Published online Jun 27, 2025. doi: 10.4240/wjgs.v17.i6.103336
Revised: March 17, 2025
Accepted: April 11, 2025
Published online: June 27, 2025
Processing time: 107 Days and 3.2 Hours
In recent years, endoscopic anti-reflux mucosal resection (ARMS) has demon
To evaluate the clinical efficacy of ARMS in patients with gastroesophageal reflux disease (GERD) and its effects on the gut microbiota.
This single-center, retrospective, self-controlled study included 80 patients with GERD. All patients underwent endoscopic ARMS and were followed for at least 3 months after surgery. The primary outcome measures were changes in the gut microbiota before and after treatment and clinical efficacy.
After surgery, the counts of Escherichia coli and Staphylococcus aureus were significantly lower than those before surgery (P < 0.05), whereas the counts of Bifidobacterium and Lactobacillus were significantly higher than those before surgery (P < 0.05). Symptoms, such as reflux and heartburn, were markedly relieved postoperatively. The average Gerd Q score prior to surgery was 11.32 ± 1.26 points, which decreased to 5.89 ± 0.52 points 3 months after surgery. All patients used proton pump inhibitors before surgery, and the proportion of patients using proton pump inhibitors declined significantly postoperatively. Sixteen patients (20.0%) experienced surgery-related adverse reactions within 2 weeks to 1 month post-surgery. The incidence rates of postoperative esophageal stricture and delayed bleeding were 15.0% and 5.0%, respectively.
Endoscopic ARMS can effectively alleviate reflux symptoms, maintain gut microbiota balance, and improve gastrointestinal function in patients with GERD.
Core Tip: In recent years, endoscopic anti-reflux mucosal resection (ARMS) has demonstrated benefits, including good efficacy, ease of operation, low cost, and fewer complications; however, it is still in the exploratory stage. This study evaluated the clinical efficacy of ARMS in patients with gastroesophageal reflux disease (GERD) and its effects on gut microbiota. It was found that endoscopic ARMS can effectively alleviate reflux symptoms, maintain gut microbiota balance, and improve gastrointestinal function in patients with GERD.
- Citation: Han Z, Jiang HB, Wang FK, Wang ZY, Pang HF, Wang YY, Wei M. Endoscopic anti-reflux mucosal resection for patients with gastroesophageal reflux disease: Clinical efficacy and impact on gut microbiota. World J Gastrointest Surg 2025; 17(6): 103336
- URL: https://www.wjgnet.com/1948-9366/full/v17/i6/103336.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i6.103336
Gastroesophageal reflux disease (GERD) results from the reflux of gastric and duodenal contents into the esophagus, causing symptoms of discomfort and/or complications. Reflux and heartburn are the most common symptoms. The prevalence of GERD varies regionally, affecting 10%-20% of the population in Europe and the Americas and approximately 12.5% in China[1]. Proton pump inhibitors (PPIs) are the first-line treatment for GERD, with a recommended treatment course of at least 8 weeks[2]. However, approximately 40% of patients are dissatisfied with the effects of standard-dose PPI treatment[3]. Refractory GERD (rGERD) is defined as persistent GERD symptoms despite 8-12 weeks of double-dose PPI treatment[4]. For patients who do not respond adequately to medication or who have difficulty tolerating long-term drug treatments, anti-reflux surgery is a treatment option. Laparoscopic fundoplication is currently the most commonly used surgical method; however, owing to high rates of postoperative complications and recurrence, the number of surgeries has declined over the past decade, with patients and doctors exercising caution in choosing surgical procedures[5]. In addition, long-term PPI use may inhibit gastric acid secretion, damaging gastric mucosal function and causing bacterial colonization in the stomach and gut microbiota disorders, which negatively affects patient prognosis[6]. Addressing gut microbiota imbalances is therefore crucial for reducing complications and improving outcomes.
Over the past two decades, endoscopic technology has rapidly advanced in GERD treatment. In 2014, Inoue et al[7] conducted the first preliminary clinical study on anti-reflux mucosal resection (ARMS) in 10 patients with rGERD without hiatal hernia, showing significant improvement in GERD symptoms post-surgery. Subsequently, a retrospective study by Hedberg et al[8] included 19 patients with GERD and analyzed their clinical data. Thirteen patients (68.4%) experienced significant improvement in GERD-related symptoms and were able to discontinue PPIs. As digestive endoscopy technology has evolved, endoscopic anti-reflux therapy has emerged as a viable alternative to both drug and surgical treatments. Endoscopic treatment is less invasive and less costly than conventional GERD surgery, making it an increasingly preferred option among patients with GERD[9].
The gut microbiota is now recognized as an important factor affecting disease occurrence and has become a research hotspot in recent years. Intestinal microorganisms may affect whole-body substance metabolism by altering the absorption and decomposition of intestinal nutrients[10]. Meanwhile, changes in the gut flora can impact systemic immune and inflammatory responses, significantly influencing disease progression[11]. After more than 20 years of continuous exploration and research, many new endoscopic anti-reflux techniques have emerged for GERD treatment. However, endoscopic therapy is not yet established as the standard treatment for GERD because of insufficient long-term efficacy data and the need for expensive specialized equipment. After minimally invasive laparoscopic anti-reflux surgery, some patients still experience gastric retention, abdominal distension, belching, and other gastrointestinal motility disorders to a certain extent, which may lead to further surgical intervention and reduce patient satisfaction[12].
As a new endoscopic anti-reflux technology, ARMS has not been widely used in clinical practice, and there are few reports on its effects on the gut microbiota of patients. Therefore, this study aimed to explore the clinical efficacy of ARMS and its impact on the gut microbiota in patients with GERD, to inform the selection of endoscopic treatments for GERD. This study is significant as it emphasizes the effects of endoscopic ARMS on the gut microbiota of GERD patients, providing insights into a critical aspect of treatment that has been underexplored. By understanding how ARMS influences the gut microbiota, we can better evaluate its potential benefits and risks, ultimately contributing to improved patient management and outcomes.
This study included 80 patients with GERD who were admitted between February 2020 and March 2024. All patients underwent endoscopic ARMS and were followed for at least 3 months after surgery. Patients’ general information, such as age, sex, body mass index, and use of drugs such as PPIs, was collected. This single-center, retrospective, self-controlled study was approved by the Clinical Research Ethics Committee of our hospital.
The history of GERD and borderline GERD was assessed by endoscopy and potential of hydrogen test results, following the Lyon consensus guidelines[13]. GERD was diagnosed when: (1) Acid exposure time > 6%; (2) Acid exposure time between 4% and 6%; and (3) The total number of reflux episodes > 80.
The inclusion criteria were: (1) Age 18-70 years old; (2) Any sex; (3) Meeting the diagnostic criteria for GERD and any of the following conditions[14,15]: Failure of medical treatment including poor symptom control, severe symptoms that cannot be controlled by acid suppressants, or the presence of drug side effects; Drug treatment was effective and long-term maintenance treatment was required, including the need to improve quality of life, unwillingness to take long-term medication, or the belief that drug treatment is too costly; GERD complications, including Barrett esophagus, esophageal stenosis, ulcer, and esophagitis (> Los Angeles grade B); Presence of hiatal hernia > 3 cm; Chronic or recurrent extraesophageal symptoms and complications, including reflux asthma, cough, otolaryngology symptoms, laryngeal spasm, and aspiration; and (4) No history of major cardiopulmonary disease and being able to tolerate anti-reflux surgery under intubation and general anesthesia.
The exclusion criteria included: (1) Patients with Helicobacter pylori infection; (2) Patients with achalasia or other primary esophageal motility disorders; (3) Patients with malignant tumors; (4) Patients with a history of upper gastrointestinal surgery; (5) Patients with inflammatory bowel disease, liver cirrhosis, diabetes, thyroid dysfunction, or immune system-related diseases; (6) Patients who used antibiotics or probiotics in the past 3 months; (7) Patients with mental illness or genetic or metabolic diseases; (8) Pregnant or lactating women; and (9) Patients who were participating in other studies and unwilling to participate in this study.
All 80 patients underwent endoscopic ARMS. The specific process was as follows: Patients underwent routine blood tests and coagulation function tests, among other tests, before surgery. They were instructed to fast and abstain from drinking. The feasibility of surgery was comprehensively evaluated, potential risks were explained, and emergency plans were formulated. Patients were placed in the left lateral decubitus position and underwent surgery under endotracheal intubation and general anesthesia. A transparent cap was placed at the front end of the endoscope, and the intended resection range was marked around the lumen using a dual knife. Isotonic sodium chloride solution (HES, Beijing Fresenius Kabi Pharmaceutical Co., Ltd.), methylene blue (Macklin Biochemical Co., Ltd.), and epinephrine (Cusabio Biotech Co., Ltd.) were injected submucosally at multiple points outside the marked point. The lifting sign was good; the dual knife was used to make a circular incision along the marked point, the submucosal layer was gradually peeled off, and 1/2 or 3/4 of the cardia mucosa was completely removed (the first case was a full circumferential resection), with 1/2 or 1/4 of the mucosa on the greater curvature retained. The resection length was approximately 3 cm (1 cm on the esophageal side and 2 cm on the gastric side), and the wound surface was crescent-shaped. During the incision and stripping process, if bleeding or exposed blood vessels were encountered, a dual knife or FD-410 LR (Olympus Corporation, Tokyo, Japan) hot biopsy forceps were used for electrocoagulation to stop the bleeding. If local stripping was deep or the muscularis propria was damaged, the wound was closed with titanium clips. After complete stripping, all visible blood vessels on the wound surface were treated with preventive electrocoagulation, and the wound surface was covered with a topical freeze-dried human fibrinogen adhesive. The scope was withdrawn after confirming the absence of active bleeding.
After surgery, patients were forbidden to eat or drink for 24-48 hours and gradually transitioned from low-temperature liquid, semi-liquid, and soft food to a normal diet according to their condition. Treatments, such as acid suppression, hemostasis, anti-infection, fluid replacement, and nutritional support, were provided, and patients were closely monitored for complications, such as bleeding, perforation, and infection. After discharge, patients were advised to take PPIs and mucosal protective agents regularly for 2 months to promote healing of the surgical wound. Patients were followed regularly through outpatient/inpatient visits and telephone calls. Gastroscopy was repeated 3 months after surgery, and the Gerd Q score was evaluated once 3 months post-surgery.
Main observation indicators: Fresh feces (mass > 1 g) were collected from patients in the morning before and after treatment and placed in sterile cryopreservation tubes at -80 °C. One gram of each sample was processed using a bacterial DNA extraction kit, and the concentration and mass of DNA were measured using microspectrophotometry (NanoDrop Technologies, Inc.) and electrophoresis (Bioanalyzer 2100, Agilent Technologies), respectively. After screening the target primers, the numbers of Bifidobacterium, Lactobacilli, Escherichia coli, and Staphylococcus aureus were determined by fluorescence quantitative polymerase chain reaction (PCR) analyzer (CFX Connect, Bio-Rad, United States)[16].
PCR primers and reaction conditions: The primer sequences used in the PCR reactions are as follows: PCR primers: 5’-GTTAAGTTTGTTGTAGGATAGGGTAGT-3’ and 5’-AAATCTACATCTAAACCCTATTATCACA-3’; Sequence primer: 5’-GTGGTGTAGATGAAGT-3’. The final volume of the PCR reaction mixture was 20 μL, which included 10 μL of 2 × PCR Master Mix, 0.5 μL of each primer (10 μmol/L), 1 μL of extracted sample DNA, and 8 μL of deionized water. The cycling conditions for PCR are: Initial denaturation at 72 °C for 5 minutes, followed by denaturation at 94 °C for 30 seconds, annealing at 55 °C for 30 seconds, and extension at 72 °C for 1 minute, with a total of 35 cycles, and a final extension at 72 °C for 5 minutes. After the reaction, the specificity and size of the PCR products were analyzed using polyacrylamide gel electrophoresis.
Clinical efficacy: According to the Gerd Q score of patients after ARMS surgery and improvement in PPI use, patients were divided into three groups[17]: Complete response (CR), partial response (PR), and no response (NR). CR was defined as a Gerd Q score dropping to < 8 points after ARMS surgery and discontinuation of PPIs. PR was defined as the Gerd Q score dropping to < 8 points after ARMS surgery, and the PPI dosage (frequency or daily dosage) was reduced compared to pre-surgery levels. NR was defined as patients who did not meet the CR or PR criteria. According to these criteria, CR + PR was considered effective, whereas NR was deemed ineffective.
Gerd Q was used to assist in the diagnosis of GERD, assess the severity of clinical symptoms, and monitor treatment efficacy[18]. This self-administered questionnaire uses a four-level score (0-3) to assess the frequency of the following six conditions: Heartburn, reflux, epigastric pain, nausea, GERD-related sleep disturbances, and use of over-the-counter drugs. The score range of the Gerd Q questionnaire is 0-18 points, and a total score of > 8 points is considered positive, indicating that the patient has GERD. The higher the score, the more severe the patient’s typical GERD symptoms and the greater the diagnostic accuracy.
Use of PPIs in patients before and after ARMS surgery and the occurrence of surgical complications, such as esophageal stenosis and bleeding, were also measured. The incidence of adverse reactions was calculated as (number of adverse reactions/total number of cases) × 100%.
SPSS 26.0 was used to analyze the data. Measurement data with a normal distribution and homogeneity of variance are expressed as the mean ± SD. Paired-sample t-tests were used for intragroup comparisons, and independent-sample t-tests were used for intergroup comparisons. Count data are expressed as cases (%) and were compared using χ2 tests. Statistical significance was set at P < 0.05.
Among the 80 patients included in the study, the average age was 52.18 ± 7.56 years, with 58 men and 22 women. Two patients underwent full circumferential resection, whereas the remaining 78 underwent crescent resection, of which 42 underwent 3/4 circumferential resection and 36 underwent 1/2 circumferential resection. All patients had successful ARMS surgery, with a median operation time of 48.5 minutes (range; 21-122 minutes). The basic clinical data of the patients are shown in Table 1.
| Variable | Patients (n = 80) | Percentage (%) |
| Sex | ||
| Male | 58 | 72.5 |
| Female | 22 | 27.5 |
| Age (years) | 52.18 ± 7.56 | |
| BMI (kg/m2) | 23.87 ± 3.25 | |
| Average Gerd Q score (points) | 11.32 ± 1.26 | |
| History of PPIs use | 80 | 100.0 |
| Mucosal resection range | ||
| Full circumference | 2 | 2.5 |
| 3/4 circumference | 42 | 52.5 |
| 1/2 circumference | 36 | 45.0 |
| Median operative time (minutes) | 48.5 (21, 122) |
After surgery, the counts of Escherichia coli and Staphylococcus aureus were significantly lower than those before treatment (P < 0.05), whereas the counts of Bifidobacterium and Lactobacillus were significantly higher (P < 0.05) (Table 2).
| Index | Before operation | Three months after surgery | t value | P value |
| Bifidobacterium | 4.95 ± 1.02 | 8.92 ± 1.03 | 24.496 | < 0.001 |
| Lactobacillus | 6.57 ± 0.81 | 8.87 ± 0.88 | 17.200 | < 0.001 |
| Escherichia coli | 7.65 ± 0.68 | 6.74 ± 0.42 | 10.184 | < 0.001 |
| Staphylococcus | 8.76 ± 1.15 | 7.32 ± 0.81 | 9.157 | < 0.001 |
The therapeutic efficacy of ARMS at 3 months after surgery was 70.0% (56/80; 50 cases in the CR group and six cases in the PR group).
After ARMS surgery, the patients’ symptoms of reflux, heartburn, and other discomforts significantly improved. The average Gerd Q score of patients before ARMS surgery was 11.32 ± 1.26 points (n = 80), which dropped to 5.89 ± 0.52 points (n = 80, P < 0.01) 3 months after surgery.
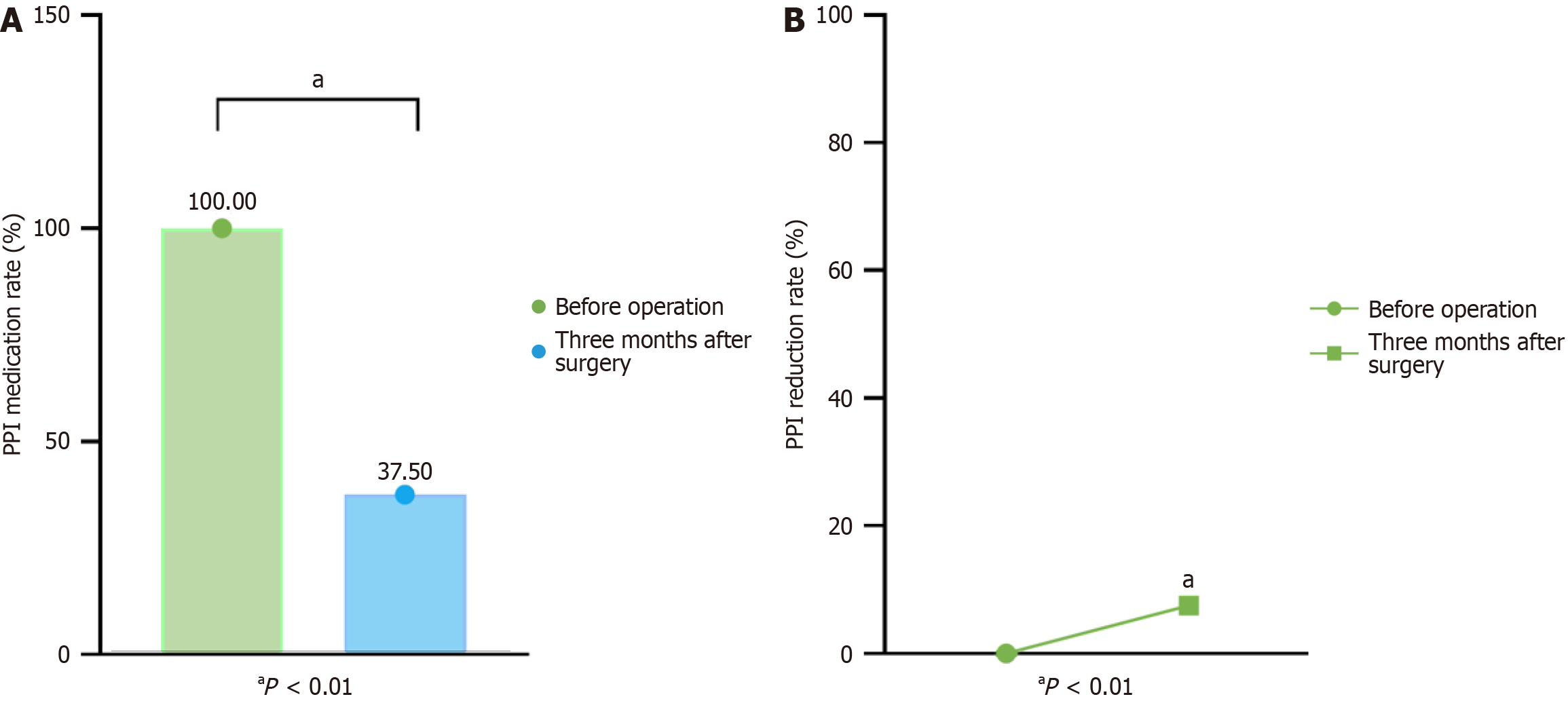
As shown in Figure 1, all 80 patients (100%) used PPIs to control symptoms before surgery, and the proportion of patients taking PPIs 3 months after surgery decreased significantly. Three months after ARMS surgery, 62.5% (50/80) of patients had discontinued PPI use, 37.5% continued to use them, and 7.5% (6/80) used PPIs less frequently than before surgery.
Sixteen patients (20.0%) experienced surgery-related adverse reactions within 2 weeks to 1 month after surgery. Among them, 12 (15.0%) experienced severe dysphagia due to postoperative esophageal stenosis. After endoscopic esophageal bougienage, symptoms were significantly relieved. Delayed bleeding occurred in four patients (5.0%), all of whom had minor bleeding from the wound surface. Bleeding was successfully controlled through endoscopic thermal biopsy forceps electrocoagulation and protective spraying to cover the wound surface. No surgery-related complications requiring further intervention occurred in any of the patients (Table 3).
| Index | Patients (n = 80) | Percentage (%) |
| Severe dysphagia | 12 | 15.0 |
| Delayed bleeding | 4 | 5.0 |
| Total incidence | 16 | 20.0 |
The long-term use of PPIs to control gastric acid secretion can lead to a decline in gastric acid barrier function and, consequently, disturbances in the gut microbiota. The gut microbiota consists of various microorganisms that settle in the gut and can be classified as beneficial, harmful, or neutral based on their effects on the human body[19]. Under normal conditions, the gut microbiota maintains a relatively balanced state, with beneficial bacteria, such as Bifidobacterium and Lactobacillus, accounting for approximately 90% of the total flora. The microorganisms, collectively known as “superorganisms”, play crucial roles in nutrition and material metabolism, immune regulation, and biological barriers; disruptions in this balance can easily lead to diseases[20]. PPIs are thought to reduce gastric acid’s ability to resist exogenous bacteria, allowing harmful pathogens to invade the intestine, affect normal flora reproduction, and alter gut microbiota homeostasis. Imbalances in the gut microbiota are a primary driver of GERD progression, inhibiting the growth of probiotics, such as Bifidobacterium and Lactobacillus, while increasing the propagation of pathogenic bacteria, such as Escherichia coli and Staphylococcus aureus. This imbalance activates inflammatory pathways, increases the expression of inflammatory factors, and aggravates gastrointestinal mucosal damage. Additionally, it can impair the lower esophageal sphincter’s function, delay gastric emptying, and promote GERD formation[21]. The results of this study showed that the numbers of Escherichia coli and Staphylococcus aureus cells after surgery were significantly lower than those before treatment (P < 0.05), whereas the numbers of Bifidobacterium and Lactobacillus cells were significantly higher after treatment (P < 0.05).
Sumi et al[22] recently published a retrospective study on ARMS, which is the largest study to date, including 109 patients with rGERD. The results showed that ARMS significantly improved symptoms such as heartburn and regurgitation, with 21 patients maintaining symptom relief at three years of follow-up. Their Gerd Q scores decreased significantly from 10.6 ± 2.9 before surgery to < 6.0 ± 1.9 at three years post-surgery (P < 0.05). After surgery, 40% to 50% of patients were able to discontinue PPIs. However, the study also highlighted some key complications, the most common of which is postoperative esophageal stenosis, defined as esophageal narrowing requiring endoscopic treatment, with an incidence of 6.1% to 15.8%[8,17,23].
Additionally, delayed postoperative bleeding and perforation have occasionally been reported, with rates ranging from 0% to 5.3%[8] and 0% to 3.2%[24], respectively. Our study showed that the effective rate at three months after ARMS was 70.0% (56 out of 80 patients in the CR group and 6 in the PR group), and the postoperative Gerd Q scores of the patients significantly decreased. This result aligns with the 40% to 50% PPI discontinuation rate reported by Sumi et al[22], indicating that patients in our study also achieved good symptom control. By examining patient characteristics, we observed that all subjects relied on PPIs to control their symptoms before undergoing ARMS, while the proportion of patients using PPIs significantly decreased after the procedure. Regarding complications, the incidence rates of esophageal stenosis and delayed bleeding in our study were 15.0% and 5.0%, respectively, which are similar to the data reported by Sumi et al[22]. However, the specific incidence rates may relate to baseline patient characteristics and differences in surgical techniques. For instance, patient age, comorbidities, and technical details during surgery may all influence the occurrence of complications. Further stratified analysis could help identify key factors affecting treatment outcomes, providing more meaningful guidance for future clinical practice.
This study has the following limitations: First, as a single-center retrospective study, there was a selection bias in case screening. Some patients failed to attend scheduled gastroscopy follow-ups after surgery, resulting in missing data, which may have affected the statistical analysis and reliability of the study conclusions. Second, this was a self-controlled study without a control group undergoing laparoscopic fundoplication; thus, the influence of confounding factors could not be entirely excluded. Third, the sample size was relatively small, and the follow-up period was limited. Given the chronic nature and recurrence of GERD, future studies should include more cases and conduct long-term follow-up to better evaluate the efficacy and safety of ARMS in GERD. Therefore, multi-center prospective long-term follow-up studies with large sample sizes should be conducted in the future to further verify the results of this study. The potential mechanisms of ARMS in the treatment of GERD also need to be further explored.
The results of this study indicate that ARMS can significantly relieve GERD symptoms, maintain gut microbiota balance, reduce PPI usage, and lower the incidence of surgery-related complications. ARMS provides an intermediate option between drug therapy and surgical anti-reflux surgery, offering a new treatment for GERD patients who experience poor outcomes with drug therapy, prefer to avoid long-term medications, or do not wish to undergo traditional surgical anti-reflux procedures.
| 1. | Edinoff AN, Wu NW, Parker K, Dudossat E, Linquest L, Flanagan CJ, Dharani A, Patel H, Willett O, Cornett EM, Kaye AM, Kaye AD. Proton Pump Inhibitors, Kidney Damage, and Mortality: An Updated Narrative Review. Adv Ther. 2023;40:2693-2709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Dutta AK, Jain A, Jearth V, Mahajan R, Panigrahi MK, Sharma V, Goenka MK, Kochhar R, Makharia G, Reddy DN, Kirubakaran R, Ahuja V, Berry N, Bhat N, Dutta U, Ghoshal UC, Jain A, Jalihal U, Jayanthi V, Kumar A, Nijhawan S, Poddar U, Ramesh GN, Singh SP, Zargar S, Bhatia S. Guidelines on optimizing the use of proton pump inhibitors: PPI stewardship. Indian J Gastroenterol. 2023;42:601-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Lakhtakia S, Singh AP, Singla N, Memon SF, Reddy DN. Efficacy and safety of pantoprazole and itopride in patients with overlap of gastroesophageal reflux disease and dyspepsia: A prospective, open-label, single-arm pilot study. JGH Open. 2024;8:e12988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Sawada A, Sifrim D, Fujiwara Y. Esophageal Reflux Hypersensitivity: A Comprehensive Review. Gut Liver. 2023;17:831-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Khan F, Maradey-Romero C, Ganocy S, Frazier R, Fass R. Utilisation of surgical fundoplication for patients with gastro-oesophageal reflux disease in the USA has declined rapidly between 2009 and 2013. Aliment Pharmacol Ther. 2016;43:1124-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Fossmark R, Olaisen M. Changes in the Gastrointestinal Microbiota Induced by Proton Pump Inhibitors-A Review of Findings from Experimental Trials. Microorganisms. 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 7. | Inoue H, Ito H, Ikeda H, Sato C, Sato H, Phalanusitthepha C, Hayee B, Eleftheriadis N, Kudo SE. Anti-reflux mucosectomy for gastroesophageal reflux disease in the absence of hiatus hernia: a pilot study. Ann Gastroenterol. 2014;27:346-351. [PubMed] |
| 8. | Hedberg HM, Kuchta K, Ujiki MB. First Experience with Banded Anti-reflux Mucosectomy (ARMS) for GERD: Feasibility, Safety, and Technique (with Video). J Gastrointest Surg. 2019;23:1274-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Liu DS, Allan Z, Wong DJ, Goh SK, Stevens S, Aly A, Bright T, Watson DI; PROTECTinG Antireflux Surgery study group. Pre-existing hiatal mesh increases morbidity during and after revisional antireflux surgery: A retrospective multicenter study. Surgery. 2023;174:549-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Ziese AL, Suchodolski JS. Impact of Changes in Gastrointestinal Microbiota in Canine and Feline Digestive Diseases. Vet Clin North Am Small Anim Pract. 2021;51:155-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 11. | Taleb S. Tryptophan Dietary Impacts Gut Barrier and Metabolic Diseases. Front Immunol. 2019;10:2113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 159] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 12. | Lee Y, Tahir U, Tessier L, Yang K, Hassan T, Dang J, Kroh M, Hong D. Long-term outcomes following Dor, Toupet, and Nissen fundoplication: a network meta-analysis of randomized controlled trials. Surg Endosc. 2023;37:5052-5064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Gyawali CP, Yadlapati R, Fass R, Katzka D, Pandolfino J, Savarino E, Sifrim D, Spechler S, Zerbib F, Fox MR, Bhatia S, de Bortoli N, Cho YK, Cisternas D, Chen CL, Cock C, Hani A, Remes Troche JM, Xiao Y, Vaezi MF, Roman S. Updates to the modern diagnosis of GERD: Lyon consensus 2.0. Gut. 2024;73:361-371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 192] [Article Influence: 192.0] [Reference Citation Analysis (0)] |
| 14. | Mastracci L, Grillo F, Parente P, Unti E, Battista S, Spaggiari P, Campora M, Scaglione G, Fassan M, Fiocca R. Gastro-esophageal reflux disease and Barrett's esophagus: an overview with an histologic diagnostic approach. Pathologica. 2020;112:117-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Richter JE, Rubenstein JH. Presentation and Epidemiology of Gastroesophageal Reflux Disease. Gastroenterology. 2018;154:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 365] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 16. | Shi YC, Cai ST, Tian YP, Zhao HJ, Zhang YB, Chen J, Ren RR, Luo X, Peng LH, Sun G, Yang YS. Effects of Proton Pump Inhibitors on the Gastrointestinal Microbiota in Gastroesophageal Reflux Disease. Genomics Proteomics Bioinformatics. 2019;17:52-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 17. | Yoo IK, Ko WJ, Kim HS, Kim HK, Kim JH, Kim WH, Hong SP, Yeniova AÖ, Cho JY. Anti-reflux mucosectomy using a cap-assisted endoscopic mucosal resection method for refractory gastroesophageal disease: a prospective feasibility study. Surg Endosc. 2020;34:1124-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 18. | Simadibrata DM, Ngadiono E, Sinuraya FAG, Damara I, Fass R, Simadibrata M. Diagnostic accuracy of gastroesophageal reflux disease questionnaire for gastroesophageal reflux disease: A systematic review and meta-analysis. Neurogastroenterol Motil. 2023;35:e14619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Shi Y, Li J, Cai S, Zhao H, Zhao H, Sun G, Yang Y. Proton pump inhibitors induced fungal dysbiosis in patients with gastroesophageal reflux disease. Front Cell Infect Microbiol. 2023;13:1205348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 20. | Koo SH, Deng J, Ang DSW, Hsiang JC, Lee LS, Aazmi S, Mohamed EHM, Yang H, Yap SY, Teh LK, Salleh MZ, Lee EJD, Ang TL. Effects of proton pump inhibitor on the human gut microbiome profile in multi-ethnic groups in Singapore. Singapore Med J. 2019;60:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Weitsman S, Celly S, Leite G, Mathur R, Sedighi R, Barlow GM, Morales W, Sanchez M, Parodi G, Villanueva-Millan MJ, Rezaie A, Pimentel M. Effects of Proton Pump Inhibitors on the Small Bowel and Stool Microbiomes. Dig Dis Sci. 2022;67:224-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 22. | Sumi K, Inoue H, Kobayashi Y, Iwaya Y, Abad MRA, Fujiyoshi Y, Shimamura Y, Ikeda H, Onimaru M. Endoscopic treatment of proton pump inhibitor-refractory gastroesophageal reflux disease with anti-reflux mucosectomy: Experience of 109 cases. Dig Endosc. 2021;33:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (1)] |
| 23. | Zhang Y, Zhang B, Wang Y, Zhang J, Wu Y, Xiao T, Liao Y, Bao Y, Qiu H, Sun S, Guo J. Advances in the Prevention and Treatment of Esophageal Stricture after Endoscopic Submucosal Dissection of Early Esophageal Cancer. J Transl Int Med. 2020;8:135-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Patil G, Dalal A, Maydeo A. Feasibility and outcomes of anti-reflux mucosectomy for proton pump inhibitor dependent gastroesophageal reflux disease: First Indian study (with video). Dig Endosc. 2020;32:745-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |