMD | T10 | 0.341 | < 0.001 |
| T11 | 0.328 | < 0.001 | ||
| T12 | 0.330 | < 0.001 | ||
| VAT/BW | BMD | T10 | 0.374 | 0.007 |
| T11 | 0.361 | 0.01 | ||
| T12 | 0.314 | 0.026 | ||
| SF area | T10 | -0.373 | 0.008 | |
| T11 | -0.383 | 0.006 | ||
| T12 | -0.373 | 0.008 | ||
| VF area | T10 | -0.430 | 0.002 | |
| T11 | -0.522 | < 0.001 | ||
| T12 | -0.520 | < 0.001 | ||
| EqO2nadir | SF area | T10 | -0.350 | 0.013 |
| T11 | -0.341 | 0.016 | ||
| T12 | -0.334 | 0.018 | ||
| Oxygen uptake efficiency slope | DMG volume | T10 | 0.449 | 0.001 |
| T11 | 0.483 | < 0.001 | ||
| T12 | 0.509 | < 0.001 | ||
| DMG area | T10 | 0.449 | 0.001 | |
| T11 | 0.435 | 0.002 | ||
| T12 | 0.451 | 0.001 |
Morphomic analysis of the DMG highlighted a clear link between muscle mass and cardiopulmonary fitness. DMG area at T10-T12 showed positive correlation with several CPET variables, including peak VO2 (r = 0.42-0.46, P < 0.001), peak WR (r = 0.34-0.39, P < 0.05), peak VO2/BW (r = 0.32-0.39, P < 0.05), VAT (r = 0.32-0.36, P = 0.01-0.02) and oxygen uptake efficiency slope (OUES) (r = 0.43-0.45, P < 0.001). Besides, DMG volume showed similar positive associations with peak VO2 (r = 0.47-0.50), peak WR (r = 0.43-0.44) and OUES (r = 0.45-0.51) (all P < 0.001) (Table 2; Figure 1B, Figure 2).
In contrast to muscular morphomics, the fat component from morphomics showed negative correlation with CPET variables. SF area exhibited a negative correlation with peak VO2/BW (r = -0.46 to -0.47, P < 0.001), VAT/BW (r = -0.37 to -0.38, P < 0.001) and EqO2 nadir (r = -0.33 to -0.35, P < 0.05). VF area demonstrated a similar correlation with peak VO2/BW (r = -0.3 to -0.43, P < 0.05) and VAT/BW (r = -0.43 to -0.52, P < 0.001).
Furthermore, BMD demonstrated a positive correlation with VAT WR (r = 0.33-0.34, P = 0.02) and VAT/BW (r = 0.31-0.37, P < 0.05).
Since all the morphomic parameters were derived from the same anatomical region, we tried to detect potential multicollinearity using variance inflation factors. We found little to moderate collinearity among all the eight morphomic predictors. (Supplementary Table 2) Therefore, all morphomic parameters were suitable for logistic regression analysis.
The multivariable logistic regression analysis indicated that a larger VF area significantly increased the likelihood of an VAT/BW ≤ 11 mL/min/kg (OR 5.01, 95%CI: 1.91-18.72, P = 0.0046). Conversely, higher BMD demonstrated a protective effect against low VAT/BW (OR 0.42, 95%CI: 0.17-0.90, P = 0.038). Similarly, although not reaching statistical significance, a higher DMG volume showed a trend toward an inverse association with low VAT/BW (OR 0.41, 95%CI: 0.15-0.94, P = 0.051) (Table 3).
| Predictor | Full model | Parsimonious model | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Bone mineral density | 0.37 (0.14-0.85) | 0.03 | 0.42 (0.17-0.90) | 0.038 |
| Subcutaneous fat area | 0.94 (0.25-4.55) | 0.93 | ||
| Subcutaneous fat density | 0.93 (0.26-2.97) | 0.90 | ||
| Visceral fat area | 5.73 (1.39-33.37) | 0.03 | 5.01 (1.91-18.72) | 0.0046 |
| Visceral fat density | 1.17 (0.45-3.09) | 0.75 | ||
| Dorsal muscle group volume | 0.27 (0.05-1.12) | 0.10 | 0.41 (0.15-0.94) | 0.051 |
| Dorsal muscle group area | 1.59 (0.38-7.52) | 0.54 | ||
| Dorsal muscle group density | 1.22 (0.48-3.16) | 0.67 | ||
Subsequently, we employed a ROC analysis to examine the efficacy of morphomics in differentiating participants with an VAT/BW ≤ 11 mL/kg/min. The results indicated a robust performance, with an apparent AUC of 0.840 (95%CI: 0.72-0.96) and an optimism-corrected AUC of 0.778 (95%CI: 0.63-0.88, P < 0.001) (Figure 3). The model's sensitivity was 64.3%, its specificity was 78.7%, and it had a positive predictive value of 66.8% as well as a negative predictive value of 76.8%.
The present study showed that T11 Level exhibits zero missing information in morphomics derived from CT in this esophageal cancer cohort. Body composition measurements obtained from morphomics are highly correlated with those from BIA, including muscle and fat composition, supporting their validity. In the muscle component, the DMG volume and area measured by morphomics exhibited strong positive correlations with BCM, FFM, LBM, SMM, and ASM obtained from BIA. Regarding the fat component, BF measured via BIA positively correlated with SF and VF areas but negatively with DMG density. Moreover, a moderate linear correlation is observed among morphomic parameters, CRF, and HGS. Specifically, BMD, VF area and DMG volume from morphomics were found to be predictive of low VAT/BW.
The measurement of core muscle size using morphomics at the lumbar level was initially introduced as a predictor of surgical outcomes[4]. Since then, most studies have focused on morphomic variables derived from a single lumbar vertebral level. However, research has demonstrated that CT-derived body composition assessments at the cervical, thoracic, and lumbar levels exhibit comparable reliability[7,8]. Furthermore, integrating CT imaging from multiple vertebral levels has been shown to improve correlation strength[22]. Given the limited availability of lumbar-level CT imaging in esophageal cancer patients and the need for more precise assessments, this study utilized multi-level morphomics to enhance feasibility and validity.
Muscle and fat are the two primary components of body composition, and numerous studies have compared different methods for their assessment. Previous research has demonstrated a positive correlation between muscle mass measured by CT-derived morphomics and BIA[6,7,23,24]. Consistent with these findings, our study revealed that the DMG area and DMG volume correlated positively with muscle-related components from BIA, including SMM, FFM, LBM, BCM, and ASM. Similarly, previous studies have reported comparable fat measurements between morphomics and BIA[7,8,22,23,25]. Our findings align with these reports, demonstrating a moderate to strong positive correlation between BF and both SF area and VF area.
Beyond muscle and fat mass, we also investigated the relationship between muscle quality and fat composition. Our results indicated a negative correlation between BF and DMG density, suggesting that higher fat accumulation may be associated with less dense muscle. These findings highlight the added value of morphomics in providing deeper insights into tissue integrity beyond traditional body composition assessments.
Low HGS is associated with increased hospitalization rates, poor nutritional status, higher overall mortality, and diminished quality of life[26]. Previous studies have reported that muscle strength is positively correlated with muscle mass and inversely correlated with fat mass[27-30]. However, our study found that although HGS was positively associated with DMG volume and area, it did not show a significant correlation with SF and VF areas. These findings suggest that, in esophageal cancer patients, muscle strength is primarily influenced by muscle mass rather than fat mass.
CRF is a critical prognostic factor in esophageal cancer, and several studies have explored its relationship with body composition[12,14,31,32]. While previous research has consistently shown a positive correlation between muscle mass and CRF, findings regarding fat mass have been inconclusive[16,33,34]. In this cohort of esophageal cancer patients, we observed a positive correlation between DMG and key CRF indicators, including peak VO2, peak WR, peak VO2/BW, VAT, and OUES. Skeletal muscle is the primary site of VO2 during exercise, and individuals with greater muscle mass demonstrate higher oxygen demand and utilization efficiency[35]. Additionally, muscle mass correlates with central circulatory factors such as blood volume and stroke volume, which are key determinants of maximal aerobic capacity[36]. Additionally, we found a negative correlation between SF and VF area and both peak VO2/BW and VAT/BW. This could be attributed to the role of adipose tissue in promoting systemic inflammation through the secretion of pro-inflammatory cytokines, which can impair endothelial function and oxygen delivery[37,38].
Apart from soft tissues, BMD was positively associated with aerobic capacity, particularly VAT/BW. This finding aligns with previous evidence suggesting that individuals with higher BMD may exhibit improved mechanical efficiency and physical performance[39,40].
Low CRF, such as VAT/BW, has been associated with poor outcomes after major surgery[14,15]. In the present study, we utilized morphomic analysis to predict low CRF and found that low BMD and high VF area were associated with reduced VAT/BW. Although low DMG volume only showed a trend toward association with low VAT/BW, these findings underscore the significant role of the musculoskeletal system in CRF. Furthermore, we demonstrated that with only three morphomic variables could predict VAT/BW ≤ 11 mL/kg/min, with an AUC of 0.778, a sensitivity of 64.3%, and a specificity of 78.7% in the model.
While this study provides valuable insights, several limitations should be acknowledged. The limited sample size, lack of external validation for the prediction model of low VAT/BW, and the homogeneity of the Taiwanese cohort may restrict the generalizability of the findings. Additionally, as the participants were initially recruited for esophageal cancer evaluation, the results may not necessarily be applicable to other diseases or the general population. These limitations highlight the need for further research to validate and expand upon our findings.
In morphomics derived from CT in patients with esophageal cancer, T11 is the only level with complete data in the present cohort, making it the most applicable in clinical practice. Body composition measurements obtained from morphomics are highly correlated with those from BIA, supporting their validity. Moreover, morphomic variables demonstrated moderate correlations with HGS and CRF, while also showing potential for predicting low VAT/BW. Future prospective studies with larger and more diverse cohorts are warranted to validate and implement morphomics-guided assessment in surgical planning.
The authors acknowledge the administrative support and statistical assistance provided by the Morphomic Analysis Group at the University of Michigan, Ann Arbor, United States.
| 1. | Boshier PR, Heneghan R, Markar SR, Baracos VE, Low DE. Assessment of body composition and sarcopenia in patients with esophageal cancer: a systematic review and meta-analysis. Dis Esophagus. 2018;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 2. | Chiu CH, Zhang P, Chang AC, Derstine BA, Ross BE, Enchakalody B, Shah NV, Wang SC, Chao YK, Lin J. Morphomic Factors Associated With Complete Response to Neoadjuvant Therapy in Esophageal Carcinoma. Ann Thorac Surg. 2020;109:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Takeoka T, Kanemura T, Sugase T, Matsuura N, Sugimura K, Yamamoto M, Shinno N, Hara H, Mukai Y, Hasegawa S, Nishimura J, Akita H, Wada H, Matsuda C, Omori T, Yasui M, Ohue M, Miyata H. Clinical impact of postoperative changes in body composition on long-term outcomes in patients with esophageal cancer. Clin Nutr. 2024;43:2188-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Englesbe MJ, Lee JS, He K, Fan L, Schaubel DE, Sheetz KH, Harbaugh CM, Holcombe SA, Campbell DA Jr, Sonnenday CJ, Wang SC. Analytic morphomics, core muscle size, and surgical outcomes. Ann Surg. 2012;256:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 5. | Chiu CH, Zhang P, Lin J, Chang AC, Ross BE, Enchakalody B, Shah NV, Liu YH, Chao YK, Wang SC. Morphomic predictors for post-esophagectomy pulmonary complications and overall survival. J Thorac Dis. 2025;17:209-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Tewari N, Awad S, Macdonald IA, Lobo DN. A comparison of three methods to assess body composition. Nutrition. 2018;47:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 7. | Zopfs D, Pinto Dos Santos D, Kottlors J, Reimer RP, Lennartz S, Kloeckner R, Schlaak M, Theurich S, Kabbasch C, Schlamann M, Große Hokamp N. Two-dimensional CT measurements enable assessment of body composition on head and neck CT. Eur Radiol. 2022;32:6427-6434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Daly A, Newman L, Thomas A, Munro A, Spence C, Long J, Arnott J, Durkin K, Layfield D, Heetun A, Wootton S, Copson ER, Cutress RI. Assessment of body composition in breast cancer patients: concordance between transverse computed tomography analysis at the fourth thoracic and third lumbar vertebrae. Front Nutr. 2024;11:1366768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Nogueira EC, Porto LG, Nogueira RM, Martins WR, Fonseca RM, Lunardi CC, de Oliveira RJ. Body Composition is Strongly Associated With Cardiorespiratory Fitness in a Large Brazilian Military Firefighter Cohort: The Brazilian Firefighters Study. J Strength Cond Res. 2016;30:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Miranda H, Bentes C, Resende M, Netto CC, Nasser I, Willardson J, Marinheiro L. Association between handgrip strength and body composition, physical fitness, and biomarkers in postmenopausal women with metabolic syndrome. Rev Assoc Med Bras (1992). 2022;68:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 11. | Levett DZH, Jack S, Swart M, Carlisle J, Wilson J, Snowden C, Riley M, Danjoux G, Ward SA, Older P, Grocott MPW; Perioperative Exercise Testing and Training Society (POETTS). Perioperative cardiopulmonary exercise testing (CPET): consensus clinical guidelines on indications, organization, conduct, and physiological interpretation. Br J Anaesth. 2018;120:484-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 226] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 12. | Benington S, Bryan A, Milne O, Alkhaffaf B. CPET and cardioesophagectomy: A single centre 10-year experience. Eur J Surg Oncol. 2019;45:2451-2456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Hagens ERC, Feenstra ML, van Egmond MA, van Laarhoven HWM, Hulshof MCCM, Boshier PR, Low DE, van Berge Henegouwen MI, Gisbertz SS. Influence of body composition and muscle strength on outcomes after multimodal oesophageal cancer treatment. J Cachexia Sarcopenia Muscle. 2020;11:756-767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Forshaw MJ, Strauss DC, Davies AR, Wilson D, Lams B, Pearce A, Botha AJ, Mason RC. Is cardiopulmonary exercise testing a useful test before esophagectomy? Ann Thorac Surg. 2008;85:294-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Wilson RJ, Davies S, Yates D, Redman J, Stone M. Impaired functional capacity is associated with all-cause mortality after major elective intra-abdominal surgery. Br J Anaesth. 2010;105:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 256] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 16. | Berkel AEM, van Wijk L, van Dijk DPJ, Prins SN, van der Palen J, van Meeteren NLU, Olde Damink SWM, Klaase JM, Bongers BC. The association between preoperative body composition and aerobic fitness in patients scheduled for colorectal surgery. Colorectal Dis. 2022;24:93-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Okura K, Suto A, Sato Y, Takahashi Y, Hatakeyama K, Nagaki Y, Wakita A, Kasukawa Y, Miyakoshi N, Minamiya Y. Preoperative inspiratory muscle weakness as a risk factor of postoperative pulmonary complications in patients with esophageal cancer. J Surg Oncol. 2023;128:1259-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Morishita Y, Kubo K, Haga Y, Miki A, Ishibashi K, Kusano E, Nagata D. Skeletal muscle loss is negatively associated with single-pool Kt/V and dialysis duration in hemodialysis patients. Ther Apher Dial. 2014;18:612-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Murakami K, Hirano H, Watanabe Y, Edahiro A, Ohara Y, Yoshida H, Kim H, Takagi D, Hironaka S. Relationship between swallowing function and the skeletal muscle mass of older adults requiring long-term care. Geriatr Gerontol Int. 2015;15:1185-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | ERS Task Force, Palange P, Ward SA, Carlsen KH, Casaburi R, Gallagher CG, Gosselink R, O'Donnell DE, Puente-Maestu L, Schols AM, Singh S, Whipp BJ. Recommendations on the use of exercise testing in clinical practice. Eur Respir J. 2007;29:185-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 366] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 21. | Huang SC, Yeh CH, Hsu CC, Lin YC, Lee CH, Hsiao CC, Chiu CH, Fu TC. Trainability for cardiopulmonary fitness is low in patients with peripheral artery disease. Eur J Cardiovasc Nurs. 2024;23:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Chan B, Yu Y, Huang F, Vardhanabhuti V. Towards visceral fat estimation at population scale: correlation of visceral adipose tissue assessment using three-dimensional cross-sectional imaging with BIA, DXA, and single-slice CT. Front Endocrinol (Lausanne). 2023;14:1211696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 23. | Kim J, Kim S, Hwang HK, Kang CM, Kim KS, Kim SH. Body composition assessment using bioelectrical impedance analysis and computed tomography in patients who underwent pancreatoduodenectomy in Korea: a before and after study. Ann Clin Nutr Metab. 2023;15:72-80. [DOI] [Full Text] |
| 24. | Zuo J, Zhou D, Zhang L, Zhou X, Gao X, Hou W, Wang C, Jiang P, Wang X. Comparison of bioelectrical impedance analysis and computed tomography for the assessment of muscle mass in patients with gastric cancer. Nutrition. 2024;121:112363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 25. | Gao B, Liu Y, Ding C, Liu S, Chen X, Bian X. Comparison of visceral fat area measured by CT and bioelectrical impedance analysis in Chinese patients with gastric cancer: a cross-sectional study. BMJ Open. 2020;10:e036335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 26. | Vaishya R, Misra A, Vaish A, Ursino N, D'Ambrosi R. Hand grip strength as a proposed new vital sign of health: a narrative review of evidences. J Health Popul Nutr. 2024;43:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 85] [Reference Citation Analysis (0)] |
| 27. | Lee DY, Oh JS, Kim JW, Kim JS, Oh M, Kim YI, Ko DH, Bae SJ, Kim HK, Ryu JS. Comparative analysis of body composition using torso CT from PET/CT with bioelectrical impedance and muscle strength in healthy adults. Sci Rep. 2024;14:21597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 28. | van Egmond MA, van der Schaaf M, Hagens ERC, van Laarhoven HWM, van Berge Henegouwen MI, Haverkort EB, Engelbert RHH, Gisbertz SS. Muscle Strength Is Associated With Muscle Mass in Patients With Esophageal Cancer Awaiting Surgery. J Geriatr Phys Ther. 2020;43:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Sugawara K, Yamashita H, Okumura Y, Yagi K, Yoshimura S, Kawasaki K, Tanabe A, Aikou S, Seto Y. Relationships among body composition, muscle strength, and sarcopenia in esophageal squamous cell carcinoma patients. Support Care Cancer. 2020;28:2797-2803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Dhar DK, Purwar B. Effect of Body Fat and BMI on Muscle Strength and Endurance in Young Adults: A Cross-sectional Study. J Clin Diagn Res. 2023;17. [DOI] [Full Text] |
| 31. | Moyes LH, McCaffer CJ, Carter RC, Fullarton GM, Mackay CK, Forshaw MJ. Cardiopulmonary exercise testing as a predictor of complications in oesophagogastric cancer surgery. Ann R Coll Surg Engl. 2013;95:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Feeney C, Reynolds JV, Hussey J. Preoperative physical activity levels and postoperative pulmonary complications post-esophagectomy. Dis Esophagus. 2011;24:489-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Karlsson P, Lind L, Michaëlsson K, Malinovschi A. Cardiopulmonary exercise testing and body composition. ERJ Open Res. 2024;10:00970-02023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Karlsson P, Strand R, Kullberg J, Michaëlsson K, Ahlström H, Lind L, Malinovschi A. A detailed analysis of body composition in relation to cardiopulmonary exercise test indices. Sci Rep. 2024;14:21633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 35. | Fleg JL, Lakatta EG. Role of muscle loss in the age-associated reduction in VO2 max. J Appl Physiol (1985). 1988;65:1147-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 368] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 36. | Hunt BE, Davy KP, Jones PP, DeSouza CA, Van Pelt RE, Tanaka H, Seals DR. Role of central circulatory factors in the fat-free mass-maximal aerobic capacity relation across age. Am J Physiol. 1998;275:H1178-H1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1478] [Cited by in RCA: 1650] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 38. | Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3103] [Cited by in RCA: 3166] [Article Influence: 226.1] [Reference Citation Analysis (0)] |
| 39. | Hernandez CJ, Keaveny TM. A biomechanical perspective on bone quality. Bone. 2006;39:1173-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 214] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 40. | Shin H, Panton LB, Dutton GR, Ilich JZ. Relationship of Physical Performance with Body Composition and Bone Mineral Density in Individuals over 60 Years of Age: A Systematic Review. J Aging Res. 2011;2011:191896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |