Published online Aug 27, 2025. doi: 10.4240/wjgs.v17.i8.104954
Revised: April 29, 2025
Accepted: June 10, 2025
Published online: August 27, 2025
Processing time: 230 Days and 1 Hours
Allied disorders of Hirschsprung diseases (ADHDs) have abnormal enteric gan
Core Tip: While allied disorders of Hirschsprung disease share clinical similarities with Hirschsprung’s disease (Q43.1), the pathologic manifestation and therapeutic appro
- Citation: Lu M. Clinicopathologic features of allied disorders of Hirschsprung disease and status update. World J Gastrointest Surg 2025; 17(8): 104954
- URL: https://www.wjgnet.com/1948-9366/full/v17/i8/104954.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i8.104954
Allied disorders of Hirschsprung diseases (ADHDs) are a group of congenital diseases that manifests various degrees of functional bowel obstruction, such as chronic constipation, abdominal distension, and intestinal obstruction, caused by the absence of enteric ganglion cells[1]. These disorders have been documented since the 1950s under various names, including “megacolon homolopathy”, “pseudomegacolon”, and “variant congenital megacolon”. These terms reflect the historic association with Hirschsprung’s disease (HD) while recognizing distinct pathologic features, highlighting the evolving perception of these conditions[1]. This ambiguity of terminology arises from the absence of a standardized nomenclature that distinguishes these conditions[1,2]. The pathologic changes of congenital megacolopathy appear in the intestinal tube without ganglion cells.
The diagnosis of ADHD currently relies on clinical manifestations, imaging, histopathology and functional examinations. However, there are differences in the operational norms of the “gold standard” (rectal biopsy) and pathological interpretation criteria, such as the extent of ganglion cell absence and the threshold of acetylcholinesterase (AchE) staining, among clinicians. In addition, short-segment HD or when combined with ganglion dysplasia is prone to missed or misdiagnosis, and delayed physiological ganglion development in premature infants or newborns may be misjudged as pathological changes. The lack of unified standards makes it difficult to integrate international multi-center research data, hindering the development of precision medicine. Therefore, this paper focuses on the classification, epidemiology, pathogenesis, pathologic diagnosis, treatment, and prognosis based on existing diagnostic criteria.
Classification of ADHDs, including enteric neuron dysplasia (IND), enteric ganglioneuromatosis, isolated hypoganglionosis, absent ganglia, internal anal sphincter achalasia, and congenital smooth muscle cell disease (such as Megabladder microcolon delayed peristalsis syndrome), is based on hematoxylin and eosin (HE) and AchE staining techniques[3]. A retrospective cohort research conducted in Japan from 2001-2010 by Taguchi et al[4] utilized the Medical Information Network Distribution System to develop Japanese clinical practice standards and offer tailored treatment methods for various diseases. The Medical Information Network Distribution System guidelines categorize ADHDs into two groups: (1) Ganglion cell abnormalities, which include ganglion immaturity (IG, Q43.1), isolated reduced enteric ganglia (IHG,Q43.1), IND (K59.8), diffuse enteric ganglioneuromatosis (GNM, Q43.8 or E34.8), and absence of the silurophilic plexus; and (2) Abnormalities of ganglion cells, which include giant bladder-colon-intestinal peristalsis delay syndrome (MMIHS), intestinal segmental distension, internal anal sphincter achalasia (IASA), and chronic idiopathic intestinal obstruction. The two most prevalent groups are IND and IASA.
The enteric nervous system (ENS) is composed of many neurons embedded in the gastrointestinal wall. ENS pro
The diagnosis techniques for ADHD are shown in Table 1. In the following section, we discuss the predominant ap
| Diagnostic method | Application purpose | Advantage | Limitation |
| Rectal mucosal biopsy | Detect the deficiency of ganglion cells | Gold standard, high accuracy | It is invasive and may require multiple samplings |
| Anorectal manometry | Evaluate the rectoanal inhibitory reflex | Non-invasive, functional assessment | Infants and toddlers have a poor cooperation for this method |
| Genetic test (for example: RET gene) | Screen for genetic mutations | Assists in the diagnosis of familial cases | High cost and limited positive rate |
A rectal mucosal biopsy is characterized by greater depth and size compared to a normal endoscopic biopsy[8-11]. Ganglion cells are typically absent near the dentate line, so the biopsy site should not be too close to the dentate line. Generally, the recommended distance for the biopsy site is between 10 mm and 25 mm along the dentate line. The biopsy site should extend from 10-25 mm for newborns and infants < 3 years of age. Because obtaining a biopsy clamp can be challenging, it is advisable to perform three separate biopsies at distances of 10 mm, 20 mm, and 30 mm. The primary objective of a biopsy is to definitively rule out or confirm the presence of congenital megacolopathy. The gold standard for diagnosing congenital megacolon in children is the combination of routine pathologic examination of rectal biopsies and fast-freezing pathologic examination with HE and AchE staining. The current fast-freezing pathologic diagnosis of a rectal mucosal biopsy specimen also uses calcium retinal protein (calretinin), which has been reported to be effective in accurately predicting prognosis[12]. However, some studies suggest that calretinin staining is not consistent with AchE activity[13].
When a full-layer biopsy is obtained from the rectal mucosa, the entire thickness of the tissue is examined[14-16]. Biopsies from ADHD patients haven shown that ganglion cells and AchE staining appear normal. However, chronic constipation and functional intestinal obstruction symptoms remain. A full-thickness biopsy necessitates a bowel specimen that is at least 10 mm in length and 5 mm in width. The immaturity of neurons can be identified using immunohistochemistry techniques. Additionally, the size and distribution of intestinal ganglion cells can be assessed using markers, including B-cell lymphoma 2 (BCL-2), soluble in 100% ammonium sulfate (S-100), synaptophysin (Syn), cluster of differentiation 56, protein gene product 9.5, intestinal interstitial cells Cajal (C-kit), and smooth muscle actin.
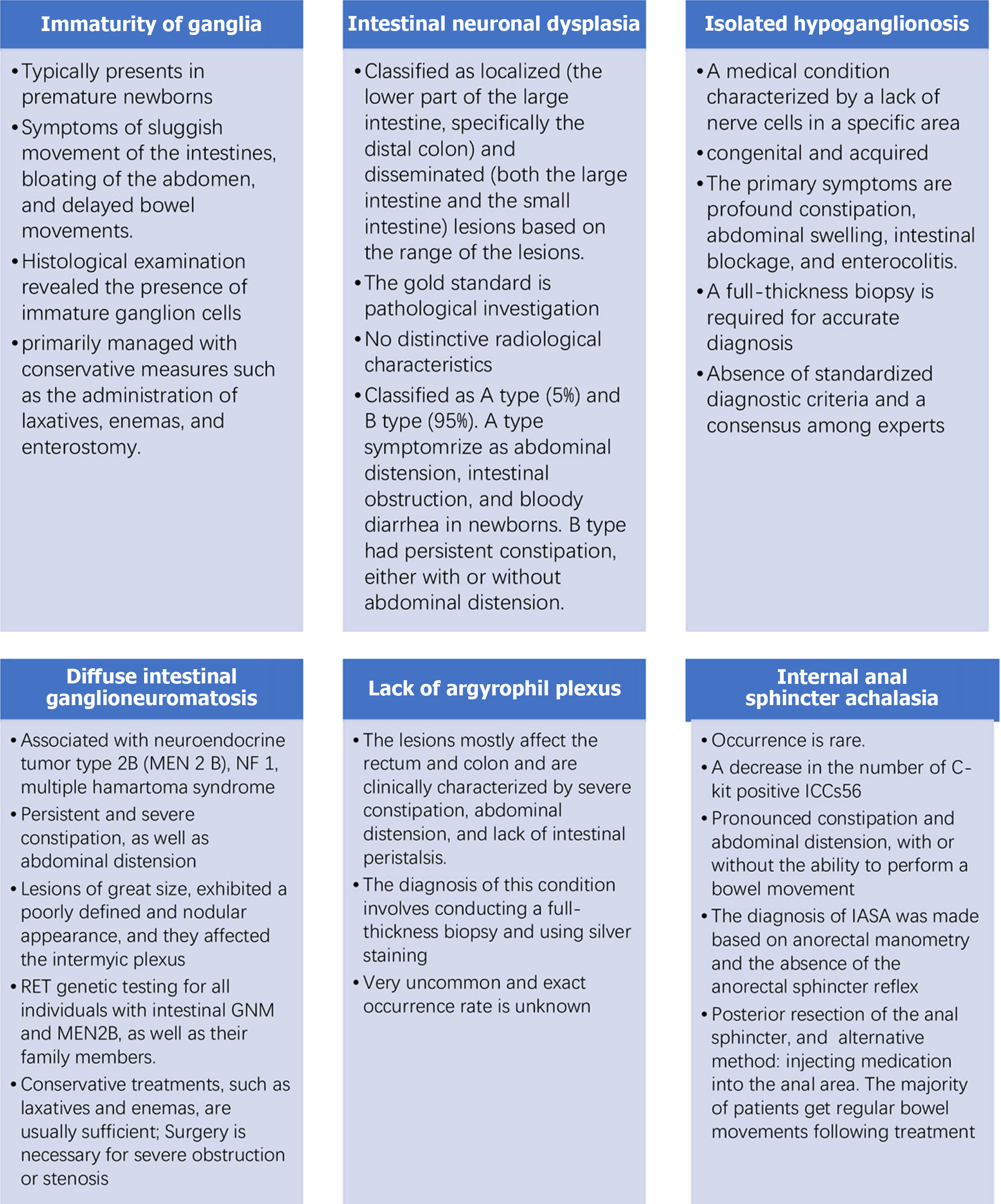
The common classification and clinicopathological characteristics of ADHD are shown in Table 2. Figure 1 summarizes the various ADHD classifications, and the details are discussed below.
| Disease | Prevalence rate | Main symptoms | Pathological characteristics |
| HD | 1:5000 in newborns | Delayed meconium expulsion, abdominal distension, intestinal obstruction | The ganglion cells in the distal intestinal segment are absent |
| IND | Account for 15%-20% of HD cases | Stubborn constipation and abdominal pain | Ganglion cells are underdeveloped and the activity of acetylcholinesterase is elevated |
| Hypoganglionosis | Account for 5%-10% of HD cases | Chronic intestinal obstruction, malnutrition | The number of ganglion cells in the intermuscular plexus decreases |
| IG | Rare (mainly seen in premature infants or patients with ADHD) | Neonatal intestinal obstruction, intractable constipation and feeding difficulties | Ganglion cells are small in volume, have a high nuclear-cytoplasmic ratio, and are accompanied by glial cell proliferation |
| GNM | Extremely rare (mostly related to MEN2B, with an incidence rate of MEN2B approximately 1:400000) | Chronic abdominal pain, intestinal obstruction, diarrhea or constipation, intussusception | The ganglion cells and nerve fibers of the submucosal and intermuscular plexus proliferate diffusely, forming tumor-like structures |
| Deficiency of silver loving nerve plexus (K59.8) | Extremely rare (with occasional case reports) | Severe intestinal motility disorder, abdominal distension and vomiting | Silver staining of the enteric intermuscular plexus (such as the Bielschowsky method) shows the loss of nerve fibers |
| IASA (K59.8) | Rare (accounting for 3%-5% of ADHD) | Difficulty in defecation, persistent constipation, and rectal dilation | Absence or dysfunction of the ganglion cells of the internal anal sphincter leads to the disappearance of the rectoanal inhibitory reflex |
There is a limited number of case reports involving IG and a lack of strong evidence for IG occurrence. Ure et al[17] detected 4 (2.8%) underdeveloped ganglion cells in a study involving 141 patients with enteric neuronal abnormalities. Typically, IG presents in premature newborns, displaying symptoms of sluggish intestinal movement, abdominal bloating, and delayed bowel movements. Histologic examination of the rectal mucosa biopsy revealed the presence of immature ganglion cells. IG cells are small, have a high nucleus-to-cytoplasm ratio, inconspicuous nucleoli, and a distinctive palisade-like structure. This particular pattern is considered a characteristic pathologic feature of IG[18]. It can be challenging to differentiate between ganglion and glial cells using HE and AchE. However, HuC/HuD can aid in identifying these small, undeveloped ganglion cells. Additionally, molecular markers, such as nicotinamide adenine dinucleotide phosphate, nerve cell adhesion molecule (NCAM), and BCL-2, are frequently used to identify IG cells. Immunohistochemical labeling of BCL-2 has been shown to have a notable impact on juvenile ganglion cells and exhibits higher expression compared to other markers, such as neuron-specific enolase and S-100. NCAM stains ganglion cells and nerve fibers[19]. It should be noted that ganglion cell development is controlled by age-related factors. Typically, ganglion cells are not fully developed during the first year of life. Therefore, it is not appropriate to perform a diagnostic diagnosis on immature ganglia. By the time children are between 3 and 4 years of age, ganglion cells have undergone a steady development in both structure and function, reaching the level of adult maturity. This finding suggests that at this developmental stage, ganglion cell condition requires more precise assessment such that the appropriate pathologic diagnosis can be determined[20,21]. IG is primarily managed with conservative measures, such as laxatives and enemas, and an enterostomy. It is important to validate ganglion cell maturity based on histologic examination when the en
IND occurs in approximately 1 of 7500 newborns. Academic studies have reported that up to 44% of patients with congenital megacolon also have IND. The rectal mucosa biopsy has a positive rate ranging from 0.3%-40%. This range can be attributed to patient age, the type of specimen provided, and staining technique used[22,23].
Fadda et al[24] classified IND into two distinct subtypes (A and B), classified as localized and disseminated based on the range of lesions. Localized lesions primarily affect the lower part of the large intestine, especially the distal colon. In contrast, diffuse lesions involve the large and small intestine. Pathologic examination has revealed dysplasia in the sympathetic and parasympathetic systems, abnormalities in the neuromuscular junction in the afflicted intestine, aberrant submucosal vasculature, and a decreased number of C-Kit-positive ICCs in the mucosa and muscularis propria. A previous study discovered a notable decrease in the level of tyrosine phosphoesterase (phosphatase and tensin homolog) expression in the submucosa and inter-muscular nerve plexuses. This reduction may be associated with intestinal motor dysfunction[25].
The diagnosis of IND is typically established based on the evaluation of clinical symptoms, anal and rectal canal manometry, barium enema or intestinal kinetic testing, and pathologic examination. The current gold standard for diagnosing IND is a pathologic evaluation. Most patients with IND do not exhibit any distinctive radiologic characteristics, except for a dilated rectal and sigmoid colon. The methylation of the Sox10 promoter has a crucial role in ENS development. Detecting the methylation status of the Sox10 promoter region in peripheral blood samples can serve as a non-invasive tool for diagnosing IND. The findings of this study offered patients prompt and efficient intervention and treatment options, which will ideally establish a strong foundation for the clinical management and treatment of IND[26,27].
The pathologic diagnosis of INDA is congenital sympathetic dysplasia, which specifically impacts the intestinal intermuscular plexus and submucosal arterial blood vessels. Intestinal mucosal biopsy reveals the presence of ulcerative colitis or inflammatory bowel disease and is characterized by complete destruction of the mucosal myometrium and a decrease in the number of C-kit-positive ICCs in the submucosal myometrium. Severe enterocolitis is present in most infants with INDA who have died.
The INDB type is responsible for nearly 95% of IND cases and is frequently related to HD. Patients have persistent constipation with or without abdominal distension. The submucosal parasympathetic plexus is hypertrophic. According to the Frankfurt diagnostic consensus, > 20% of submucosal giant plexus is observed along with ≥ 7 ganglion cells per 1 nerve plexus at > 1 year of age. Additionally, there may be ectopic ganglion cells, as well as increased AchE activity in the lamina propria and mucosal vascular tissues. The criteria for diagnosing intestinal ganglion hyperplasia using HE and immunohistochemical methods are currently uncertain. The recommended diagnostic procedure is a rectal mucosal biopsy with AchE staining on 15-μm thick frozen sections. This staining technique reveals intestinal ganglion cell proliferation, including the presence of giant ganglion cells. It is important to note that the interpretation of AchE expression in relation to staining technology and age is controversial, both qualitatively and quantitatively, and has evolved over time. Consequently, additional staining techniques are required to assess the ENS. Fadda et al[24] suggested the use of enzymatic immunohistochemistry to determine lactate dehydrogenase, succinate dehydrogenase, and nitric oxide levels as a diagnostic tool for INDB type. Additionally, several markers for ganglion cells and glia have been used, including nicotinamide adenine dinucleotide phosphate diaphorase, NCAM, human spinal cord extract, cathepsin D, protein gene product 9.5 S-100, peripheral protein, Syn, and acrylate blue[24]. The results showed that only one patient met the numerical criteria proposed by Fadda et al[24]. Additionally, children > 1 year of age exhibited neuronal immaturity similar to younger children. Therefore, numerical criteria have limited utility in routine histopathologic diagnoses, and further investigation into age criteria is warranted.
INDB is often managed with conservative measures, such as laxatives and enemas. If symptoms persist for > 6 months, anal sphincter resection or long-term follow-up with internal injection of Botox may be considered. Appropriate measures should be selected based on the pathologic examination and the individual patient's condition. Furthermore, anal sphincter resection is rarely performed on infants, whereas adolescents or adults choose surgery to eliminate dilated bowel segments. Furthermore, the application of enteric neural stem cell and small intestine transplantation has been used as a therapeutic approach for treating enteric neuronal dysplasia[27-29]. Enteric neural stem cells (ENSCs) are the optimal choice for seed cells. Nevertheless, the practical use of neural stem cell transplantation for IND remains challenging due to the scarcity of ENSCs, the brief lifespan of ENSCs in the recipients, the compromised biology of ENSCs, and the inadequate reconstruction of the intestinal nervous system[30-32]. Disseminated IND tumors have an unfavorable outlook when the intestine is surgically removed, and an anastomosis is performed due to the presence of several types of abnormalities. Small bowel transplantation may be considered for children with diffuse IND tumors[33,34].
IHG is a medical condition characterized by a lack of nerve cells in a specific area. IHG is categorized into two groups (congenital and acquired). The occurrence is still uncertain and only a limited number of cases have been documented. Biopsy specimens have shown a low incidence of 0.3%-6.4%. Since 1978, there have been 92 recorded cases of IHG in the United Kingdom, with 32% of these cases being diagnosed in the neonatal period. The median age at diagnosis is 4.8 years with a few cases diagnosed in adolescence. No alterations were detected in the Ret proto-oncogene (RET) gene[35,36]. The primary symptoms are profound constipation, abdominal swelling, intestinal blockage, and enterocolitis. Frozen section diagnosis is not appropriate, and a full-thickness biopsy is required for accurate diagnosis[37]. Additionally, the procedure involves examining three specific parts (the narrow segment, sigmoid colon, and terminal ileum)[38].
The variables that impact ganglion cell density include the following: (1) A negative correlation with age; (2) A lower number of IHG cells in the small intestine compared to the colon; (3) The extent of intestinal expansion; (4) The type of biopsy; and (5) The presence of immunohistochemical markers. Hence, diagnosing a decrease or increase in the size of the intermuscular ganglia is challenging. The examination reveals a limited number of ganglion cells with low or no AchE activity. Additionally, studies have indicated a 40% decrease in ganglion cell number, a 3-fold decrease in the spacing between ganglion cells and inadequate expression of C-kit-positive ICCs[39]. Furthermore, there was a reduced number or absence of NCAM-positive nerve fibers in the lamina propria and mucosal muscularis. The normal intestinal ganglion and plexus of congenital megacolon cannot be measured or assessed. HuC/HuD staining can be utilized to assess alterations in the quantity of ganglion cells and the remaining degenerated ganglion cells in acquired IHG[40]. Cluster of differentiation 56 staining can be used to identify the preserved myenteric plexus, while also distinguishing the myenteric plexus from the myometrium in congenital IHG. Diagnosing IHG is challenging due to the absence of standardized diagnostic criteria and a consensus among experts. Surgical treatment has a positive outcome[21,41]. The majority of deceased neonatal patients had severe enterocolitis[21,39-41].
Enteric gangliomatosis is classified into the following three subtypes: (1) Polypoid gangliomatosis; (2) Knocytic neurolimatosis polyposis; and (3) Diffuse enteric gangliomatosis. Previous studies summarized the clinical characteristics of 43 GNM cases. These included polypoid gangliocyte neuromas in 65.1% of cases, diffuse gangliocyte neuromatosis in 18.6% of cases, and gangliocyte neuromatous polyposis in 16.3% of cases[42,43]. The precise prevalence of diffuse intestinal GNM remains uncertain and is associated with multiple endocrine neoplasia 2B (MEN2B), neurofibromatosis 1, and multiple hamartoma syndrome. In numerous instances, GNM serves as the initial manifestation of these disorders. The prevalence of MEN2B in live births is approximately 1 in 4 million, and approximately 90% of MEN2B patients have intestinal GNM. The development of GNM is associated with the activity of peptidergic, cholinergic, or potentially a complicated proliferation of adrenergic nerve fibers and neurons[42,43]. The clinical symptoms include persistent and severe constipation, as well as abdominal distension. Additionally, there may be alternating episodes of constipation and diarrhea[44]. The lesions observed during the pathologic examination are characterized by their size, reaching up to 17 cm. These lesions exhibit a poorly defined and nodular appearance and are affected by the inter- myenteric plexus. The submucosal and muscular layers show a notable increase in ganglion cell abundance, including gigantic nerve cells. Typically, each ganglion has 15-40 ganglion cells. There is diffuse hyperplasia and dispersed mature ganglion cells. As a result, the intestinal wall becomes thicker and there is increased AchE activity. Additionally, neuron-specific enolase, Syn, and S-100 can be utilized for diagnostic purposes[45].
While mucosal lesions are more prevalent in adults, children tend to have both mucosal and submucosal lesions[46]. Research has indicated that the RET proto-oncogene on chromosome 10 is amplified in MEN2B, causing an upsurge in growth and differentiation signals in several tissues, including those derived from the neural crest. This process ultimately leads to the development of GNM[47-50]. It is advisable to conduct RET genetic testing for all individuals with intestinal GNM and MEN2B, as well as their family members. If there is a strong suspicion of a GNM-related symptom, it is important to detect the accompanying syndromes or systemic disease[51,52]. This can be performed by monitoring calcitonin levels in MEN2B. Patients with GNM paired with MEN2B should undergo prompt examination by endocrine and cancer specialists.
Surgery is not typically required and conservative treatments, such as laxatives and enemas, are usually sufficient. However, if there is severe obstruction or stenosis, surgical intervention may be necessary. Close monitoring and follow-up are essential. Patients with MEN2B receive peptide hormone treatment. If a mutation in the RET gene is present, it is recommended to perform prophylactic total thyroid resection to prevent the development of medullary thyroid carcinoma. Additionally, regular monitoring of plasma calcitonin and carcinoembryonic antigen levels should be performed to detect any tumor recurrence[53,54].
This disease is uncommon and the exact occurrence rate is unknown because published data are limited. There are two types of enteric ganglion cells (agrophilic and non-agstrophilic). Astrophilic ganglion cells typically make up 5%-20% of enteric neurons and cam release neurotransmitters, which leads to the contraction and relaxation of the muscles in the gut wall[55,56]. The lesions mostly affect the rectum and colon and are clinically characterized by severe constipation, abdominal distension, and lack of intestinal peristalsis. These symptoms are uncommon causes of severe constipation and functional intestinal blockage in infants and young children. Currently, the diagnosis of this condition involves a full-thickness biopsy and silver staining. It is not possible to diagnose the disease using mucosal biopsy along with AchE staining and immunohistochemistry[57].
The exact cause of IASA is not fully understood, but research has shown that there is a decrease in the number of C-kit-positive ICCs[58]. The underlying mechanisms of the disease involve two factors: (1) A lack or significant reduction in nitrogenergic innervation, which leads to increased spasticity or tension; and (2) A deficiency in both nitrogenergic innervation and ICCs, which may contribute to impaired relaxation function. Patients with IASA typically have pro
| Disease | Unique characteristics | Overlapping features with ADHD |
| Meconium intestinal obstruction | Thick meconium blocks the terminal ileum | Neonatal intestinal obstruction and abdominal distension |
| Chronic idiopathic constipation | The ganglion cells are normal | Stubborn constipation and abdominal pain |
| Congenital intestinal malrotation | Midintestinal torsion, compression by Ladd cords | Symptoms of vomiting and intestinal obstruction |
ADHD is a congenital enteric neuropathic disorder characterized by high heritability of over 80% and a polygenic inheritance of more than 20 genes[65]. A report identified PLK5 (one novel gene) and suggested 45 additional novel putative genes based on Genome-Wide Association Study Data Validation and Statistical Testing Framework datasets and suggested significant regional differences between Chinese and European patients[65]. RET is the primary gene factors in syndromic families[66]. Incomplete dominance and close relevance to RET are observed in most families with a 56% penetrance of RET mutation in familial ADHD. However, the incidence in offspring does not depend on whether the parent suffers from ADHD[66]. Endothelin B receptor is another mutated associated with increased penetrance in succeeding generations[66,67]. A study identified 103 common differentially expressed genes, of which 50 are downregulated and 53 are upregulated[68]. CXCL10, ICAM1, IL1B, IL10, EGR1, FCGR3A, FPR1, S100A12 and S100A9, which are mainly enriched in immune- and inflammation-related pathways, can aid in the genetic diagnosis of ADHD[68]. However, deep analysis of the genes and mutations in ADHD is lacking, requiring further exploration.
The international consensus and guidelines have been updated. Core recommendations of the 2022 European Society for Pediatric Gastroenterology and Nutrition Guidelines were as follows: (1) Diagnosis requires the combination of rectal aspiration biopsy (at least two layers beneath the mucosa, 2-3 cm or more from the dentate line) and anorectal manometric measurement (rectoanal inhibitory reflex absence); (2) Pathology required to clarify the extent of ganglion cell absence and distinguish between simple HD and combined lesions (such as ganglion dysplasia); and (3) New technology: Genetic panel testing (recommended for screening genes such as RET, Endothelin B receptor gene, and GDNF, especially for familial cases). High-resolution anorectal manometry: Quantifies anal sphincter relaxation function and assists in differentiating ultra-short segment ADHD. The Position Statement of the International Pediatric Endoscopic Surgery Group 2023 recommends a standardized process as follows: (1) The stepwise diagnostic process for suspected HD in newborns: Abdominal X-ray - contrast enema - rectal biopsy - genetic testing; and (2) The pathological report should include ganglion cell density, the degree of nerve fiber hypertrophy and AchE activity score.
Deep learning models (such as convolutional neural networks) can automatically identify the missing areas of ganglion cells in biopsy sections, reducing the differences in artificial interpretation. Exploration of non-invasive biomarkers such as fecal calprotectin (FC > 200 μg/g), could be used for screening and postoperative monitoring. Abnormal expression of miR-206 and miR-218 are associated with HD and could replace invasive examinations in the future.
There has also been a development in treatment strategies: (1) Transanal endoscopic single incision pull-out is suitable for short - and mid-segment HD, with less trauma and better recovery of defecation function than traditional surgical methods (2023 meta-analysis); (2) Robot-assisted surgery: The Da Vinci system for long-segment HD improves the accuracy of pelvic nerve protection. Clinical trials have been conducted at Children’s Hospitals in the United States; (3) Individualized surgical plan: Intestinal preservation strategy - Intraoperative frozen pathology is used to determine the normal boundary of the proximal ganglion to avoid excessive resection, reducing postoperative constipation or incontinence; and (4) Neural crest stem cell transplantation: Successful repair of ganglion-free intestinal segment function in animal models has been reported in Stem Cells Translational Medicine in 2015[69]. 3D bioprinting: Construction of intestinal tube substitutes containing ganglion cells is still in the development stage. However, the challenge in implementing diagnostic criteria lies in the uneven distribution of medical resources. For example, low-income countries lack the capacity for pathological and genetic testing. An international training network needs to be established to promote standardized operations. The therapeutic bottleneck lies in the fact that stem cell therapy faces the problems of immune rejection and functional integration. The quality of life after long-segment HD surgery still needs to be significantly improved. Immediate explorations in future years should focus on the development of low-cost and rapid diagnostic tools, such as portable anorectal manometry, and to explore the role of epigenetic regulation in ADHD pathogenesis.
This study was a review of ADHD, which has the following limitations. First, many statements are concluded from few reported cases of ADHD, especially those rare diseases (such as enteric gangliosis). The sample size is limited, and the data rely on retrospective historical medical records, which could lead to data loss. Second, there are differences in the pathological diagnostic criteria for ADHD among different centers, which introduces diagnostic heterogeneity. Third, for these cases, follow-up is limited and the long-term prognostic data, such as intestinal function in adulthood, is unknown. Fourth, the genetic mechanism has not been fully clarified, and some cases cannot be explained by known gene mutations.
The etiology of ADHDs remains uncertain, and additional research is required to establish a reliable taxonomy. The unification of global diagnostic criteria is the cornerstone of precise diagnosis and treatment of ADHD, which needs to be dynamically updated by combining traditional methods with emerging technologies. Therapeutic strategies are ad
To address the challenges in pathologic diagnosis, it is still necessary to develop an international consensus on a reliable classification method. First, the global standard diagnostic criteria should be established and artificial intelligence technology can be used to perform deep learning on the global data. These advances will help overcome diagnostic obstacles and enhance the accuracy of pathologic diagnosis. To produce clinical guidelines, additional data collecting, follow-up, and data analysis are necessary to acquire improved treatment methods, prognosis, etiology, and genetic investigations.
| 1. | Romero P, Burger A, Wennberg E, Schmitteckert S, Holland-Cunz S, Schwab C, Günther P. Clinical Relevance of Pathological Diagnosis of Hirschsprung's Disease with Acetylcholine-Esterase Histochemistry or Calretinin Immunohistochemistry. Children (Basel). 2024;11:428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Kläber HG, Erler T, Weigt G, Papsdorf H, Gurski A. [Hirschsprung disease. Discussion of a disease picture also relevant to neonatology]. Kinderarztl Prax. 1993;61:139-143. [PubMed] |
| 3. | Qiu J, Yang G, Lin A. Allied disorders of Hirschsprung's disease. Tech Coloproctol. 2019;23:509-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Taguchi T, Ieiri S, Miyoshi K, Kohashi K, Oda Y, Kubota A, Watanabe Y, Matsufuji H, Fukuzawa M, Tomomasa T. The incidence and outcome of allied disorders of Hirschsprung's disease in Japan: Results from a nationwide survey. Asian J Surg. 2017;40:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Brosens E, Burns AJ, Brooks AS, Matera I, Borrego S, Ceccherini I, Tam PK, García-Barceló MM, Thapar N, Benninga MA, Hofstra RM, Alves MM. Genetics of enteric neuropathies. Dev Biol. 2016;417:198-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Heiss CN, Olofsson LE. The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J Neuroendocrinol. 2019;31:e12684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 189] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 7. | Moore SW. Advances in understanding functional variations in the Hirschsprung disease spectrum (variant Hirschsprung disease). Pediatr Surg Int. 2017;33:285-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Yoshimaru K, Kinoshita Y, Yanagi Y, Obata S, Jimbo T, Iwanaka T, Takahashi Y, Esumi G, Miyata JA, Matsuura T, Izaki T, Taguchi T. The evaluation of rectal mucosal punch biopsy in the diagnosis of Hirschsprung's disease: a 30-year experience of 954 patients. Pediatr Surg Int. 2017;33:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Hirose R, Hirata Y, Yamada T, Kawana T, Taguchi T, Suita S. The simple technique of rectal mucosal biopsy for the diagnosis of Hirschsprung's disease. J Pediatr Surg. 1993;28:942-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Sholadoye TT, Aliyu HO, Mshelbwala PM. A Modified Rectal Mucosal Biopsy for the Diagnosis of Hirschsprung's Disease: Zaria Experience. J West Afr Coll Surg. 2023;13:36-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Redkar R, Chigicherla S, Tewari S, Sharma RD. Comparison between Suction Rectal Biopsy and Full-Thickness Rectal Biopsy in the Diagnosis of Hirschsprung's Disease. J Indian Assoc Pediatr Surg. 2021;26:144-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Gonzalo DH, Plesec T. Hirschsprung disease and use of calretinin in inadequate rectal suction biopsies. Arch Pathol Lab Med. 2013;137:1099-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Serafini S, Santos MM, Aoun Tannuri AC, Zerbini MCN, de Mendonça Coelho MC, de Oliveira Gonçalves J, Tannuri U. Is hematoxylin-eosin staining in rectal mucosal and submucosal biopsies still useful for the diagnosis of Hirschsprung disease? Diagn Pathol. 2017;12:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Kadowaki H, Kitano F, Takeuchi S, Tamate S. Full-thickness rectal punch biopsy for the diagnosis of Hirschsprung's disease. J Pediatr Surg. 1979;14:165-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Bjørn N, Rasmussen L, Qvist N, Detlefsen S, Ellebæk MB. Full-thickness rectal biopsy in children suspicious for Hirschsprung's disease is safe and yields a low number of insufficient biopsies. J Pediatr Surg. 2018;53:1942-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Korsager LEH, Bjørn N, Ellebæk MB, Christensen LG, Qvist N. Full-Thickness Rectal Biopsy in Children Suspected of Having Hirschsprung's Disease: The Inconclusive Biopsy. Children (Basel). 2023;10:1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Ure BM, Holschneider AM, Meier-Ruge W. Neuronal intestinal malformations: a retro- and prospective study on 203 patients. Eur J Pediatr Surg. 1994;4:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Yoshimaru K, Tamaki A, Matsuura T, Kohashi K, Kajihara K, Irie K, Hino Y, Uchida Y, Toriigahara Y, Kawano Y, Shirai T, Oda Y, Tajiri T, Taguchi T. Palisading-like arrangement of immature ganglion cell in myenteric ganglia is a unique pathological feature of immaturity of ganglia. J Pediatr Surg. 2022;57:1269-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Galazka P, Szylberg L, Bodnar M, Styczynski J, Marszalek A. Diagnostic Algorithm in Hirschsprung's Disease: Focus on Immunohistochemistry Markers. In Vivo. 2020;34:1355-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Ieiri S, Miyoshi K, Nagata K, Miyata J, Kohashi K, Oda Y, Taguchi T. Current clinical features in diagnosis and treatment for immaturity of ganglia in Japan: analysis from 10-year nationwide survey. Pediatr Surg Int. 2015;31:949-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Yamada Y, Mori T, Takahashi N, Fujimura T, Kano M, Kato M, Takahashi M, Shimojima N, Watanabe T, Yoshioka T, Kanamori Y, Kuroda T, Fujino A. Historical Cohort Study of Congenital Isolated Hypoganglionosis of the Intestine: Determining the Best Surgical Interventions. Biomolecules. 2023;13:1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 22. | Granero Cendón R, Millán López A, Moya Jiménez MJ, López Alonso M, De Agustín Asensio JC. [Intestinal neuronal dysplasia: association with digestive malformations]. Cir Pediatr. 2007;20:166-168. [PubMed] |
| 23. | Serafini S, Santos MM, Tannuri ACA, Di Loreto C, Gonçalves JO, Tannuri U. A new systematization of histological analysis for the diagnosis of Hirschsprung's disease. Clinics (Sao Paulo). 2023;78:100198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Fadda B, Maier WA, Meier-Ruge W, Schärli A, Daum R. [Neuronal intestinal dysplasia. Critical 10-years' analysis of clinical and biopsy diagnosis]. Z Kinderchir. 1983;38:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Wang D, Gao N, Zhou T, Zhang Q, Wang J, Li A. Effect of Neuroligin1 and Neurexin1 on the Colonic Motility in a Mouse Model of Neuronal Intestinal Dysplasia. Gastroenterol Res Pract. 2020;2020:9818652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Liu YR, Ba F, Cheng LJ, Li X, Zhang SW, Zhang SC. Efficacy of Sox10 Promoter Methylation in the Diagnosis of Intestinal Neuronal Dysplasia From the Peripheral Blood. Clin Transl Gastroenterol. 2019;10:e00093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Li D, Wang Q, Bayat A, Battig MR, Zhou Y, Bosch DG, van Haaften G, Granger L, Petersen AK, Pérez-Jurado LA, Aznar-Laín G, Aneja A, Hancarova M, Bendova S, Schwarz M, Kremlikova Pourova R, Sedlacek Z, Keena BA, March ME, Hou C, O'Connor N, Bhoj EJ, Harr MH, Lemire G, Boycott KM, Towne M, Li M, Tarnopolsky M, Brady L, Parker MJ, Faghfoury H, Parsley LK, Agolini E, Dentici ML, Novelli A, Wright M, Palmquist R, Lai K, Scala M, Striano P, Iacomino M, Zara F, Cooper A, Maarup TJ, Byler M, Lebel RR, Balci TB, Louie R, Lyons M, Douglas J, Nowak C, Afenjar A, Hoyer J, Keren B, Maas SM, Motazacker MM, Martinez-Agosto JA, Rabani AM, McCormick EM, Falk MJ, Ruggiero SM, Helbig I, Møller RS, Tessarollo L, Tomassoni Ardori F, Palko ME, Hsieh TC, Krawitz PM, Ganapathi M, Gelb BD, Jobanputra V, Wilson A, Greally J, Jacquemont S, Jizi K, Bruel AL, Quelin C, Misra VK, Chick E, Romano C, Greco D, Arena A, Morleo M, Nigro V, Seyama R, Uchiyama Y, Matsumoto N, Taira R, Tashiro K, Sakai Y, Yigit G, Wollnik B, Wagner M, Kutsche B, Hurst AC, Thompson ML, Schmidt R, Randolph L, Spillmann RC, Shashi V, Higginbotham EJ, Cordeiro D, Carnevale A, Costain G, Khan T, Funalot B, Tran Mau-Them F, Fernandez Garcia Moya L, García-Miñaúr S, Osmond M, Chad L, Quercia N, Carrasco D, Li C, Sanchez-Valle A, Kelley M, Nizon M, Jensson BO, Sulem P, Stefansson K, Gorokhova S, Busa T, Rio M, Hadj Habdallah H, Lesieur-Sebellin M, Amiel J, Pingault V, Mercier S, Vincent M, Philippe C, Fatus-Fauconnier C, Friend K, Halligan RK, Biswas S, Rosser J, Shoubridge C, Corbett M, Barnett C, Gecz J, Leppig K, Slavotinek A, Marcelis C, Pfundt R, de Vries BB, van Slegtenhorst MA, Brooks AS, Cogne B, Rambaud T, Tümer Z, Zackai EH, Akizu N, Song Y, Hakonarson H. Spliceosome malfunction causes neurodevelopmental disorders with overlapping features. J Clin Invest. 2024;134:e171235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 28. | Friedmacher F, Puri P. Classification and diagnostic criteria of variants of Hirschsprung's disease. Pediatr Surg Int. 2013;29:855-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Terra SA, de Arruda Lourenção PL, G Silva M, A Miot H, Rodrigues MAM. A critical appraisal of the morphological criteria for diagnosing intestinal neuronal dysplasia type B. Mod Pathol. 2017;30:978-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Yoshimaru K, Matsuura T, Uchida Y, Sonoda S, Maeda S, Kajihara K, Kawano Y, Shirai T, Toriigahara Y, Kalim AS, Zhang XY, Takahashi Y, Kawakubo N, Nagata K, Yamaza H, Yamaza T, Taguchi T, Tajiri T. Cutting-edge regenerative therapy for Hirschsprung disease and its allied disorders. Surg Today. 2024;54:977-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 31. | López Sanclemente MC, Castellvi J, de Zárate LO, Barrios P. Intestinal Neuronal Dysplasia in a Patient With Chronic Colonic Pseudo-Obstruction. Cirugía Española (English Edition). 2014;92:e59. [DOI] [Full Text] |
| 32. | Hotta R, Rahman A, Bhave S, Stavely R, Pan W, Srinivasan S, de Couto G, Rodriguez-Borlado L, Myers R, Burns AJ, Goldstein AM. Transplanted ENSCs form functional connections with intestinal smooth muscle and restore colonic motility in nNOS-deficient mice. Stem Cell Res Ther. 2023;14:232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 33. | Kulkarni S, Pasricha PJ. Detecting Adult Enteric Neurogenesis in the Context of Adult ENS Homeostasis. Cell Mol Gastroenterol Hepatol. 2022;14:967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Wu G. Intestinal autotransplantation. Gastroenterol Rep (Oxf). 2017;5:258-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Obata S, Yoshimaru K, Kirino K, Izaki T, Ieiri S, Yamataka A, Koshinaga T, Iwai J, Ikeda H, Matsufuji H, Oda Y, Taguchi T. Acquired isolated hypoganglionosis as a distinct entity: results from a nationwide survey. Pediatr Surg Int. 2019;35:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Watanabe Y, Kanamori Y, Uchida K, Taguchi T. Isolated hypoganglionosis: results of a nationwide survey in Japan. Pediatr Surg Int. 2013;29:1127-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Zhang HY, Feng JX, Huang L, Wang G, Wei MF, Weng YZ. Diagnosis and surgical treatment of isolated hypoganglionosis. World J Pediatr. 2008;4:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Dingemann J, Puri P. Isolated hypoganglionosis: systematic review of a rare intestinal innervation defect. Pediatr Surg Int. 2010;26:1111-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Rolle U, Yoneda A, Solari V, Nemeth L, Puri P. Abnormalities of C-Kit-positive cellular network in isolated hypoganglionosis. J Pediatr Surg. 2002;37:709-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Yoshimaru K, Taguchi T, Obata S, Takemoto J, Takahashi Y, Iwanaka T, Yanagi Y, Kuda M, Miyoshi K, Matsuura T, Kinoshita Y, Yoshioka T, Nakazawa A, Oda Y. Immunostaining for Hu C/D and CD56 is useful for a definitive histopathological diagnosis of congenital and acquired isolated hypoganglionosis. Virchows Arch. 2017;470:679-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Watanabe Y, Sumida W, Takasu H, Oshima K, Kanamori Y, Uchida K, Taguchi T. Early jejunostomy creation in cases of isolated hypoganglionosis: verification of our own experience based on a national survey. Surg Today. 2015;45:1509-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Mauro A, Zenzeri L, Esposito F, Gaglione G, Strisciuglio C, Pilozzi E, Corleto VD, Ziparo C, Di Nardo G. Isolated intestinal Ganglioneuromatosis: case report and literature review. Ital J Pediatr. 2021;47:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Bahmad HF, Trinh S, Qian L, Terp K, Alloush F, Elajami MK, Kilinc E, Poppiti R. Colonic Ganglioneuroma: A Combined Single-Institution Experience and Review of the Literature of Forty-Three Patients. Diseases. 2023;11:69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 44. | Charagundla SR, Levine MS, Torigian DA, Campbell MS, Furth EE, Rombeau J. Diffuse intestinal ganglioneuromatosis mimicking Crohn's disease. AJR Am J Roentgenol. 2004;182:1166-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Gfroerer S, Theilen TM, Fiegel H, Harter PN, Mittelbronn M, Rolle U. Identification of intestinal ganglioneuromatosis leads to early diagnosis of MEN2B: role of rectal biopsy. J Pediatr Surg. 2017;52:1161-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Fortea-Sanchis C, Gómez-Quiles L. Diffuse intestinal ganglioneuromatosis in the adult. Cir Esp. 2015;93:665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 47. | Bennaceur-Griscelli A, Hadoux J, Féraud O, Opolon P, Divers D, Gobbo E, Schlumberger M, Griscelli F, Turhan AG. Generation of an induced pluripotent stem cell line from a patient with hereditary multiple endocrine neoplasia 2B (MEN2B) syndrome with "highest risk" RET mutation. Stem Cell Res. 2017;23:154-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 48. | Mathew A, Latteyer S, Frank-Raue K, Moeller LC, Zwanziger D, Mengel M, Führer D, Tiedje V. A Novel Double RET E768D/L790F Mutation Associated with a MEN2B-Like Phenotype. Thyroid. 2021;31:327-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Castinetti F, Moley J, Mulligan L, Waguespack SG. A comprehensive review on MEN2B. Endocr Relat Cancer. 2018;25:T29-T39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 50. | Nakatani T, Iwasaki M, Yamamichi A, Yoshioka Y, Uesaka T, Bitoh Y, Maeda K, Fukumoto T, Takemoto T, Enomoto H. Point mutagenesis in mouse reveals contrasting pathogenetic effects between MEN2B- and Hirschsprung disease-associated missense mutations of the RET gene. Dev Growth Differ. 2020;62:214-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Herranz Bachiller MT, Barrio Andrés J, Pons F, Alcaide Suárez N, Ruiz-Zorrilla R, Sancho Del Val L, Lorenzo Pelayo S, De La Serna Higuera C, Atienza Sánchez R, Perez Miranda M. Diffuse intestinal ganglioneuromatosis an uncommon manifestation of Cowden syndrome. World J Gastrointest Oncol. 2013;5:34-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Rosenfeld EH, Chumpitazi BP, Castro E, Naik-Mathuria B. Diffuse Intestinal Ganglioneuromatosis Causing Severe Intestinal Dysmotility in a Child With a PTEN Mutation. J Pediatr Gastroenterol Nutr. 2019;68:e35-e37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 53. | Fernando AR, Samarasekera DN, Bulathsinghela RP. Constipation with megacolon in a 36-year-old man: a rare presentation of MEN2B from Sri Lanka. BMJ Case Rep. 2019;12:bcr-2018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 54. | Giani C, Ramone T, Romei C, Ciampi R, Tacito A, Valerio L, Agate L, Ugolini C, Marinò M, Basolo F, Franchi A, Borsari S, Michelucci A, Selli C, Materazzi G, Cetani F, Elisei R. A New MEN2 Syndrome with Clinical Features of Both MEN2A and MEN2B Associated with a New RET Germline Deletion. Case Rep Endocrinol. 2020;2020:4147097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 55. | Tanner MS, Smith B, Lloyd JK. Functional intestinal obstruction due to deficiency of argyrophil neurones in the myenteric plexus. Familial syndrome presenting with short small bowel, malrotation, and pyloric hypertrophy. Arch Dis Child. 1976;51:837-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 61] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 56. | Smith B. Disorders of the myenteric plexus. Gut. 1970;11:271-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 57. | Krishnamurthy S, Heng Y, Schuffler MD. Chronic intestinal pseudo-obstruction in infants and children caused by diverse abnormalities of the myenteric plexus. Gastroenterology. 1993;104:1398-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 41] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Piotrowska AP, Solari V, Puri P. Distribution of interstitial cells of Cajal in the internal anal sphincter of patients with internal anal sphincter achalasia and Hirschsprung disease. Arch Pathol Lab Med. 2003;127:1192-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | Baaleman DF, Malamisura M, Benninga MA, Bali N, Vaz KH, Yacob D, Di Lorenzo C, Lu PL. The not-so-rare absent RAIR: Internal anal sphincter achalasia in a review of 1072 children with constipation undergoing high-resolution anorectal manometry. Neurogastroenterol Motil. 2021;33:e14028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 60. | Hirakawa H, Kobayashi H, O'Briain DS, Puri P. Absence of NADPH-diaphorase activity in internal anal sphincter (IAS) achalasia. J Pediatr Gastroenterol Nutr. 1995;20:54-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Church JT, Gadepalli SK, Talishinsky T, Teitelbaum DH, Jarboe MD. Ultrasound-guided intrasphincteric botulinum toxin injection relieves obstructive defecation due to Hirschsprung's disease and internal anal sphincter achalasia. J Pediatr Surg. 2017;52:74-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 62. | Ciamarra P, Nurko S, Barksdale E, Fishman S, Di Lorenzo C. Internal anal sphincter achalasia in children: clinical characteristics and treatment with Clostridium botulinum toxin. J Pediatr Gastroenterol Nutr. 2003;37:315-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 63. | Doodnath R, Puri P. Long-term outcome of internal sphincter myectomy in patients with internal anal sphincter achalasia. Pediatr Surg Int. 2009;25:869-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 64. | Friedmacher F, Puri P. Comparison of posterior internal anal sphincter myectomy and intrasphincteric botulinum toxin injection for treatment of internal anal sphincter achalasia: a meta-analysis. Pediatr Surg Int. 2012;28:765-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 65. | Xiao J, Feng C, Zhu T, Zhang X, Chen X, Li Z, You J, Wang Q, Zhuansun D, Meng X, Wang J, Xiang L, Yu X, Zhou B, Tang W, Tou J, Wang Y, Yang H, Yu L, Liu Y, Jiang X, Ren H, Yu M, Chen Q, Yin Q, Liu X, Xu Z, Wu D, Yu D, Wu X, Yang J, Xiong B, Chen F, Hao X, Feng J. Rare and common genetic variants underlying the risk of Hirschsprung's disease. Hum Mol Genet. 2025;34:586-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 66. | Xiao J, Hao LW, Wang J, Yu XS, You JY, Li ZJ, Mao HD, Meng XY, Feng JX. Comprehensive characterization of the genetic landscape of familial Hirschsprung's disease. World J Pediatr. 2023;19:644-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 67. | Moore SW, Zaahl M. Clinical and genetic correlations of familial Hirschsprung's disease. J Pediatr Surg. 2015;50:285-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 68. | Wang J, Li Z, Xiao J, Wu L, Chen K, Zhu T, Feng C, Zhuansun D, Meng X, Feng J. Identification and validation of the common pathogenesis and hub biomarkers in Hirschsprung disease complicated with Crohn's disease. Front Immunol. 2022;13:961217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 69. | McMahill BG, Spriet M, Sisó S, Manzer MD, Mitchell G, McGee J, Garcia TC, Borjesson DL, Sieber-Blum M, Nolta JA, Sturges BK. Feasibility Study of Canine Epidermal Neural Crest Stem Cell Transplantation in the Spinal Cords of Dogs. Stem Cells Transl Med. 2015;4:1173-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |