Published online May 27, 2025. doi: 10.4240/wjgs.v17.i5.102799
Revised: February 17, 2025
Accepted: March 31, 2025
Published online: May 27, 2025
Processing time: 135 Days and 0.6 Hours
The application of perioperative disinfection and isolation measures to patients undergoing gastrointestinal surgery with postoperative infection can provide a data reference for reducing the postoperative infection rate, improving post
To explore the effectiveness of perioperative disinfection and isolation measures in controlling postoperative infection following gastrointestinal surgery. It also sought to compare infection rates and biochemical markers between the obser
A retrospective analysis was conducted. Ninety-six patients who underwent gastrointestinal surgery between January 2022 and December 2023 were selected and divided into an observation group and a control group, with 48 cases in each. The observation group received disinfection and isolation measures during the perioperative period, whereas the control group received standard nursing care. The incidence of infection, white blood cell count, C-reactive protein levels, hemoglobin levels, and liver function markers (alanine aminotransferase, aspartate aminotransferase, creatinine, and blood urea nitrogen) were monitored postoperatively in both groups.
The postoperative infection rate in the observation group was significantly lower than that in the control group (P < 0.05). White blood cell and C-reactive protein levels decreased significantly after surgery in the observation group and were significantly lower than those in the control group (P < 0.05). Alanine aminotransferase, aspartate aminotransferase, creatinine, and blood urea nitrogen levels in the observation group were lower than those in the control group on postoperative days 1 and 3, showing a significant difference (P < 0.05).
Perioperative disinfection and isolation measures effectively reduce postoperative infection rates in gastrointestinal surgery patients and improve biochemical markers, thereby enhancing recovery outcomes. This study provides a valuable basis for postoperative infection control and has significant clinical applications.
Core Tip: Perioperative disinfection and isolation measures are crucial for infection control in gastrointestinal surgery. This study demonstrates that implementing these measures significantly reduces postoperative infection rates, lowers inflammatory markers (white blood cell count, C-reactive protein), and improves liver function and recovery outcomes. A comprehensive approach, including preoperative skin preparation, intraoperative aseptic techniques, and strict postoperative wound care, enhances patient safety, reduces complications, shortens hospital stays, and mitigates antibiotic resistance. Str
- Citation: Wang HY, Zou Y, Shi LY, Qin X, Hong LJ. Effect of perioperative disinfection and isolation measures in infection control after gastrointestinal surgery: A retrospective analysis. World J Gastrointest Surg 2025; 17(5): 102799
- URL: https://www.wjgnet.com/1948-9366/full/v17/i5/102799.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i5.102799
Postoperative infection is a common and serious complication in gastrointestinal surgery, and its incidence directly affects patients’ recovery speed, hospital stay, and medical expenses[1]. Although advances in modern medical technology have significantly reduced its occurrence, the risk of postoperative infection in gastrointestinal surgery remains high due to the exposure of gastrointestinal contents and the open nature of surgical wounds. According to statistics, the infection rate after gastrointestinal surgery ranges from 10% to 20%, including abdominal infection, incision infection, and organ space infection[2,3]. This high infection rate not only increases patients’ suffering but also places a significant burden on the medical system[4]. To effectively prevent and reduce postoperative infections, perioperative disinfection and isolation measures are essential. The perioperative period refers to the entire process from preoperative preparation to postoperative recovery and includes preoperative skin preparation, intraoperative aseptic techniques, and postoperative nursing management[5].
Studies have shown that scientifically designed disinfection and isolation protocols can significantly reduce infection rates. These protocols include preoperative bathing with antibacterial soap, the use of high-efficiency broad-spectrum disinfectants for skin disinfection, standardized sterile operating procedures in the operating room, comprehensive preventive measures, and strict postoperative wound care - all of which play a crucial role in infection control[6]. In Hainan, due to its unique geographical environment and climatic conditions, the types and drug resistance profiles of infectious pathogens after gastrointestinal surgery exhibit distinct regional variations. According to numerous clinical studies and surveillance data[7], the most common pathogens associated with postoperative infections in Hainan include Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, and Enterococcus spp. These pathogens cause infections through intraoperative contamination, wound exposure, or improper postoperative care. As one of the predominant pathogenic bacteria, Escherichia coli accounts for a large proportion of postoperative infections in gastrointestinal surgery. The strain’s drug resistance is increasingly severe, particularly against ampicillin, cephalo
This study is a retrospective analysis, and the sample size was determined based on the expected statistical power and research objectives. First, by referencing previous similar studies and clinical data, and considering the potential impact of perioperative disinfection and isolation measures on postoperative infections, the primary endpoint was set as the incidence of postoperative infection. To ensure sufficient statistical power, sample size calculations were performed using statistical software based on the expected differences in infection rates and treatment effects. Considering potential sample loss and unexpected factors, and taking into account the actual patient population in our hospital, data from 96 patients who underwent gastrointestinal surgery between January 2022 and June 2024 were collected. The patients were divided into an observation group and a control group, with 48 patients in each, ensuring reliable and representative results. Disinfection and isolation measures were implemented in the observation group during the perioperative period, while standard nursing care was provided to the control group.
The inclusion criteria were as followed: (1) Patients were aged between 18 and 75 years, with an average age of 56.82 ± 10.43 years in the observation group and 55.91 ± 9.72 years in the control group; (2) All patients underwent gastrointestinal surgery for the first time and had no severe heart, liver, or renal dysfunction before surgery; and (3) None had a history of severe infections at other sites. The exclusion criteria were as followed: (1) A total of 12 cases were excluded for being outside the eligible age range (under 18 or over 75 years old); (2) Eight patients were excluded due to serious underlying conditions such as malignant tumors or chronic kidney disease; and (3) Six patients were excluded for having received antibiotic therapy before surgery or for prior use of antibacterial drugs before the diagnosis of postoperative infection.
The observation group consisted of 28 males and 20 females, while the control group consisted of 27 males and 21 females. There was no significant difference in gender ratio between the two groups (P = 0.837). Similarly, there was no significant difference in age between the two groups (P = 0.635). Regarding surgical procedures, 29 patients in the observation group underwent open abdominal surgery, while 19 underwent laparoscopic surgery. In the control group, 31 patients underwent open abdominal surgery, and 17 underwent laparoscopic surgery. There was no significant difference in the surgical type between the two groups (P = 0.652). As for preoperative complications, 12 cases were reported in the observation group and 11 in the control group, with no statistically significant difference (P = 0.804). The results indicate that there were no significant difference in baseline characteristics between the two groups, making them comparable. All patients signed an informed consent form, and the study was approved by the Hospital Ethics Committee.
Patients in the observation group received perioperative disinfection and isolation measures. Preoperatively, antibacterial soap was used for bathing, and the entire body was disinfected with a 10% povidone-iodine solution, which has a broad-spectrum antibacterial effect capable of eliminating gram-positive and gram-negative bacteria, fungi, and some viruses. This solution is suitable for large-area skin disinfection. The 10% concentration was selected based on clinical experience, providing an effective antibacterial effect while minimizing skin irritation. The disinfection procedure lasted at least five minutes to ensure sufficient contact time for the antiseptic to act on the skin surface and penetrate the stratum corneum for optimal disinfection. A 10000-level laminar flow system was used in the operating room to maintain air cleanliness and reduce bacterial contamination. During surgery, a high-frequency electrocautery device (Valleylab) was used for surgical cutting and coagulation. To prevent contamination from gastrointestinal contents, a surgical film and incision protection device were utilized. All surgical instruments adhered to aseptic protocols, and surgeons regularly changed sterile gloves. Postoperatively, the wound was covered with sterile 3M dressings, which were changed daily. For patients at high risk of infection, the incision was washed with povidone-iodine solution, and antibacterial sutures were used. Intravenous cefmetazole (1 g every 12 hours for 2-3 days) was administered to prevent infection, as it is a broad-spectrum antibiotic effective against common pathogens such as Staphylococcus aureus and Escherichia coli, with a favorable safety profile. Additionally, patients with no risk of bleeding were administered 4000 IU of low-molecular-weight heparin sodium via subcutaneous injection once daily to prevent postoperative thrombosis. Patients were monitored daily for body temperature, white blood cell count (WBC), and C-reactive protein (CRP) levels to assess infection.
Patients in the control group received routine disinfection and isolation measures during the perioperative period. Preoperatively, the local skin was disinfected using a solution containing 75% alcohol for 2-3 minutes. This concentration has strong cell membrane permeability, effectively disrupting bacterial and viral outer membranes, leading to microbial death. The 75% alcohol solution is widely used in clinical practice due to its efficacy and minimal skin irritation. The 2-3 minutes disinfection time ensures adequate contact with the skin to eliminate pathogenic microorganisms. During surgery, the abdominal cavity was rinsed with normal saline, but no incision film or incision protection device was used. Standard sutures were applied for wound closure. Postoperatively, wounds were covered with standard gauze dressings, which were replaced once daily. In some high-risk patients, intravenous cefmetazole (1 g every 24 hours for two days) was administered to prevent infection. Antithrombotic drugs were not routinely used postoperatively. Postoperative infection was assessed based on clinical symptoms, including elevated body temperature, wound swelling, and the presence of pathogenic microorganisms. Routine monitoring of CRP and WBC levels was not performed.
Clinical observations: Patients were clinically evaluated daily, beginning on the first postoperative day, by skilled nursing staff. Monitoring parameters included body temperature, pulse, respiratory rate, and blood pressure. Additionally, signs of infection, such as fever, chills, redness, pain, and exudation, were closely observed. Postoperative recovery was documented daily through detailed nursing records, with particular attention to incision changes. The incision site was regularly assessed for pus exudation and localized swelling.
Laboratory examinations: On postoperative days 1, 3, and 7, routine blood tests were conducted. A total of 5 mL of venous blood was collected and stored in an EDTA anticoagulation tube, and WBC count was analyzed using an automated hematology analyzer. CRP detection was: A 3 mL blood sample was obtained, centrifuged, and serum was separated. CRP levels were measured using immunoturbidimetry, and microbiological examination of the samples was performed as necessary.
Imaging examination: Abdominal ultrasound combined with computed tomography was performed to evaluate postoperative complications on postoperative weeks 1, 2, and 4. Patients were required to fast for four hours before the examination to minimize the effect of intestinal gas on imaging results. Ultrasound examination was: A high-frequency ultrasonic probe was used with an applied coupling agent. An appropriate probe was selected, and the operator systematically scanned all regions of the abdomen to assess for fluid accumulation or masses. Any abnormalities, particularly ascites and abscesses, were recorded. If necessary, contrast media was administered according to the physician’s instructions. Multi-slice abdominal images were acquired using spiral computed tomography, with special attention to postoperative changes. A radiologist analyzed the images to identify complications, including ascites, abscess formation, and organ damage.
Other biochemical indicators: On postoperative days 1 and 3, liver and renal function tests were conducted to evaluate systemic conditions. Levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total protein (TP), albumin (ALB), creatinine (Cr), and blood urea nitrogen (BUN) were measured using a fully automated biochemical analyzer.
SPSS 25.0 software was used for t-tests and χ2-tests to analyze differences in treatment indicators between groups. A significance level of α = 0.05 was set. Statistical methods were employed to assess the reliability of the experimental results and provide a scientific basis for the study’s conclusions.
The average body temperature of patients in the observation group was significantly lower than that in the control group. Pulse and respiratory rate measurements further indicated that postoperative recovery in the observation group was more stable, with faster recovery across clinical indicators. The incidence of infection in the observation group was significantly lower than that in the control group (P < 0.05) (Table 1).
| Time period | Group | Body temperature, °C | Pulse, beats/minutes | Respiratory rate, breaths/minutes | Incision infection, % | P value |
| Postoperative day 1 | Observation group | 37.52 ± 0.43 | 85.11 ± 10.23 | 18.32 ± 3.17 | 10.42 | < 0.05 |
| Control group | 37.83 ± 0.51 | 90.23 ± 12.11 | 20.11 ± 4.24 | 25.13 | < 0.05 | |
| Postoperative day 3 | Observation group | 37.22 ± 0.30 | 80.15 ± 9.17 | 17.01 ± 2.31 | 4.17 | < 0.05 |
| Control group | 37.41 ± 0.42 | 85.32 ± 11.01 | 19.20 ± 3.15 | 16.67 | < 0.05 | |
| Postoperative day 7 | Observation group | 36.82 ± 0.21 | 78.23 ± 8.10 | 16.09 ± 2.17 | 4.17 | < 0.05 |
| Control group | 37.13 ± 0.36 | 82.02 ± 9.03 | 18.33 ± 3.24 | 10.42 | < 0.05 |
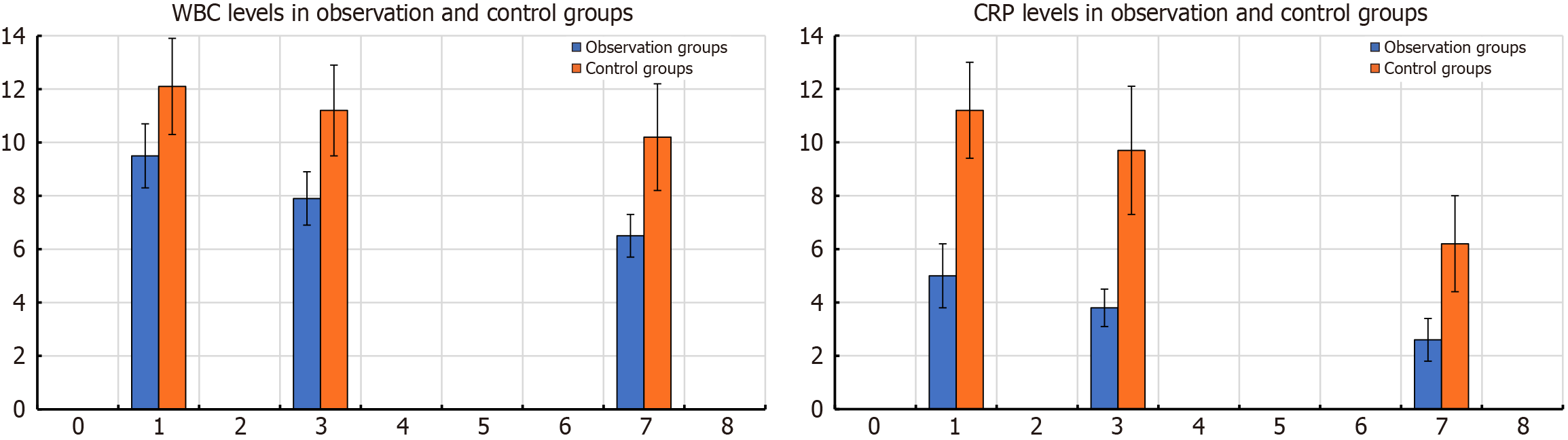
The laboratory results of the observation and control groups at each postoperative time point showed significant differences (P < 0.001). The WBC count in the observation group was significantly lower than that in the control group on postoperative days 1, 3, and 7, suggesting a significantly reduced risk of infection (Table 2 and Figure 1).
| Indicators | Observation group (n = 48) | Control group (n = 48) | P value | t |
| WBC on postoperative day 1, × 109/L | 9.25 ± 1.14 | 12.04 ± 1.55 | < 0.001 | 10.046 |
| WBC on postoperative day 3, × 109/L | 7.82 ± 1.03 | 11.02 ± 1.47 | < 0.001 | 12.352 |
| WBC on postoperative day 7, × 109/L | 6.41 ± 0.85 | 9.67 ± 1.13 | < 0.001 | 15.973 |
| CRP on postoperative day 1, mg/L | 4.83 ± 1.52 | 11.03 ± 2.06 | < 0.001 | 16.779 |
| CRP on postoperative day 3, mg/L | 3.52 ± 1.06 | 9.52 ± 2.51 | < 0.001 | 15.257 |
| CRP on postoperative day 7, mg/L | 2.50 ± 0.73 | 6.19 ± 1.55 | < 0.001 | 14.922 |
The imaging results of the observation and control groups at each postoperative time point demonstrated significant differences (P < 0.05). In the first postoperative week, the incidence of complications in the observation group was 6.25% (3/48), compared to 14.58% (7/48) in the control group, with a P value < 0.05, indicating a significant advantage in postoperative complication control in the observation group (Table 3).
| Indicators | Observation group (n = 48) | Control group (n = 48) | P value | χ2 |
| Incidence of complications in the first postoperative week | 6.25 (3/48) | 14.58 (7/48) | 0.181 | |
| Incidence of complications in the second postoperative week | 4.17 (2/48) | 10.42 (5/48) | 0.432 | |
| Incidence of complications in the fourth postoperative week | 2.08 (1/48) | 6.25 (3/48) | 0.610 | |
| 0.037 | 4.350 | |||
| Incidence of intraperitoneal effusion in the first week after surgery | 2.08 (1/48) | 12.5 (6/48) | 0.049 | |
| Incidence of intraperitoneal effusion in the second week after surgery | 0.0 (0/48) | 6.25 (3/48) | 0.083 | |
| Incidence of intraperitoneal effusion in the fourth week after surgery | 0.0 (0/48) | 4.17 (2/48) | 0.153 | |
| 0.007 | 7.265 | |||
| Abscess formation rate in the first postoperative week | 0.0 (0/48) | 8.33 (4/48) | 0.041 | |
| Abscess formation rate in the second postoperative week | 0.0 (0/48) | 4.17 (2/48) | 0.153 | |
| Abscess formation rate in the 4th postoperative week | 0.0 (0/48) | 2.08 (1/48) | 0.312 | |
| 0.008 | 7.137 |
The levels of ALT, AST, Cr, and BUN in the observation group were significantly lower than those in the control group on postoperative days 1 and 3 (P < 0.05), suggesting a significantly reduced risk of postoperative complications. The levels of TP and ALB in the observation group were also relatively higher, reflecting better nutritional status in these patients (Table 4).
| Indicators | Observation group, n = 48, the first day after surgery | Control group, n = 48, the first day after surgery | P value | t | Observation group, n = 48, the third day after surgery | Control group, n = 48, the third day after surgery | P value | t |
| ALT (U/L) | 22.03 ± 3.02 | 28.05 ± 4.09 | < 0.001 | -8.204 | 19.05 ± 2.53 | 25.03 ± 5.03 | < 0.001 | -7.358 |
| AST (U/L) | 30.03 ± 4.05 | 40.04 ± 5.02 | < 0.001 | -10.752 | 27.08 ± 3.53 | 35.00 ± 6.04 | < 0.001 | -7.843 |
| TP (g/L) | 70.12 ± 6.34 | 65.34 ± 7.12 | 0.001 | 3.474 | 68.45 ± 5.89 | 63.20 ± 8.00 | < 0.001 | 3.661 |
| ALB (g/L) | 40.25 ± 4.12 | 35.75 ± 5.67 | < 0.001 | 4.448 | 39.00 ± 3.85 | 34.50 ± 6.00 | < 0.001 | 4.373 |
| Cr (μmol/L) | 68.07 ± 9.08 | 82.02 ± 10.07 | < 0.001 | -7.128 | 62.03 ± 7.02 | 75.02 ± 9.03 | < 0.001 | -7.868 |
| BUN (mmol/L) | 4.54 ± 0.65 | 5.81 ± 0.84 | < 0.001 | -8.284 | 4.06 ± 0.53 | 5.54 ± 0.77 | < 0.001 | -10.969 |
The incidence of postoperative infection, as well as WBC and CRP levels, were significantly lower in the observation group than in the control group. Studies have shown that postoperative infection is closely associated with the body’s immune response, particularly because surgical trauma can induce both local and systemic inflammation[9,10]. Disinfection and isolation measures play a crucial role in reducing the risk of wound infection by decreasing the microbial load in the surgical area[11]. Strict perioperative disinfection, adherence to aseptic techniques, and postoperative isolation significantly limit the spread and proliferation of pathogenic bacteria at the surgical site, thereby reducing the likelihood of infection. This result aligns with existing research and reinforces the importance of perioperative bacterial infection control[12,13]. The changes in TP and ALB levels further reflected the overall health status of patients in the observation group. During postoperative recovery, immune suppression is a common complication, which not only slows the recovery process but also increases the risk of infection[14]. In the observation group, TP and ALB levels improved significantly after surgery, suggesting that disinfection and isolation measures effectively reduced postoperative complications such as infection and bleeding, thereby minimizing nutritional depletion and supporting red blood cell production. Moreover, studies have demonstrated that postoperative infection is directly associated with suppressed leukocyte production[15]. Therefore, infection control through disinfection and isolation measures helps maintain normal leukocyte levels, promoting a more effective recovery. Changes in liver function indicators revealed that postoperative ALT and AST levels were significantly lower in the observation group than in the control group, suggesting that disinfection and isolation measures played a protective role in liver function after surgery[16]. Postoperative infection and the associated systemic inflammatory response syndrome are known contributors to liver damage. The key mechanism underlying this effect is that the systemic inflammatory response induced by infection can activate hepatocyte apoptotic pathways, leading to liver injury. Strict perioperative disinfection measures reduce the incidence of postoperative infection and, consequently, the risk of liver damage[17,18]. Relevant studies have reported that controlling postoperative infection not only alleviates hepatic burden but also promotes liver function recovery by suppressing liver inflammation[19,20]. Cr and BUN are key indicators of renal function, and both markers were significantly lower in the observation group than in the control group after surgery, indicating better renal function in this group. Research has demonstrated that early fluid management and the appropriate use of diuretics can significantly enhance renal perfusion, reducing the risk of postoperative renal injury[21]. As a result, these interventions alleviate postoperative symptoms and improve infection control.
Studies have demonstrated that strict preoperative disinfection significantly reduces the microbial load on the skin and in the surgical area. For instance, antibacterial soap baths, which commonly contain antimicrobial agents such as chlorhexidine or povidone-iodine, are effective in reducing bacterial colonization on the skin surface. Antibacterial soap inhibits bacterial growth and replication by disrupting bacterial cell membranes. Additionally, its prolonged antimicrobial activity helps prevent the transmission of exogenous pathogens during surgery. Research has shown that the mechanism of antibacterial soap action involves inhibiting bacterial surface protein synthesis, compromising bacterial membrane integrity, and altering cell permeability, thereby significantly reducing the risk of intraoperative and postoperative infections[22]. Perioperative disinfection and isolation measures not only reduce the risk of local infection by eliminating microorganisms but also mitigate the systemic inflammatory response caused by bacterial infections. Studies have shown that postoperative infections exacerbate the body’s metabolic burden and contribute to organ dysfunction by triggering systemic inflammatory response syndrome[23]. The significant reduction in postoperative infection rates, WBC count, and CRP levels in the observation group highlights the critical role of strict disinfection and isolation measures in controlling systemic inflammatory responses. Further mechanistic studies are needed to investigate how disinfection and isolation measures alleviate the postoperative immune system burden, thereby promoting recovery and reducing complications. Although this study ensured a relatively balanced research sample through strict inclusion and exclusion criteria, potential result deviations may still exist due to incomplete data records or selection bias. Additionally, retrospective analyses cannot control for potential confounding factors such as preoperative health status, immune function, or variations in surgical techniques, all of which may significantly influence the incidence of postoperative infections. Future research should incorporate prospective randomized controlled trials to more accurately assess the impact of different perioperative disinfection and isolation strategies on postoperative infection. By randomly assigning patients to distinct disinfection and isolation protocols, the effect of each intervention can be evaluated with greater precision. Furthermore, a prospective study design would allow better control of confounding factors and enable long-term follow-up to assess the sustained effects of disinfection and isolation measures on postoperative recovery, complications, and quality of life. This approach would enhance the generalizability and effectiveness of these measures across different surgical procedures and patient populations.
Based on existing literature and the findings of this study, perioperative disinfection and isolation measures play a crucial role in infection control following gastrointestinal surgery. These measures effectively reduce microbial load, mitigate systemic inflammatory responses, protect liver function, and maintain platelet stability. Future research should focus on optimizing disinfection and isolation strategies tailored to different surgical types and individual patient variations to further enhance postoperative management and improve patient outcomes.
| 1. | Han C, Chen W, Ye XL, Cheng F, Wang XY, Liu AB, Mu ZH, Jin XJ, Weng YH. Risk factors analysis of surgical site infections in postoperative colorectal cancer: a nine-year retrospective study. BMC Surg. 2023;23:320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Bassetti M, Eckmann C, Giacobbe DR, Sartelli M, Montravers P. Post-operative abdominal infections: epidemiology, operational definitions, and outcomes. Intensive Care Med. 2020;46:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 3. | Liu S, Miao J, Wang G, Wang M, Wu X, Guo K, Feng M, Guan W, Ren J. Risk factors for postoperative surgical site infections in patients with Crohn's disease receiving definitive bowel resection. Sci Rep. 2017;7:9828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Hutfless S. Endoscope infection transmission state-of-the-art: beyond duodenoscopes to a culture of infection prevention. Curr Opin Gastroenterol. 2020;36:366-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Jin B, Hu Y, Huang L, Cheng X, Zhao J, Yang X, Sun X, Gan T, Lu B. Effectiveness Between Daily and After-Each-Case Room Disinfection of the Endoscopy Unit. Front Public Health. 2021;9:700041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 6. | Lascaris B, Thorne AM, Lisman T, Nijsten MWN, Porte RJ, de Meijer VE. Long-term normothermic machine preservation of human livers: what is needed to succeed? Am J Physiol Gastrointest Liver Physiol. 2022;322:G183-G200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | GlobalSurg Collaborative. Surgical site infection after gastrointestinal surgery in high-income, middle-income, and low-income countries: a prospective, international, multicentre cohort study. Lancet Infect Dis. 2018;18:516-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 301] [Cited by in RCA: 306] [Article Influence: 43.7] [Reference Citation Analysis (1)] |
| 8. | Owusu E, Asane FW, Bediako-Bowan AA, Afutu E. Bacterial Contamination of Surgical Instruments Used at the Surgery Department of a Major Teaching Hospital in a Resource-Limited Country: An Observational Study. Diseases. 2022;10:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Wilhelm D, Vogel T, Jell A, Brunner S, Kranzfelder M, Wantia N, Feussner H, Ostler D, Koller S. MIEO: a micro-invasive endoscopic operation port system for transluminal interventions-an acute and survival porcine study. Surg Endosc. 2020;34:2814-2823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 10. | Mozgova Y, Mishyna M, Syplyviy V, Ievtushenko O, Ievtushenko D, Marchenko I, Mishyn Y. Microbiological analysis of abdominal cavity exudate, blood and affected tissues samples from patients with intra-abdominal abscesses in complicated infection of abdominal cavity. Wiad Lek. 2023;76:1717-1724. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Xu Y, Shi C, Liu Y. Application effect of PDCA circulation on nursing quality management and risk control in digestive endoscopy room. Medicine (Baltimore). 2023;102:e35885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 12. | Baek SH, Kang JM, Ihn K, Han SJ, Koh H, Ahn JG. The Epidemiology and Etiology of Cholangitis After Kasai Portoenterostomy in Patients With Biliary Atresia. J Pediatr Gastroenterol Nutr. 2020;70:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Huang Q, Liu G, Wang J, Duan T, Feng Y, Lin X, Zhu Y, Wang H, Cui Y, He S, Zhu Y, Li P, Rong L, Liu Y. Control measures to prevent Coronavirus disease 2019 pandemic in endoscopy centers: Multi-center study. Dig Endosc. 2020;32:914-920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Torrance HDT, Longbottom ER, Vivian ME, Lalabekyan B, Abbott TEF, Ackland GL, Hinds CJ, Pearse RM, O'Dwyer MJ. Post-operative immune suppression is mediated via reversible, Interleukin-10 dependent pathways in circulating monocytes following major abdominal surgery. PLoS One. 2018;13:e0203795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Ferrie S, Webster A, Wu B, Tan C, Carey S. Gastrointestinal surgery and the gut microbiome: a systematic literature review. Eur J Clin Nutr. 2021;75:12-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Vining CC, Skowron KB, Hogg ME. Robotic gastrointestinal surgery: learning curve, educational programs and outcomes. Updates Surg. 2021;73:799-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Brajcich BC, Ko CY, Liu JB, Ellis RJ, D'Angelica MI. A NSQIP-Based Randomized Clinical Trial Evaluating Choice of Prophylactic Antibiotics for Pancreaticoduodenectomy. Cancer Treat Res. 2024;192:131-145. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Longbottom ER, Torrance HD, Owen HC, Fragkou PC, Hinds CJ, Pearse RM, O'Dwyer MJ. Features of Postoperative Immune Suppression Are Reversible With Interferon Gamma and Independent of Interleukin-6 Pathways. Ann Surg. 2016;264:370-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Prochazka Zá Rate RA, Cabrera Cabrejos MC, Piscoya A, Vera Calderón AF. [Recommendations of the Society of Gastroenterology of Peru to avoid the spread of SARS-CoV-2 through digestive endoscopy procedures]. Rev Gastroenterol Peru. 2020;40:95-99. [PubMed] |
| 20. | Jia W, Liu W, Qiao X. Chinese Expert Consensus on Enhanced Recovery After Hepatectomy (Version 2017). Asian J Surg. 2019;42:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Sakamoto T, Goto T, Fujiogi M, Kawarai Lefor A. Machine learning in gastrointestinal surgery. Surg Today. 2022;52:995-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Lepelletier D, Maillard JY, Pozzetto B, Simon A. Povidone Iodine: Properties, Mechanisms of Action, and Role in Infection Control and Staphylococcus aureus Decolonization. Antimicrob Agents Chemother. 2020;64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |