Published online Aug 27, 2025. doi: 10.4240/wjgs.v17.i8.108418
Revised: May 20, 2025
Accepted: June 16, 2025
Published online: August 27, 2025
Processing time: 133 Days and 5 Hours
KRAS mutation status and primary tumor location serve as critical prognostic factors for colorectal liver metastases (CLMs). Emerging evidence suggests a potential interaction between these two variables that may influence clinical outcomes.
To investigate the association of KRAS mutations with recurrence in patients with CLM who underwent radiofrequency ablation (RFA) according to the primary tumor location.
This retrospective study analyzed 164 patients with KRAS-determined CLM treated with percutaneous RFA between January 2012 and December 2018. The clinicopathological characteristics, recurrence patterns, and survival outcomes were systematically evaluated.
A total of 164 patients (mean age: 58.0 ± 9.8 years, range: 34-83 years) who underwent percutaneous RFA of 325 CLMs (mean size: 2.2 ± 1.0 cm, range: 0.7-5.0 cm) were included in the study. Eighty-nine (54.3%) patients had wild-type KRAS, and 75 (45.7%) patients had mutated KRAS. Compared with wild-type patients, patients with KRAS mutations presented significantly higher local tumor progression rates (30.7% vs 14.6%, P = 0.013). Among 126 patients (76.8%) who experienced post-RFA recurrence, 61.6% developed intrahepatic me
KRAS mutation status predicts differential recurrence patterns after CLM ablation, with significant prognostic implications, specifically in left-sided CRCs. These findings underscore the importance of integrating molecular profiling and primary tumor characteristics in therapeutic decision-making for patients with metastatic CRC.
Core Tip: The mutation status of the KRAS differentially impacts intrahepatic recurrence after radiofrequency ablation (RFA) for colorectal liver metastases (CLMs) based on primary tumor location. Patients with left-sided colorectal cancer harboring KRAS mutations have significantly worse intrahepatic recurrence-free survival post-RFA, whereas no such association exists in right-sided patients. KRAS status was not correlated with extrahepatic recurrence, regardless of tumor origin. These findings highlight the clinical value of incorporating both primary tumor location and KRAS genotyping to optimize post-RFA management strategies, enabling personalized approaches to reduce recurrence risk and improve long-term outcomes in CLM patients.
- Citation: Jiang BB, Wang JC, Yan K, Zhang ZY, Wang S, Wu W, Yang W. Differential effects of the KRAS gene on recurrence in right- vs left-sided colorectal liver metastases undergoing radiofrequency ablation. World J Gastrointest Surg 2025; 17(8): 108418
- URL: https://www.wjgnet.com/1948-9366/full/v17/i8/108418.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i8.108418
Radiofrequency ablation (RFA) has been recommended as a pivotal local treatment for colorectal liver metastases (CLMs), particularly in managing patients with multiple, bilobar, or recurrent lesions[1-4]. The minimally invasive nature of RFA enables preservation of functional liver volume and reduces the risk of hepatic failure, making it a preferred option for elderly patients or those with comorbidities[5]. Accumulating evidence from clinical studies supports the safety and efficacy of RFA in CLM management[6,7]. Notably, a recent phase II randomized trial demonstrated that RFA signi
Despite these advances, post-RFA recurrence remains a major obstacle to achieving long-term therapeutic success[9,10]. Although studies have explored the association between KRAS status and recurrence after CLM resection, their conclusions are contradictory[11-14]. Notably, recurrent lesions after RFA may compromise subsequent treatments and ultimately affect survival outcomes[15]. These observations collectively highlight the need to elucidate the relationship between KRAS and recurrence, which could refine clinical decision-making throughout the RFA treatment continuum.
The complexity of this issue is further compounded by the influence of primary tumor location. Growing evidence indicates that primary colorectal tumor sidedness independently affects CLM prognosis after RFA[16,17]. Notably, the prevalence of KRAS mutations is significantly different: right-sided tumors have higher KRAS mutation rates than their left-sided counterparts do[18]. This collinearity between primary tumor location and KRAS status creates a potential confounding effect, as both factors are independently associated with prognosis[19]. Recent studies have revealed that the prognostic value of KRAS mutations in CLM patients undergoing hepatic resection varies according to primary tumor location[20,21]. Furthermore, differential treatment responses have been observed between KRAS wild-type tumors of right- and left-sided origin[22,23]. These findings underscore the importance of accounting for primary tumor location when investigating KRAS-related recurrence mechanisms.
To address these knowledge gaps, the aim of this study was to investigate the association between KRAS mutations and post-RFA recurrence in CLM patients, with specific stratification by primary tumor location. Elucidating this relationship may provide biomarkers for risk stratification and inform personalized therapeutic strategies to improve clinical outcomes.
The need for informed consent was waived because of the retrospective study design, and the study was approved by the Hospital Medical Ethics Committee. Between January 2012 and December 2018, 164 consecutive patients with surgical resection of the primary tumor and known KRAS gene status who underwent percutaneous RFA of 325 CLMs were included in our study. The inclusion criteria for this study were as follows: (1) Surgical resection of a primary tumor; (2) Known KRAS gene status; (3) Metastatic diameter ≤ 5 cm; and (4) Extrahepatic metastases with stable control before RFA. Patients with incomplete medical records or a follow-up duration of ≤ 3 months were excluded from the study.
Before RFA, all patients were examined via ultrasound (US) and contrast-enhanced computed tomography (CT)/magnetic resonance imaging (MRI) to obtain baseline data. Treatments were planned according to the size, shape, and border of the tumor. Local anesthesia and general anesthesia were performed by the anesthesiologist. Local infiltration anesthesia was induced with 5-15 mL of 1% lidocaine (Liduokayin; Yimin, Beijing, China), after which the RFA electrode was placed when the patient was conscious. General intravenous anesthesia was then induced with 2.5-5.0 mg of midazolam (Roche; Basel, Switzerland) and 50-100 μg of fentanyl (Fentaini; Renfu, Yichang, China). During the procedure, the vital signs of the patients were continuously monitored.
RFA procedures were performed by two of four radiologists, with > 10 years of experience in US-guided interventional procedures. Real-time US and enhanced US, if necessary, were used to target, monitor, and modify the ablation. If the tumors were > 3 cm, overlapping ablations were used to ensure a sufficient ablation zone[24]. Ablative margins covering > 0.5 cm beyond the original tumor, with the exception of tumors close to important structures such as the diaphragm, gastrointestinal tract, or gallbladder, did not have a sufficient ablative margin around the lesion. Therefore, an individualized protocol is needed for patients with CLM.
The ablation electrodes used included multipolar electrodes (Celon Lab Power, Germany), multitined RITA electrodes (RITA Medical Systems, Martinez, CA, United States), and a Valleylab system (Tyco Health care, North Haven, CT, United States). Real-time US systems, such as the Aloka SSD-4000, SSD-5500, α-10 (Tokyo, Japan) and GE Logic-9 (CT) US systems, were used for scanning and guidance with 3.5-5.0 MHz convex probes with needle guide devices. After RFA, CEUS was immediately performed to evaluate the ablated tumor size and whether the target lesion was completely covered.
KRAS mutations were measured via polymerase chain reaction amplification fluorescence. A pathologist analyzed hematoxylin and eosin-stained sections and extracted DNA from surgically resected primary tumor samples. The main reason is that the KRAS mutational status between primary and metastatic lesions has a high concordance rate[25]. After specific primers and probes are combined with DNA, Taq DNA polymerase uses deoxynucleotide as a substrate to amplify the internal reference gene and mutant genes in specific regions of codons 12, 13, and 61 of the KRAS gene in vitro. The pathologists who performed the KRAS gene analyses were blinded to the patients’ clinical outcomes.
To evaluate technique efficacy, contrast-enhanced CT was performed at 1 month after RFA. The patients were then followed up via an abdominal CEUS and enhanced CT/MRI every 2-3 months in the first 2 years and every 4-6 months in the following years. Radiologists were blinded to the clinical data to ensure the evaluation of the treatment effect.
OS[26] was calculated from the date of RFA to the date of death or last follow-up. Local tumor progression (LTP)[26] was measured from the date of RFA to the date when new lesions occurred around the prior ablation site or at the last follow-up. Intrahepatic recurrence was measured from the date of ablation to the date of detection of new hepatic lesions without contact with the prior ablation zone in the liver or to the last follow-up data. Extrahepatic recurrence was measured from the date of ablation to the date of detection of new extrahepatic lesions or to the last follow-up date. Recurrence-free survival (RFS) was calculated from the time of ablation to the time of intra- or extrahepatic recurrence or to the last follow-up date. The right-sided colon was from the cecum to the transverse colon, whereas the left-sided colon was from the splenic flexure to the rectum.
The clinicopathologic characteristics, LTP, intrahepatic and extrahepatic recurrence, RFS and OS of patients in the two KRAS gene groups were compared. Categorical variables were analyzed via the χ2 test or Fisher’s exact test. Continuous variables were analyzed via the Wilcoxon rank sum test or student's t test. The RFS and OS rates were analyzed via Cox regression models via univariable and multivariable analyses. All the data were analyzed via SPSS software (SPSS Inc., Chicago, IL, United States) and R version 4.2.3 (R Foundation for Statistical Computing, Vienna, Austria). P ≤ 0.05 was considered significant.
Among the 164 patients (98 men and 66 women; mean age: 58.0 ± 9.8 years, range: 34-83 years) with CLM, 89 (54.3%) had wild-type KRAS, and 75 (45.7%) had KRAS mutations. The baseline characteristics of the patients in the KRAS wild-type and KRAS mutation groups were compared before RFA and are shown in Table 1. The prevalence of KRAS mutations in right-sided colorectal cancer (CRC) patients was greater than that in left-sided CRC patients (66.7% vs 41.6%, P = 0.017). No other significant differences in clinical characteristics were observed between the two groups.
| Characteristics | No. of patients (n = 164) | KRAS | ||
| Wild (n = 89) | Mutation (n = 75) | P value | ||
| Age (year), mean | 58.0 ± 9.8 | 57.0 ± 9.5 | 59.1 ± 10.0 | 0.167 |
| ≤ 60 | 90 (54.9) | 51 (56.7) | 39 (43.3) | 0.497 |
| > 60 | 74 (45.1) | 38 (51.4) | 36 (48.6) | |
| Sex | 0.794 | |||
| Male | 98 (59.8) | 54 (55.1) | 44 (44.9) | |
| Female | 66 (40.2) | 35 (53.0) | 31 (47.0) | |
| Primary tumor site | 0.017 | |||
| Right colon | 27 (16.5) | 9 (33.3) | 18 (66.7) | |
| Left colon | 137 (83.5) | 80 (58.4) | 57 (41.6) | |
| Primary tumor invasion | 0.203 | |||
| T1-2 | 11 (6.7) | 8 (72.7) | 3 (27.3) | |
| T3-4 | 153 (93.3) | 81 (52.9) | 72 (47.1) | |
| Nodal status of primary tumor | 0.561 | |||
| Positive | 128 (78.0) | 71 (55.5) | 57 (44.5) | |
| Negative | 36 (22.0) | 18 (50.0) | 18 (50.0) | |
| Time of CLM | 0.504 | |||
| Synchronous | 85 (51.8) | 44 (51.8) | 41 (48.2) | |
| Heterochronous | 79 (48.2) | 45 (57.0) | 34 (43.0) | |
| Extrahepatic metastases | 0.139 | |||
| Yes | 75 (45.7) | 36 (48.0) | 39 (52.0) | |
| No | 89 (54.3) | 53 (59.6) | 36 (40.4) | |
| Tumor size (cm) | ||||
| Median diameter, cm (quartile) | 2.2 (1.7-2.9) | 2.0 (1.7-2.7) | 2.2 (1.6-3.3) | 0.236 |
| ≤ 3 | 123 (75.0) | 71 (57.8) | 52 (42.2) | 0.124 |
| > 3 | 41 (25.0) | 18 (43.9) | 23 (56.1) | |
| DFI (months) | 0.485 | |||
| < 12 | 137 (83.5) | 76 (55.5) | 61 (44.5) | |
| ≥ 12 | 27 (16.5) | 13 (48.1) | 14 (51.9) | |
| CEA level (ng/mL) | 0.914 | |||
| ≤ 30 | 135 (82.3) | 73 (54.1) | 62 (45.9) | |
| > 30 | 29 (17.7) | 16 (55.2) | 13 (44.8) | |
| CRS | 0.313 | |||
| 0-2 | 77 (47.0) | 45 (58.4) | 32 (41.6) | |
| 3-5 | 87 (53.0) | 44 (50.6) | 43 (49.4) | |
| Liver metastases resection pre-RFA | 0.485 | |||
| Yes | 66 (40.2) | 38 (57.6) | 28 (42.4) | |
| No | 98 (59.8) | 51 (52.0) | 47 (48.0) | |
| Preoperative chemotherapy | 0.221 | |||
| Yes | 148 (90.2) | 78 (52.7) | 70 (47.3) | |
| No | 16 (9.8) | 11 (68.8) | 5 (31.2) | |
| Ablative margin (mm) | 0.156 | |||
| < 5 | 17 (10.4) | 7 (41.2) | 10 (58.8) | |
| 5-10 | 101 (61.6) | 52 (51.5) | 49 (48.5) | |
| > 10 | 46 (28.0) | 30 (65.2) | 16 (34.8) | |
A total of 325 CLM lesions (mean size ± standard deviation: 2.2 ± 1.0 cm, range: 0.7-5.0 cm) were analyzed in this study. The technique efficacy rate was 98.8% (321/325). Among the patients, 22.0% (36/164) developed LTP; 23 of 75 patients (30.7%) in the KRAS mutation group developed LTP, which was significantly greater than that in patients with wild-type KRAS (13/89, 14.6%) (P = 0.013). The 3-year cumulative incidence of LTP was also significantly greater in the KRAS mutation group (40.5% vs 17.0%, P = 0.008). Major complications included liver abscess (n = 1) and acute biliary infection (n = 2). All patients who received active treatment improved. There were no treatment-related deaths.
In this study, 126 (76.8%) patients experienced recurrence after RFA; 101 patients (61.6%) experienced intrahepatic recurrence, and 88 (53.7%) experienced extrahepatic recurrence.
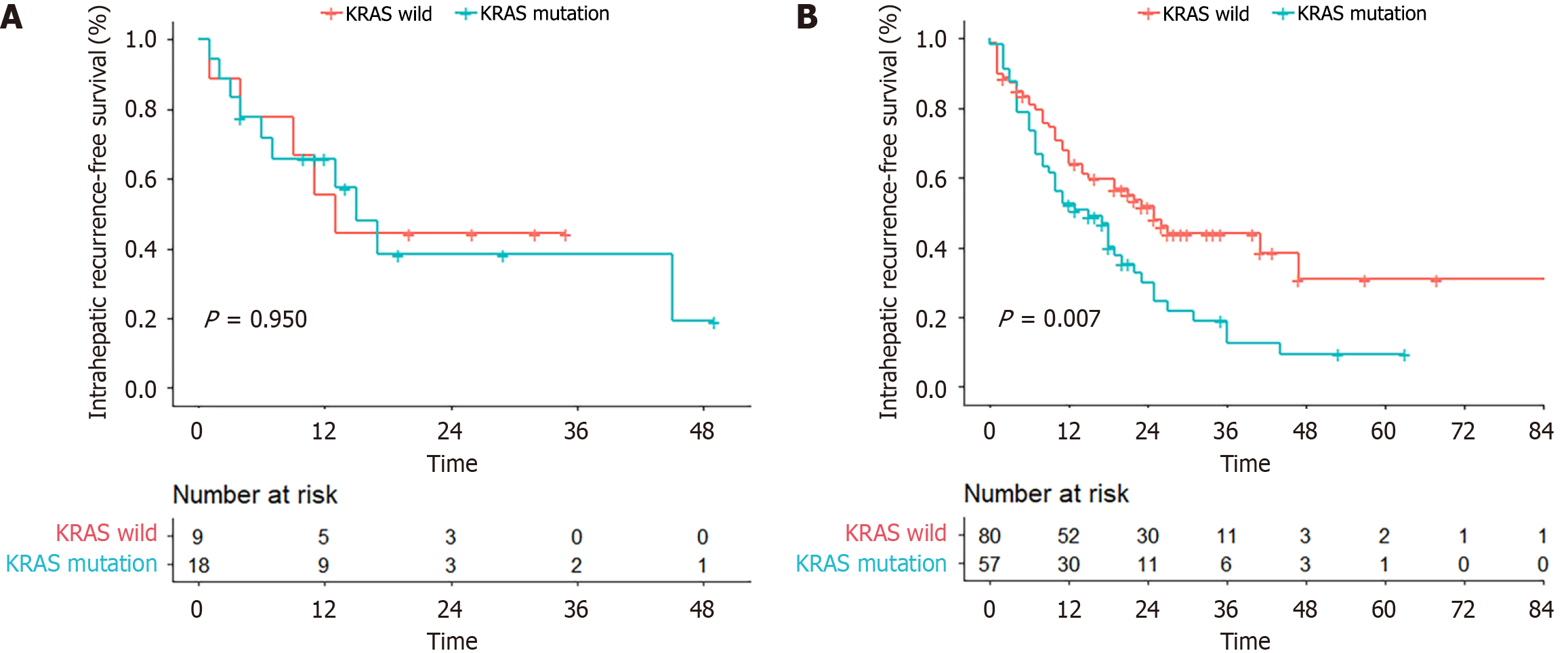
A total of 47 (46.5%) of the 101 patients with intrahepatic recurrence had wild-type KRAS, whereas 54 (53.5%) had KRAS mutations. There was a significant difference between the two groups (P = 0.012). In the left-sided CRC group, patients with KRAS mutations had greater intrahepatic recurrence than did those with wild-type KRAS (77.2% vs 52.5%, P = 0.003). For right-sided CRC, there were no clear differences in the incidence of intrahepatic recurrence (P > 0.05) (Table 2).
| Characteristic | All patients (n = 164) | Right colon | Left colon | ||||
| Wild (n = 9) | Mutation (n = 18) | P value | Wild (n = 80) | Mutation (n = 57) | P value | ||
| Intrahepatic recurrence | 101 (61.6) | 5 (55.6) | 10 (55.6) | 1.000 | 42 (52.5) | 44 (77.2) | 0.003 |
| Extrahepatic recurrence | 88 (53.7) | 5 (55.6) | 7 (38.9) | 0.448 | 43 (53.7) | 33 (57.9) | 0.630 |
| Number | 1.000 | 0.460 | |||||
| Single | 79 (89.8) | 4 (80.0) | 6 (85.7) | 40 (93.0) | 29 (87.9) | ||
| Multiple | 9 (10.2) | 1 (20.0) | 1 (14.3) | 3 (7.0) | 4 (12.1) | ||
| Site of single extrahepatic recurrence | |||||||
| Lung | 38 (48.1) | 2 (50.0) | 2 (33.3) | 0.582 | 20 (50.0) | 14 (48.3) | 1.000 |
| Bone | 11 (13.9) | 0 | 2 (33.3) | 0.538 | 5 (10.0) | 4 (13.8) | 1.000 |
| Peritoneal | 8 (10.1) | 0 | 0 | - | 4 (8.0) | 4 (13.8) | 0.719 |
| Ovary | 4 (5.1) | 1 (25.0) | 0 | 0.333 | 2 (4.0) | 1 (3.4) | 1.000 |
| Adrenal | 2 (2.5) | 0 | 0 | - | 0 | 2 (6.9) | 0.171 |
| Lymph node | 15 (19.0) | 1 (25.0) | 2 (33.3) | 1.000 | 8 (16.0) | 4 (13.8) | 0.761 |
| Other | 1 (1.3) | 0 | 0 | - | 1 (2.0) | 0 | 1.000 |
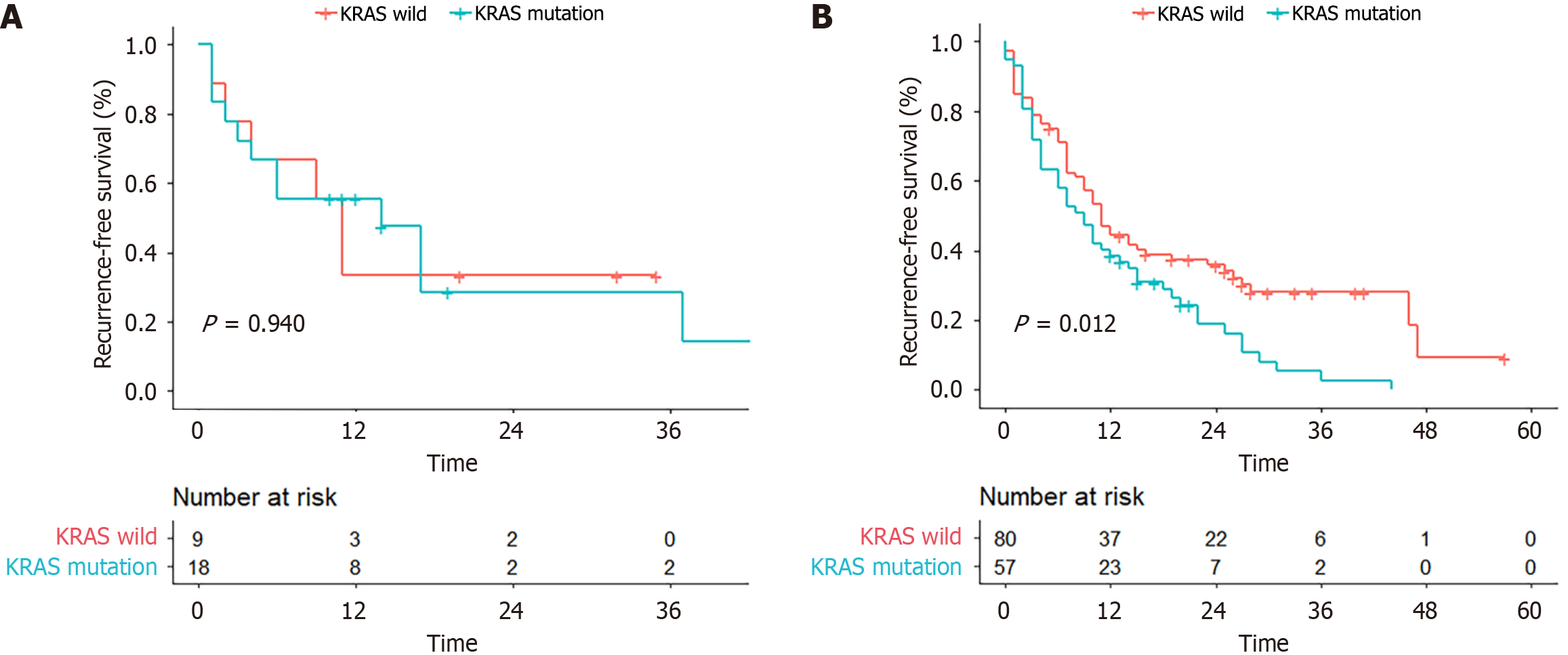
| RFS1 | 10 (0-57) | 11 (1-35) | 14 (1-45) | 0.944 | 11 (0-57) | 9 (0-44) | 0.012 |
| Intrahepatic RFS1 | 19 (0-86) | 13 (1-35) | 15 (1-49) | 0.950 | 25 (0-86) | 15 (0-63) | 0.007 |
| Extrahepatic RFS1 | 23 (0-95) | 22 (2-35) | 37 (1-47) | 0.515 | 27 (0-57) | 22 (0-95) | 0.467 |
Forty-eight of the 88 patients with extrahepatic recurrence had wild-type KRAS, and 40 had KRAS mutations (54.5% vs 45.5%, P = 0.939). There were no clear differences in the proportion of patients with extrahepatic recurrence regardless of the primary site (P > 0.05). Initial extrahepatic recurrence presented as single-site recurrence in 89.8% (79/88) and multiple-site recurrence in 10.2% (9/88) of patients. The initial locations of extrahepatic recurrence after RFA were as follows: Lungs (n = 38, 48.1%), bone (n = 11, 13.9%), peritoneal dissemination (n = 8, 10.1%), ovaries (n = 4, 5.1%), adrenal glands (n = 2, 2.5%), lymph nodes (n = 15, 19.0%) and other (n = 1, 1.3%) (Table 2). No significant difference in the initial recurrence site was detected between the KRAS wild-type and mutant groups, regardless of the primary tumor location (P > 0.05) (Table 2).
The median 1-, 3-, and 5-year intrahepatic RFS rates were 59.6%, 31.0%, and 20.9%, respectively (median: 19 months). The intrahepatic RFS in the KRAS wild-type group (median: 25 months; 3-year intrahepatic RFS: 44.2%) was longer than that in the KRAS mutation group (median: 19 months; 3-year intrahepatic RFS: 22.5%, P = 0.014). Among patients with right-sided CRC, the intrahepatic RFS in the KRAS mutation group seemed consistent with the effects observed in patients with wild-type KRAS (median: 13 vs 15 months; 2-year intrahepatic RFS: 44.4% vs 38.4%, P = 0.950). However, in the left-sided CRC group, intrahepatic RFS differed between KRAS wild-type patients (median: 25 months and 3-year intrahepatic RFS: 44.1%) and KRAS mutation patients (median: 15 months and 3-year intrahepatic RFS: 12.7%, P = 0.007) (Figure 1).
Extrahepatic RFS did not differ significantly between the KRAS wild-type and mutation groups (median: 26 months vs 22 months; 3-year extrahepatic RFS: 42.7% vs 32.8%, P = 0.663). In addition, similar results were observed for extrahepatic RFS between the two groups irrespective of the primary lesion location (left-sided CRC: Median: 27 vs 23 months, P = 0.467; right-sided CRC, median: 22 vs 37 months, P = 0.515).
The RFS in the KRAS wild-type group was longer than that in the KRAS mutation group (median: 11 vs 9 months; 3-year RFS: 28.0% vs 2.7%, P = 0.012) for left-sided CRC patients. However, the two groups had similar RFS rates for right-sided CRC patients (P = 0.944) (Figure 2). Univariable and multivariable analyses revealed that KRAS mutation and positive lymph nodes were independent factors associated with worse RFS (HR: 1.526, 95%CI: 1.056-2.207, P = 0.025; HR: 1.602, 95%CI: 1.008-2.54, P = 0.046) (Table 3).
| Prognostic factor | Univariable analysis | Multivariable analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (year) | 0.890 (0.622-1.273) | 0.523 | ||
| Tumor size > 3 cm | 1.070 (0.715-1.602) | 0.743 | ||
| Sex | 1.112 (0.776-1.593) | 0.563 | ||
| Right colon | 1.072 (0.657-1.748) | 0.781 | ||
| Pathological differentiation/poorly | 0.766 (0.430-1.364) | 0.366 | ||
| KRAS mutation | 1.507 (1.058-2.148) | 0.023 | 1.526 (1.056-2.207) | 0.025 |
| T stage | 0.910 (0.461-1.796) | 0.786 | ||
| Lymph node metastasis | 1.674 (1.064-2.635) | 0.026 | 1.602 (1.008-2.545) | 0.046 |
| Synchronous liver metastasis | 0.824 (0.580–1.169) | 0.278 | ||
| No. of liver metastases | 1.414 (0.992-2.014) | 0.055 | 1.241 (0.857-1.797) | 0.253 |
| Liver metastasis resection pre-RFA | 1.414 (0.990-2.019) | 0.057 | 1.418 (0.988-2.034) | 0.058 |
| Extrahepatic metastases | 1.066 (0.745-1.524) | 0.727 | ||
| CRS | 1.238 (0.869-1.762) | 0.236 | ||
| CEA | 0.893 (0.563-1.417) | 0.631 | ||
| DFI | 1.043 (0.645-1.686) | 0.863 | ||
| Preoperative chemotherapy | 1.111 (0.611-2.017) | 0.731 | ||
The overall median follow-up period was 42 months. The median follow-up times for patients with wild-type and mutated KRAS genes were 40 months and 51 months, respectively (P = 0.099). The 1-, 3-, 5-, and 7-year OS rates were 89.9%, 52.0%, 16.0%, and 16.0%, respectively, in the KRAS wild-type group and 81.3%, 35.2%, 10.3%, and 5.2%, respectively, in the KRAS mutation group (P = 0.012). The KRAS gene had no impact on OS, irrespective of the primary tumor site (P > 0.05). Multivariable analysis revealed that a right-sided primary tumor (HR: 2.234, 95%CI: 1.298-3.845, P = 0.004), a tumor size ≥ 3 cm (HR: 1.829, 95%CI: 1.124-2.972, P = 0.015), and extrahepatic metastases pre-RFA (HR: 1.700, 95%CI: 1.113-2.598, P = 0.014) were independent risk factors for OS in patients with CLM (Table 4).
| Prognostic factor | Univariable analysis | Multivariable analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (year) | 1.057 (0.707-1.579) | 0.787 | ||
| Tumor size > 3 cm | 1.598 (1.037-2.464) | 0.034 | 1.829 (1.124-2.972) | 0.015 |
| Sex | 0.898 (0.599-1.347) | 0.604 | ||
| Right colon | 2.098 (1.258-3.498) | 0.005 | 2.234 (1.298-3.845) | 0.004 |
| Pathological differentiation/poorly | 1.465 (0.813-2.638) | 0.203 | ||
| KRAS mutation | 1.681 (1.123-2.515) | 0.012 | 1.325 (0.865-2.031) | 0.196 |
| T stage | 1.338 (0.583-3.070) | 0.492 | ||
| Lymph node metastases | 1.317 (0.787-2.201) | 0.294 | ||
| Synchronous liver metastasis | 1.267 (0.846-1.898) | 0.252 | ||
| No. of liver metastases | 1.708 (1.124-2.594) | 0.012 | 1.469 (0.839-2.570) | 0.178 |
| Liver metastasis resection pre-RFA | 0.803 (0.526-1.226) | 0.310 | ||
| Extrahepatic metastases | 1.849 (1.229-2.782) | 0.003 | 1.700 (1.113-2.598) | 0.014 |
| CRS | 1.690 (1.115-2.561) | 0.013 | 1.174 (0.658-2.095) | 0.587 |
| CEA | 1.069 (0.636-1.797) | 0.802 | ||
| DFI | 1.279 (0.755-2.167) | 0.360 | ||
| Margin | 1.216 (0.843-1.755) | 0.295 | ||
| Preoperative chemotherapy | 1.623 (0.751-3.508) | 0.218 | ||
This study demonstrated that the prognostic impact of KRAS mutations on recurrence in CLM patients after RFA significantly differs based on primary tumor location. This spatial heterogeneity may be closely associated with em
In left-sided CRC, KRAS mutations significantly increase recurrence risk, a phenomenon mechanistically linked to: (1) Enhanced dysregulation of major gene pathways; (2) Coordinated up-/downregulation of genes associated with corresponding hypo-/hypermethylation events; and (3) Prominent overexpression of CDKN2A[30], collectively contributing to tumor aggressiveness. In contrast, while right-sided CRC has a higher baseline prevalence of KRAS mutations than left-sided tumors before RFA-which is consistent with prior reports[18]-these patients exhibit universally high recurrence rates irrespective of KRAS status. This observation suggests that the intrinsic biological aggressiveness of right-sided tumors may supersede the isolated prognostic influence of KRAS[31].
This study revealed a 76.8% recurrence rate in CRC patients with liver metastases after RFA, which may be associated with the following mechanisms. First, while RFA effectively eliminates radiographically detectable lesions as a localized therapy, it fails to eradicate preexisting occult micrometastases and circulating tumor cells, leading to residual tumor progression. Second, although RFA activates systemic CD8+ T-cell immune responses through tumor antigen release, it simultaneously induces immunosuppressive adaptations within the ablation microenvironment, characterized by PD-L1 upregulation and regulatory T-cell (Treg) infiltration[32]. Emerging evidence suggests that combining PD-1 inhibitors synergistically enhances CD8+ T-cell antitumor activity, significantly improving disease control and survival outcomes[32]. Therefore, integrating RFA with immune checkpoint inhibitors may achieve more sustained therapeutic efficacy.
Notably, this study revealed no significant association between KRAS status and extrahepatic recurrence risk, which contrasts with conclusions from surgical resection studies[11,33,34]. This discrepancy may stem from RFA-specific microenvironmental regulation of tumor biological behavior. Additionally, the inherent biological characteristics of right-sided CRC might drive more extensive early micrometastases, potentially masking the prognostic signal of KRAS. Consequently, recurrence surveillance after RFA should establish stratification criteria distinct from those of surgical resection protocols. We recommend increasing the frequency of systemic radiological evaluation for right-sided CRC patients regardless of their KRAS status during post-RFA follow-up.
This study has several limitations. First, the retrospective design introduces inherent selection biases, particularly regarding treatment allocation. Second, the limited representation of right-sided CRC (16.5% vs 83.5% left-sided) reflects both its lower incidence and aggressive biology, which often precludes RFA eligibility, which is consistent with epidemiological data reporting 18.1% right-sided CRC prevalence[21]. Third, while BRAF/NRAS mutations were not analyzed, their low prevalence (< 5% incidence in CRC) likely minimally affects the generalizability of the results. These limitations highlight the need for prospective multicenter trials that integrate comprehensive molecular profiling to validate our findings.
KRAS mutation status has distinct prognostic effects on intrahepatic RFS following RFA for CLM patients, with stratification by primary tumor location. In left-sided CRC, KRAS mutations independently predict reduced intrahepatic RFS, whereas they are not significantly associated with post-RFA intrahepatic recurrence in right-sided CRC.
| 1. | Takahashi H, Berber E. Role of thermal ablation in the management of colorectal liver metastasis. Hepatobiliary Surg Nutr. 2020;9:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 2. | Yoshino T, Cervantes A, Bando H, Martinelli E, Oki E, Xu RH, Mulansari NA, Govind Babu K, Lee MA, Tan CK, Cornelio G, Chong DQ, Chen LT, Tanasanvimon S, Prasongsook N, Yeh KH, Chua C, Sacdalan MD, Sow Jenson WJ, Kim ST, Chacko RT, Syaiful RA, Zhang SZ, Curigliano G, Mishima S, Nakamura Y, Ebi H, Sunakawa Y, Takahashi M, Baba E, Peters S, Ishioka C, Pentheroudakis G. Pan-Asian adapted ESMO Clinical Practice Guidelines for the diagnosis, treatment and follow-up of patients with metastatic colorectal cancer. ESMO Open. 2023;8:101558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 3. | Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, Seligmann J, De Baere T, Osterlund P, Yoshino T, Martinelli E; ESMO Guidelines Committee. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:10-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 817] [Article Influence: 408.5] [Reference Citation Analysis (34)] |
| 4. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Gunn A, Hoffe S, Hubbard J, Hunt S, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Johnson-Chilla A, Gurski LA. NCCN Guidelines Insights: Rectal Cancer, Version 6.2020. J Natl Compr Canc Netw. 2020;18:806-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 332] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 5. | Gillams A, Goldberg N, Ahmed M, Bale R, Breen D, Callstrom M, Chen MH, Choi BI, de Baere T, Dupuy D, Gangi A, Gervais D, Helmberger T, Jung EM, Lee F, Lencioni R, Liang P, Livraghi T, Lu D, Meloni F, Pereira P, Piscaglia F, Rhim H, Salem R, Sofocleous C, Solomon SB, Soulen M, Tanaka M, Vogl T, Wood B, Solbiati L. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, The Interventional Oncology Sans Frontières meeting 2013. Eur Radiol. 2015;25:3438-3454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 227] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 6. | Shady W, Petre EN, Gonen M, Erinjeri JP, Brown KT, Covey AM, Alago W, Durack JC, Maybody M, Brody LA, Siegelbaum RH, D'Angelica MI, Jarnagin WR, Solomon SB, Kemeny NE, Sofocleous CT. Percutaneous Radiofrequency Ablation of Colorectal Cancer Liver Metastases: Factors Affecting Outcomes--A 10-year Experience at a Single Center. Radiology. 2016;278:601-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 281] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 7. | Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265:958-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 262] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 8. | Ruers T, Van Coevorden F, Punt CJ, Pierie JE, Borel-Rinkes I, Ledermann JA, Poston G, Bechstein W, Lentz MA, Mauer M, Folprecht G, Van Cutsem E, Ducreux M, Nordlinger B; European Organisation for Research and Treatment of Cancer (EORTC); Gastro-Intestinal Tract Cancer Group; Arbeitsgruppe Lebermetastasen und tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); National Cancer Research Institute Colorectal Clinical Study Group (NCRI CCSG). Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. J Natl Cancer Inst. 2017;109:djx015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 455] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 9. | Kron P, Linecker M, Jones RP, Toogood GJ, Clavien PA, Lodge JPA. Ablation or Resection for Colorectal Liver Metastases? A Systematic Review of the Literature. Front Oncol. 2019;9:1052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Zhang Z, Yan K. The current application of thermal ablation in colorectal liver metastasis. Hepatobiliary Surg Nutr. 2021;10:872-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Shindoh J, Nishioka Y, Yoshioka R, Sugawara T, Sakamoto Y, Hasegawa K, Hashimoto M, Kokudo N. KRAS Mutation Status Predicts Site-Specific Recurrence and Survival After Resection of Colorectal Liver Metastases Irrespective of Location of the Primary Lesion. Ann Surg Oncol. 2016;23:1890-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Narayan RR, Harris JW, Chou JF, Gönen M, Bao F, Shia J, Allen PJ, Balachandran VP, Drebin JA, Jarnagin WR, Kemeny NE, Kingham TP, D'Angelica MI. Prediction of Recurrence Patterns from Hepatic Parenchymal Disease After Resection of Colorectal Liver Metastases. Ann Surg Oncol. 2020;27:188-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Margonis GA, Kim Y, Sasaki K, Samaha M, Amini N, Pawlik TM. Codon 13 KRAS mutation predicts patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Cancer. 2016;122:2698-2707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Kemeny NE, Chou JF, Capanu M, Gewirtz AN, Cercek A, Kingham TP, Jarnagin WR, Fong YC, DeMatteo RP, Allen PJ, Shia J, Ang C, Vakiani E, D'Angelica MI. KRAS mutation influences recurrence patterns in patients undergoing hepatic resection of colorectal metastases. Cancer. 2014;120:3965-3971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 15. | Stang A, Donati M, Weilert H, Oldhafer KJ. Impact of Systemic Therapy and Recurrence Pattern on Survival Outcome after Radiofrequency Ablation for Colorectal Liver Metastases. J Cancer. 2016;7:1939-1949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Buisman FE, Galjart B, Buettner S, Groot Koerkamp B, Grünhagen DJ, Verhoef C. Primary tumor location and the prognosis of patients after local treatment of colorectal liver metastases: a systematic review and meta-analysis. HPB (Oxford). 2020;22:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Gu Y, Huang Z, Gu H, Gao F, Zhang T, Huang S, Huang J. Does the Site of the Primary Affect Outcomes When Ablating Colorectal Liver Metastases with Radiofrequency Ablation? Cardiovasc Intervent Radiol. 2018;41:912-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Bylsma LC, Gillezeau C, Garawin TA, Kelsh MA, Fryzek JP, Sangaré L, Lowe KA. Prevalence of RAS and BRAF mutations in metastatic colorectal cancer patients by tumor sidedness: A systematic review and meta-analysis. Cancer Med. 2020;9:1044-1057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 19. | Yoo W, Mayberry R, Bae S, Singh K, Peter He Q, Lillard JW Jr. A Study of Effects of MultiCollinearity in the Multivariable Analysis. Int J Appl Sci Technol. 2014;4:9-19. [PubMed] |
| 20. | Yan XL, Wang K, Bao Q, Wang HW, Jin KM, Su YM, Xing BC. Prognostic value of the combination of primary tumor location and RAS mutational status on patients with colorectal liver metastasis undergoing hepatectomy. J Surg Oncol. 2022;125:1002-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 21. | Kim HS, Lee JM, Kim HS, Yang SY, Han YD, Cho MS, Hur H, Min BS, Lee KY, Kim NK. Prognosis of Synchronous Colorectal Liver Metastases After Simultaneous Curative-Intent Surgery According to Primary Tumor Location and KRAS Mutational Status. Ann Surg Oncol. 2020;27:5150-5158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Chen CC, Chang SC, Chang YY, Lin BW, Chen HH, Hsieh YY, Hsu HC, Hsieh MC, Ke TW, Kuan FC, Wu CC, Lu WC, Su YL, Liang YH, Chen JB, Huang HY, Tsai HL, Wang JY. Survival benefit of metastasectomy in first-line cetuximab therapy in patients with RAS wild-type metastatic colorectal cancer: a nationwide registry. Am J Cancer Res. 2023;13:6333-6345. [PubMed] |
| 23. | Fernández Montes A, Alonso Orduña V, Asensio Martínez E, Rodríguez Salas N, Torres E, Cacho Lavín D, Rodríguez Alonso RM, Falcó E, Oliva JC, Cirera L, García Gómez J, Pericay C. The Frequency of Specific KRAS Mutations, and Their Impact on Treatment Choice and Survival, in Patients With Metastatic Colorectal Cancer. Oncologist. 2023;28:e902-e909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 24. | Chen MH, Yang W, Yan K, Zou MW, Solbiati L, Liu JB, Dai Y. Large liver tumors: protocol for radiofrequency ablation and its clinical application in 110 patients--mathematic model, overlapping mode, and electrode placement process. Radiology. 2004;232:260-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 182] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 25. | Santini D, Loupakis F, Vincenzi B, Floriani I, Stasi I, Canestrari E, Rulli E, Maltese PE, Andreoni F, Masi G, Graziano F, Baldi GG, Salvatore L, Russo A, Perrone G, Tommasino MR, Magnani M, Falcone A, Tonini G, Ruzzo A. High concordance of KRAS status between primary colorectal tumors and related metastatic sites: implications for clinical practice. Oncologist. 2008;13:1270-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 26. | Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, Chen MH, Choi BI, de Baère T, Dodd GD 3rd, Dupuy DE, Gervais DA, Gianfelice D, Gillams AR, Lee FT Jr, Leen E, Lencioni R, Littrup PJ, Livraghi T, Lu DS, McGahan JP, Meloni MF, Nikolic B, Pereira PL, Liang P, Rhim H, Rose SC, Salem R, Sofocleous CT, Solomon SB, Soulen MC, Tanaka M, Vogl TJ, Wood BJ, Goldberg SN; International Working Group on Image-guided Tumor Ablation; Interventional Oncology Sans Frontières Expert Panel; Technology Assessment Committee of the Society of Interventional Radiology,; Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology. 2014;273:241-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 897] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 27. | Arnold D, Lueza B, Douillard JY, Peeters M, Lenz HJ, Venook A, Heinemann V, Van Cutsem E, Pignon JP, Tabernero J, Cervantes A, Ciardiello F. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28:1713-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 628] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 28. | Missiaglia E, Jacobs B, D'Ario G, Di Narzo AF, Soneson C, Budinska E, Popovici V, Vecchione L, Gerster S, Yan P, Roth AD, Klingbiel D, Bosman FT, Delorenzi M, Tejpar S. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014;25:1995-2001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 494] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 29. | Petrelli F, Tomasello G, Borgonovo K, Ghidini M, Turati L, Dallera P, Passalacqua R, Sgroi G, Barni S. Prognostic Survival Associated With Left-Sided vs Right-Sided Colon Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2017;3:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 538] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 30. | Jasmine F, Almazan A, Khamkevych Y, Bissonnette M, Ahsan H, Kibriya MG. Association of KRAS Mutation and Gene Pathways in Colorectal Carcinoma: A Transcriptome- and Methylome-Wide Study and Potential Implications for Therapy. Int J Mol Sci. 2024;25:8094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 31. | Chibaudel B, André T, Tournigand C, Louvet C, Benetkiewicz M, Larsen AK, de Gramont A. Understanding the Prognostic Value of Primary Tumor Location and KRAS in Metastatic Colorectal Cancer: A Post Hoc Analysis of the OPTIMOX3 DREAM Phase III Study. Clin Colorectal Cancer. 2020;19:200-208.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Shi L, Chen L, Wu C, Zhu Y, Xu B, Zheng X, Sun M, Wen W, Dai X, Yang M, Lv Q, Lu B, Jiang J. PD-1 Blockade Boosts Radiofrequency Ablation-Elicited Adaptive Immune Responses against Tumor. Clin Cancer Res. 2016;22:1173-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 233] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 33. | Frankel TL, Vakiani E, Nathan H, DeMatteo RP, Kingham TP, Allen PJ, Jarnagin WR, Kemeny NE, Solit DB, D'Angelica MI. Mutation location on the RAS oncogene affects pathologic features and survival after resection of colorectal liver metastases. Cancer. 2017;123:568-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Okuno M, Goumard C, Kopetz S, Vega EA, Joechle K, Mizuno T, Omichi K, Tzeng CD, Chun YS, Vauthey JN, Conrad C. RAS Mutation is Associated with Unsalvageable Recurrence Following Hepatectomy for Colorectal Cancer Liver Metastases. Ann Surg Oncol. 2018;25:2457-2466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |