Published online Mar 27, 2025. doi: 10.4240/wjgs.v17.i3.99782
Revised: December 9, 2024
Accepted: January 11, 2025
Published online: March 27, 2025
Processing time: 116 Days and 1.2 Hours
Colorectal cancer (CRC) is a malignant tumor with high morbidity and mortality rates worldwide. With the development of medical imaging technology, imaging features are playing an increasingly important role in the prognostic evaluation of CRC. Laparoscopic radical resection is a common surgical approach for treating CRC. However, research on the link between preoperative imaging and short-term prognosis in this context is limited. We hypothesized that specific preope
To investigate the imaging features of CRC and analyze their correlation with the short-term prognosis of laparoscopic radical resection.
This retrospective study conducted at the Affiliated Cancer Hospital of Shandong First Medical University included 122 patients diagnosed with CRC who under
Among 122 patients, 22 had irregular, low-intensity tumors with adjacent high signals. In 55, tumors were surrounded by alternating signals in the muscle layer. In 32, tumors extended through the muscular layer and blurred boundaries with perienteric adipose tissue. Tumor signals appeared in the adjacent tissues in 13 patients with blurred gaps. Logistic regression revealed differences in longitudinal tumor length, axial tumor length, volume transfer constant, plasma volume fraction, and apparent diffusion coefficient among patients with varying prognostic results. ROC analysis indicated that the areas under the curve for these parameters were 0.648, 0.927, 0.821, 0.809, and 0.831, respectively. Sensitivity values were 0.643, 0.893, 0.607, 0.714, and 0.714, and specificity 0.702, 0.904, 0.883, 0.968, and 0.894 (P < 0.05).
The imaging features of CRC correlate with the short-term prognosis following laparoscopic radical resection. These findings provide valuable insights for clinical decision-making.
Core Tip: Colorectal cancer (CRC) is recognized as a highly prevalent and deadly malignant tumor that affects many patients globally. This study analyzed preoperative magnetic resonance imaging (MRI) data and 30-day postoperative prognostic indicators by using logistic regression and receiver operating characteristic curves. The key findings highlight the role of MRI in predicting short-term outcomes after laparoscopic radical resection, emphasizing the importance of imaging-derived indicators. These insights enhance the prognostic accuracy and guide tailored treatment approaches for patients with CRC.
- Citation: Fang ZH, Hao AH, Qi YG. Imaging features and correlation with short-term prognosis in laparoscopic radical resection of colorectal cancer. World J Gastrointest Surg 2025; 17(3): 99782
- URL: https://www.wjgnet.com/1948-9366/full/v17/i3/99782.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i3.99782
Colorectal cancer (CRC), a prevalent malignant tumor of the digestive system, often initially manifests as subtle changes in bowel habits, diarrhea, and hematochezia. As the condition progresses, patients may exhibit systemic symptoms, including weight loss and anemia. Poor lifestyle, genetic predisposition, and intestinal inflammation contribute to its development[1,2]. With recent developments and the popularization of laparoscopic technology, laparoscopic radical resection has become a common surgical method for treatment[3,4]. However, the prognoses of these patients vary signi
Between January 2021 and February 2024, 122 patients diagnosed with CRC who underwent radical laparoscopic surgery at the Affiliated Cancer Hospital of Shandong First Medical University were included in this study. Postoperative ima
Eligibility for the study was determined using the following criteria: First, participants had not undergone radio
A 1.5T MRI system equipped with an 8-channel phased-array body coil was used in this study. The participants were placed in the supine position with their feet forward during the procedure. Initial tumor localization was performed, followed by sequential imaging in both the cross-sectional and coronal planes with the tumor as the focal point. The technical parameters for the scans were as follows: 5 mm slice thickness with a corresponding 5 mm gap. For T1-weighted imaging, the parameters included a repetition time (TR) of 620 millisecond and an echo time (TE) of 6 millisecond within a scanning field of view (FOV) of 32 cm × 32 cm. Axial T2-weighted imaging (T2WI) without fat suppression featured a TR of 2600 millisecond and (TE) of 80 millisecond, with a FOV of 32 cm × 32 cm. Sagittal T2WI without fat suppression had a TR of 3400 millisecond and a TE of 120 millisecond within a FOV of 26 cm × 26 cm. Finally, coronal and oblique axial T2WI without fat suppression were acquired with a TR of 3400 millisecond and TE of 120 millisecond, with a FOV of 20 cm × 20 cm.
The axial tumor length (ATL), longitudinal tumor length (LTL), ATL/LTL ratio, apparent diffusion coefficient (ADC), lymph node metastasis, and tumor circumferential resection margin (CRM) were measured and recorded. Lymph nodes with short diameters greater than 8 mm or less than 8 mm but with irregular morphology, rough edges, uneven signals, and high DWI signals were defined as positive for lymph node metastasis (+); otherwise, lymph node metastasis was defined as negative (-). When the distance between the primary tumor and malignant structures, such as metastatic lymph nodes in the mesorectum, cancer nodules, extra-rectal vascular invasion, mesorectal fascia, and adjacent struc
Prognostic indicators included perioperative indicators (operation time, perioperative bleeding, first exhaust time, first defecation time, eating recovery time, and hospital stay) and postoperative complications (incision infection, anastomotic fistula, anastomotic bleeding, ileus, abdominal infection, and pulmonary infection).
Statistical analyses were performed using SPSS version 23.0. Categorical data are presented as the frequency of occurrence (n) and percentage (%). The χ2 test was used to examine the differences in proportions between the groups. Additionally, we performed a t-test to evaluate and compare the quantitative data between the two groups. To assess the predictive value of the imaging characteristics for the outcomes of patients diagnosed with CRC, we performed receiver operating characteristic (ROC) curve analysis. Statistical significance was set at P < 0.05.
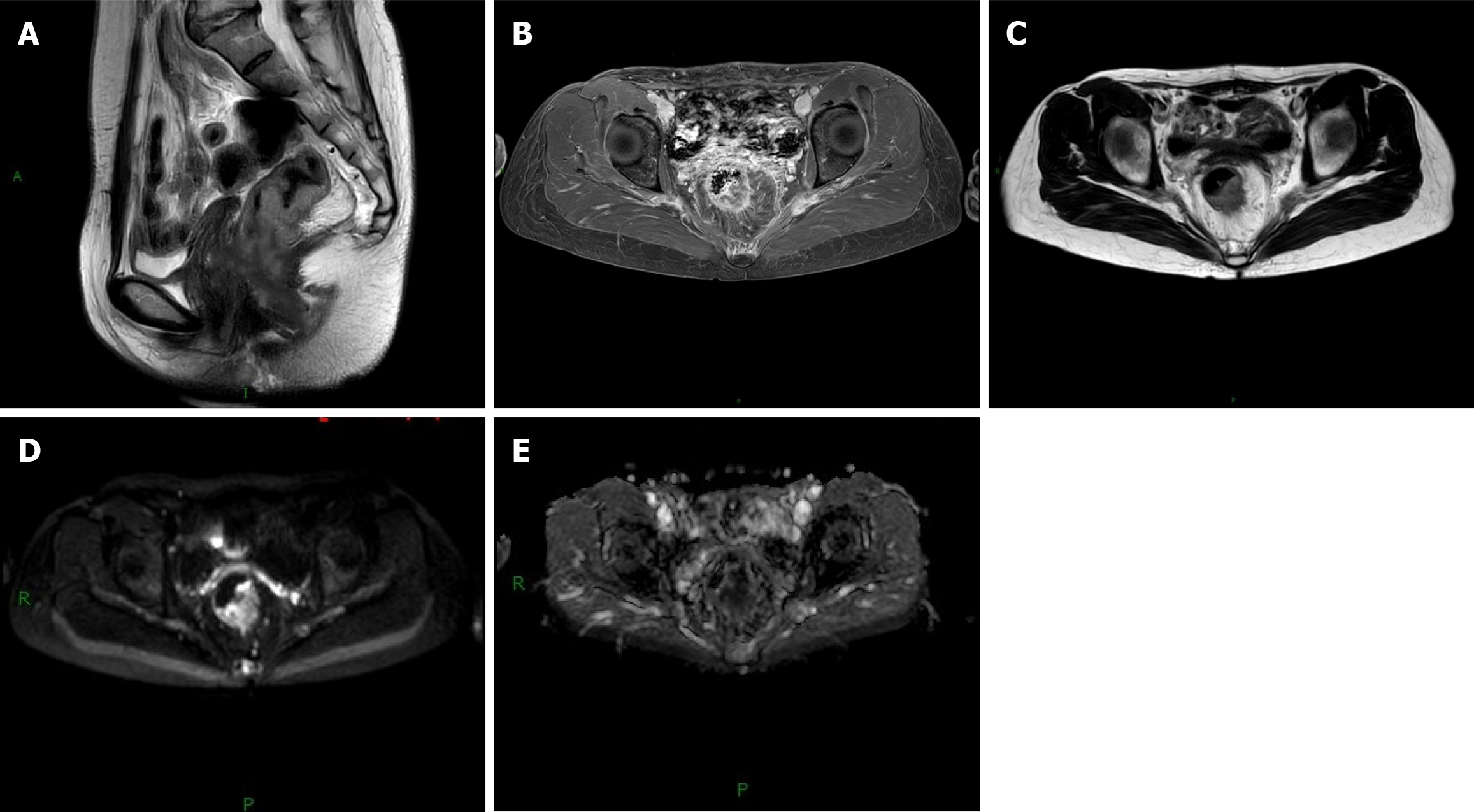
Among 122 cases of CRC, 22 showed low signal intensity and irregularly shaped tumor lesions, whereas the adjacent mucosa and submucosa had relatively high signal intensities. In 55 cases, alternating low and high signals of the muscle layer surrounded the tumor lesions, with the low signal of the muscle layer appearing as a complete continuous ring (of these, 46 cases of tumor lesions had rough edges or uneven signals, and nine cases of lymph nodes on MRI images showed obvious high signals). In 32 cases, the tumor signal passed through the muscular layer, and the boundary between the muscular layer and the perienteric adipose tissue was blurred (among these cases, 7 showed a high signal in the perienteric adipose tissue, which was mostly tortuous or nodular). Tumor signals were found in 13 adjacent tissues and organs, and the fat space between the adjacent tissues and organs was blurred (among these cases, three lymph nodes showed obvious high signals on MRI). Figure 1 shows the MRI findings of the patient diagnosed with rectal cancer.
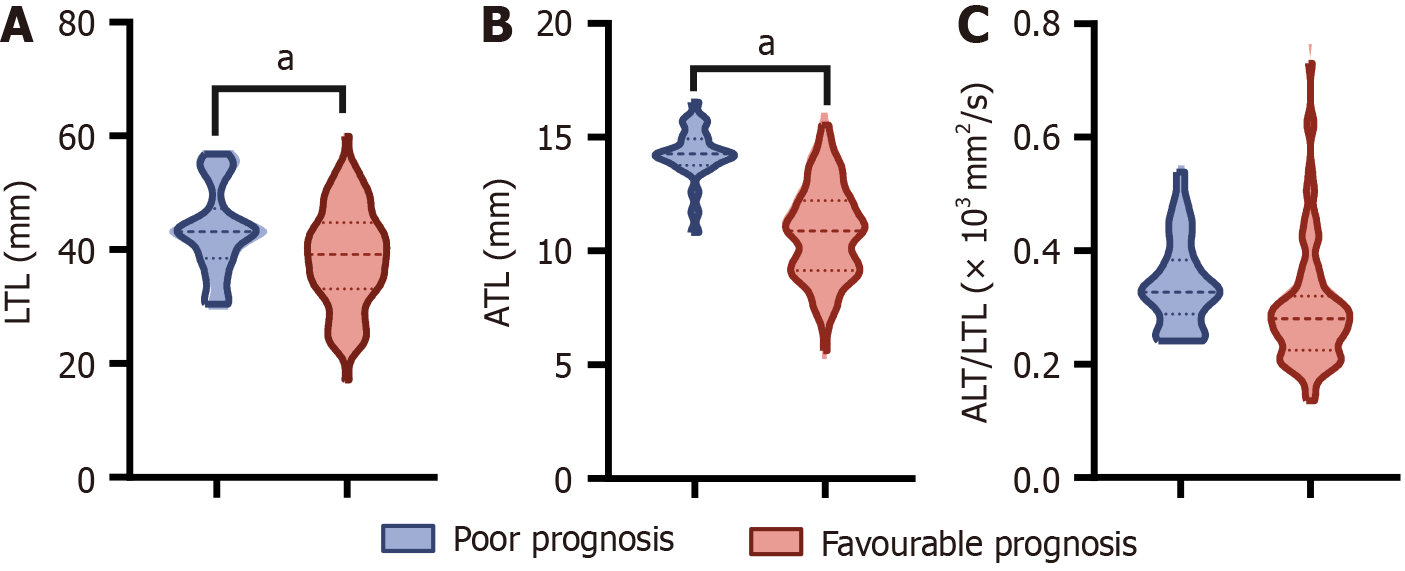
In the present study, we evaluated the radiographic characteristics of patients and discovered a significant difference in the average LTL and ATL between patients with poor and good prognoses, with the former exhibiting higher values (P < 0.05). In addition, although we also compared ATL/LTL, lymph node metastasis, and CRM between the two groups of patients, the differences between these indicators were not statistically significant (P > 0.05). This finding may indicate that these parameters play a limited role in predicting the prognosis of the disease or that more in-depth research is needed in future studies to reveal their potential relationship with disease prognosis (Figures 2 and 3).
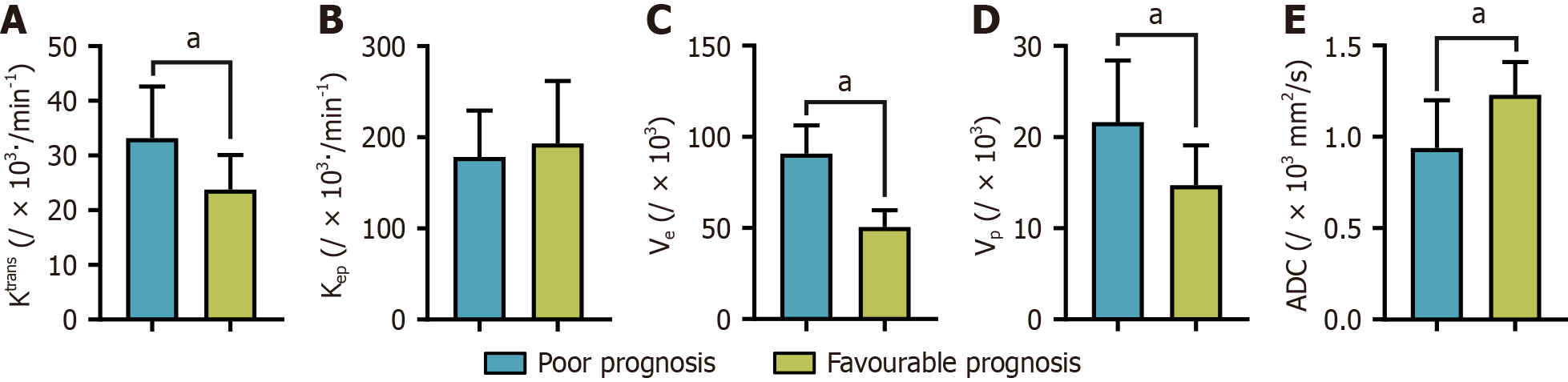
We conducted an in-depth analysis of the imaging parameters across the two patient groups to identify any potential disparities associated with varying disease prognoses. However, Kep did not show statistically significant variation between the different prognostic groups (P > 0.05), suggesting its limited efficacy in differentiating the prognosis of patients with CRC. Nonetheless, we observed substantial differences in the other imaging parameters, specifically Ktrans, Ve, Vp, and ADC (P < 0.05). These significant variations imply that these parameters may be crucial for the prognostic assessment of CRC. Figure 4 provides a visual representation of the disparities in Ktrans, Ve, Vp, and ADC values between the two patient groups to present these findings clearly.
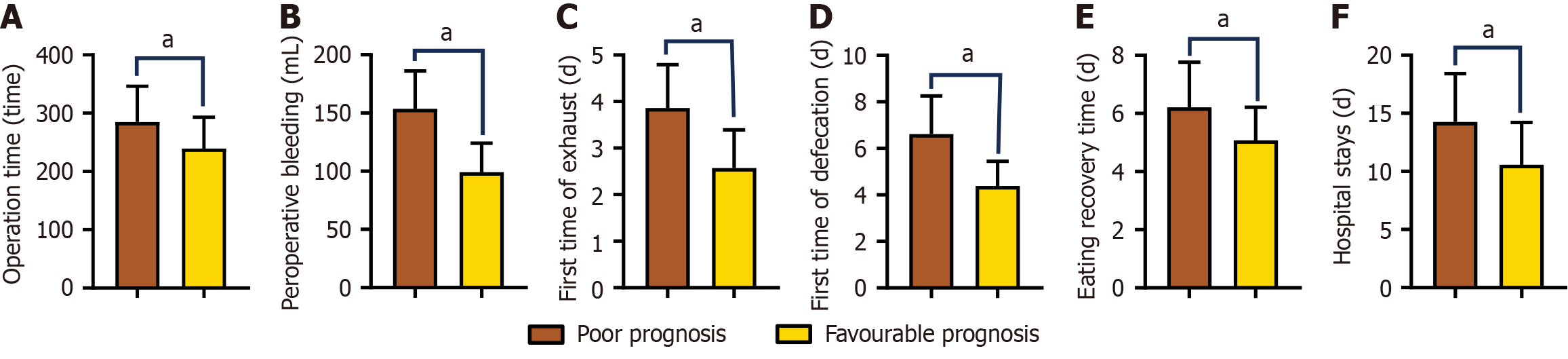
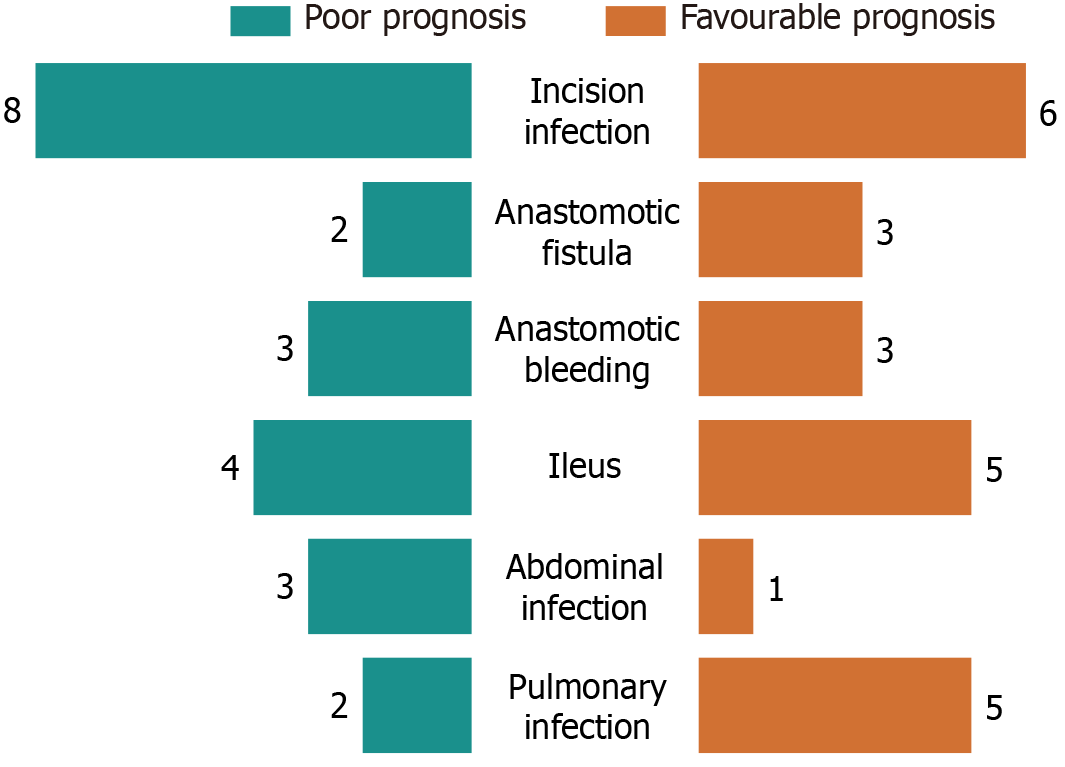
In our analysis of the prognostic factors in patients with CRC, we identified significant disparities in various clinical indicators related to patient outcomes. Specifically, there were pronounced differences in operation time, perioperative bleeding, first exhaust, first defecation, eating recovery period, and hospital stay between patients with contrasting prognoses (P < 0.05). Patients with a favorable prognosis had an average hospital stay of approximately 10.57 days, whereas those with an unfavorable prognosis experienced prolonged hospitalization, averaging 14.25 days. Furthermore, the rate of postoperative complications varied significantly between the two groups. Patients with good prognosis had an average complication rate of approximately 3.95%, whereas those with poor prognosis had a markedly higher rate of 12.64%. To illustrate these differences more vividly, Figures 5 and 6 depict the variations in the perioperative indices and complication rates among patients with different prognoses.
In the logistic regression analysis, the primary outcome of interest was the patient prognosis at the 30-day mark after surgery. For this analysis, a favorable outcome was recorded as ‘0’, whereas an unfavorable outcome was recorded as ‘1’. The independent variables considered included LTL, ATL, ADC, Ktrans, Ve, and Vp, all of which showed significant variance in the initial assessment of imaging features in the univariate analysis. A binary logistic regression model was constructed using these variables to examine their associations with the dependent variable. These findings indicate that LTL, ATL, ADC, Ktrans, and Vp are predictive factors for short-term postoperative prognosis following laparoscopic radical resection. For the detailed results, please refer to Tables 1 and 2.
| Index | Assignment method |
| LTL | Actual value entry |
| ATL | Actual value entry |
| ADC | Actual value entry |
| Ktrans | Actual value entry |
| Ve | Actual value entry |
| Vp | Actual value entry |
| Index | β | SE | Wald | P value | OR | 95%CI |
| LTL | -0.190 | 0.067 | 8.093 | 0.004 | 0.827 | 0.726-0.943 |
| ATL | 1.397 | 0.372 | 14.065 | < 0.001 | 4.042 | 1.948-8.386 |
| ADC | -7.359 | 1.737 | 17.959 | < 0.001 | 0.001 | 0.000-0.019 |
| Ktrans | 0.185 | 0.063 | 8.598 | 0.003 | 1.203 | 1.063-1.361 |
| Ve | -0.043 | 0.047 | 0.840 | 0.359 | 0.958 | 0.873-1.051 |
| Vp | -.260 | 0.116 | 5.050 | 0.025 | 0.771 | 0.614-0.967 |
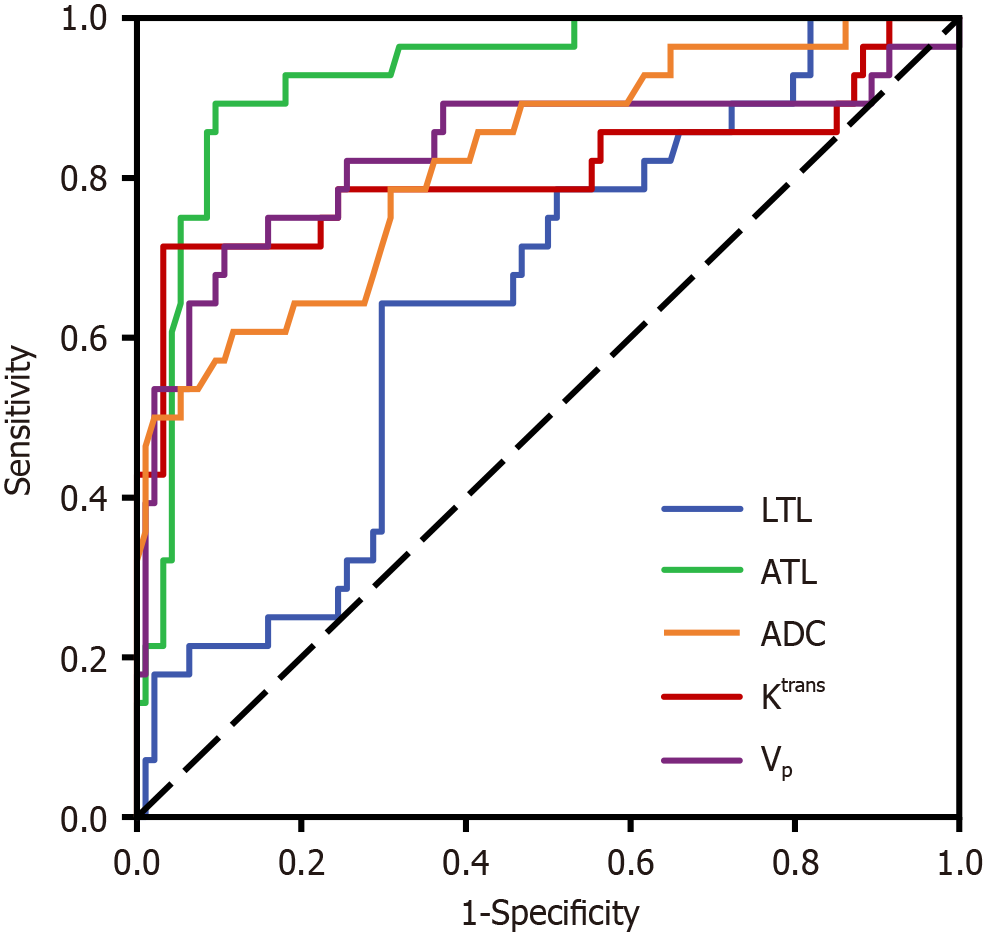
The ROC curve results showed that LTL, ATL, ADC, Ktrans, and Vp had predictive values for short-term prognosis of laparoscopic radical resection (P < 0.05), with AUC of 0.648, 0.927, 0.821, 0.809, and 0.831, respectively. The optimal cutoff value for LTL was 43.120, with a predictive sensitivity of 0.643 and specificity of 0.702 (P = 0.017). For ATL, the optimal cutoff value was 13.580, predictive sensitivity was 0.893, and specificity was 0.904 (P < 0.001). ADC had an optimal cutoff value of 0.370, with a predictive sensitivity of 0.607 and specificity of 0.883 (P < 0.001). Ktrans had an optimal cutoff value of 32.090, with a predictive sensitivity of 0.714 and specificity of 0.968 (P < 0.001). The optimal cutoff value of Vp was 19.950, with a predictive sensitivity of 0.714 and specificity of 0.894 (P < 0.001). Further details are provided in Table 3 and Figure 7.
| Index | AUC | Cutoff | SE | Sensitivity | Specificity | P value | 95%CI |
| LTL | 0.648 | 43.120 | 0.057 | 0.643 | 0.702 | 0.017 | 0.538-0.759 |
| ATL | 0.927 | 13.580 | 0.027 | 0.893 | 0.904 | < 0.001 | 0.875-0.979 |
| ADC | 0.821 | 0.370 | 0.048 | 0.607 | 0.883 | < 0.001 | 0.727-0.915 |
| Ktrans | 0.809 | 32.090 | 0.061 | 0.714 | 0.968 | < 0.001 | 0.689-0.928 |
| Vp | 0.831 | 19.950 | 0.056 | 0.714 | 0.894 | < 0.001 | 0.721-0.940 |
CRC is the third most prevalent malignancy globally and poses a significant threat to human health. In 2020, approximately 1.93 million individuals were diagnosed with the disease globally. The mortality rate associated with CRC is surpassed by that of lung cancer, making it the second leading cause of cancer-related deaths globally[7]. Patients often do not exhibit distinct symptoms during the initial stages of CRC development. However, as the tumor progresses and enlarges, patients may experience a growing frequency of bowel movements, a sensation of incomplete bowel evacuation, and potentially develop conditions such as intestinal narrowing or complete bowel obstruction. Additionally, it is common for patients to present with general symptoms such as diminished appetite and significant weight loss[8,9]. Clinical research indicates that nearly 85% of individuals with early-stage rectal cancer present with hematochezia as the sole clinical sign. However, these symptoms are frequently disregarded by patients, leading to delays in seeking medical intervention[10]. As imaging diagnostics continues to advance, the spatial resolution achievable using MRI technology has steadily improved. Consequently, an increasing array of functional MRI techniques are being integrated into clinical diagnostic practices[11,12].
Variations in MRI parameters can profoundly affect study outcomes, including the potential to degrade image quality and data reliability, produce artifacts and measurement errors, compromise the accuracy of tumor microvascular assess
Laparoscopic surgery is a prevalent and minimally invasive approach for the treatment of CRC that offers benefits such as reduced patient trauma and quicker postoperative recovery. In cases of early to moderately advanced CRC, this surgical method has the potential to achieve a complete cure[15]. Despite the effectiveness of laparoscopic procedures in enhancing patient survival rates, the risks of postoperative metastasis and tumor recurrence persist and can significantly affect patient outcomes[16]. Our study findings indicate that patients with poor prognoses had notably higher LTL and ATL measurements than those with favorable prognoses. This finding suggests that elevated LTL and ATL values detected on MRI are indicative of a higher risk of unfavorable prognosis in patients with CRC. Elevated LTL and ATL levels may also reflect a more extensive tumor burden, which could be associated with rapid tumor growth and lower tumor differentiation grade, among other factors. Results of Hajibandeh et al[17] showed that, in patients with colon cancer, tumor size was an independent predictor of the number of harvested lymph nodes, positive lymph nodes, and lymphocytic infiltration. In tumor biology, the volume and growth rate of tumors is intrinsically linked to their proliferation. A larger tumor volume may suggest a higher tumor cell load and more complex tumor microenvironment, which can increase the complexity of surgery and extend postoperative recovery times. Tumor size is a crucial indicator of tumor cell proliferation. Increased tumor volume often implies heightened proliferation activity among tumor cells, typically associated with the invasiveness and metastatic potential of the tumor[18]. The results of Dai et al[19] showed that tumor size is an independent factor for overall survival and disease-free survival in patients with infiltrative colo
Within the scope of this study, we observed that patients with a poor prognosis exhibited significantly elevated levels of Ktrans, Ve, and Vp than those with a more favorable outcome. Conversely, the ADC values were markedly lower in the former group. Our analysis suggests that patients with CRC with a negative outlook tend to have less mature vascular development and a reduced muscular component, resulting in increased vascular permeability of the tumor. This heightened permeability allows for rapid infiltration of contrast medium into the interstitial space of the tumor. In contrast, patients with a favorable prognosis have tumor vascular permeability closer to normal levels, accounting for the relatively higher Ktrans, Ve, and Vp values observed in patients with poor prognoses[20,21]. Ktrans is an indicator of vascular permeability, blood flow, and microvascular density and provides insight into the rate at which the contrast agent diffuses from the vascular space into the extravascular extracellular space. Elevated Ktrans levels indicated a higher malignant grade of the lesion. The Ktrans value is intricately linked to tumor angiogenesis, which is the key connection between tumor growth and metastasis. Generally, higher Ktrans values indicate increased angiogenic activity, which may affect the invasiveness of tumors and, by extension, patient prognosis[22]. Ve is a measure of the extracellular volume fraction, which represents the metabolic capacity of the contrast agent within the tumor.
Conversely, Vp signifies average vascular density and is particularly responsive to changes in perfusion within the tumor vasculature. An increase in these parameters is associated with enhanced blood perfusion to the tumor, suggesting a more aggressive tumor behavior and potentially poorer patient outcomes[23,24]. ADC quantifies the diffusion rate and extent of movement of water molecules. This result serves as a descriptor of the diffusional mobility of water molecules in various spatial orientations. The cellular density of tissue influences the ADC value. With an increase in cell density, intercellular spaces become narrow, the intracellular environment undergoes alterations, and the mobility of water molecules is constrained. This restriction leads to heightened signals in diffusion-weighted MRI scans and a corresponding decrease in ADC values[25]. The findings of this study indicate that parameters such as LTL, ATL, ADC, Ktrans, Ve, and Vp can predict short-term outcomes after laparoscopic radical resection surgery. The areas under the ROC curves for these parameters were 0.648, 0.927, 0.821, 0.809, and 0.831, with sensitivity values of 0.643, 0.893, 0.607, 0.714, and 0.714 and specificities of 0.702, 0.904, 0.883, 0.968, and 0.894, respectively. These results suggest a correlation between imaging biomarkers and the short-term prognosis of laparoscopic radical resection. The current investigation was a retrospective analysis conducted at a single medical center, and the relatively brief follow-up period could have intro
In our study, we analyzed the radiographic characteristics of CRC and their association with short-term outcomes in patients undergoing laparoscopic radical resection. These findings suggest that these imaging biomarkers have predictive significance and are correlated with short-term prognoses. This insight can serve as a valuable reference point for clinical decision-making and may contribute to the refinement of individualized treatment strategies. It is essential to adopt a holistic approach when applying these findings and thoroughly assessing the prognosis of each patient, considering their unique circumstances and medical histories.
| 1. | Wang RC, Wang JQ, Zhou XY, Zhong CL, Chen JX, Chen JS. Survival benefits of para-aortic lymphadenectomy in colorectal cancer with clinically suspected para-aortic lymph node metastasis: a meta-analysis and systematic review. World J Surg Oncol. 2023;21:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Li J, Ma X, Chakravarti D, Shalapour S, DePinho RA. Genetic and biological hallmarks of colorectal cancer. Genes Dev. 2021;35:787-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 300] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 3. | Feng Q, Yuan W, Li T, Tang B, Jia B, Zhou Y, Zhang W, Zhao R, Zhang C, Cheng L, Zhang X, Liang F, He G, Wei Y, Xu J; REAL Study Group. Robotic versus laparoscopic surgery for middle and low rectal cancer (REAL): short-term outcomes of a multicentre randomised controlled trial. Lancet Gastroenterol Hepatol. 2022;7:991-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 221] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 4. | Bohne A, Grundler E, Knüttel H, Fürst A, Völkel V. Influence of Laparoscopic Surgery on Cellular Immunity in Colorectal Cancer: A Systematic Review and Meta-Analysis. Cancers (Basel). 2023;15:3381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Grimm P, Loft MK, Dam C, Pedersen MRV, Timm S, Rafaelsen SR. Intra- and Interobserver Variability in Magnetic Resonance Imaging Measurements in Rectal Cancer Patients. Cancers (Basel). 2021;13:5120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med. 2016;15:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9979] [Cited by in RCA: 15768] [Article Influence: 1752.0] [Reference Citation Analysis (0)] |
| 7. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64307] [Article Influence: 16076.8] [Reference Citation Analysis (174)] |
| 8. | Patel SG, Karlitz JJ, Yen T, Lieu CH, Boland CR. The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol Hepatol. 2022;7:262-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 379] [Article Influence: 126.3] [Reference Citation Analysis (6)] |
| 9. | Zhao W, Dai S, Yue L, Xu F, Gu J, Dai X, Qian X. Emerging mechanisms progress of colorectal cancer liver metastasis. Front Endocrinol (Lausanne). 2022;13:1081585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 10. | Patel SG, Murphy CC, Lieu CH, Hampel H. Early age onset colorectal cancer. Adv Cancer Res. 2021;151:1-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Mao Y, Chen B, Wang H, Zhang Y, Yi X, Liao W, Zhao L. Diagnostic performance of magnetic resonance imaging for colorectal liver metastasis: A systematic review and meta-analysis. Sci Rep. 2020;10:1969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Zhang K, Ren Y, Xu S, Lu W, Xie S, Qu J, Wang X, Shen B, Pang P, Cai X, Sun J. A clinical-radiomics model incorporating T2-weighted and diffusion-weighted magnetic resonance images predicts the existence of lymphovascular invasion / perineural invasion in patients with colorectal cancer. Med Phys. 2021;48:4872-4882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Westberg K, Othman B, Suzuki C, Blomqvist L, Martling A, Iversen H. Magnetic resonance imaging as a predictor of surgical outcome in patients with local pelvic recurrence of colorectal cancer. Eur J Surg Oncol. 2021;47:2119-2124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Xiao Y, Li J, Zhong J, Chen D, Shi J, Jin H. Diagnostic Performance of Diffusion-Weighted Imaging for Colorectal Cancer Detection: An Updated Systematic Review and Meta-Analysis. Front Oncol. 2022;12:656095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 15. | Jia W, Cheng X, Zhao R. Editorial: Laparoscopic surgery in colorectal cancer. Front Oncol. 2022;12:960730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | Passuello N, Polese L, Ometto G, Grossi U, Mammano E, Vittadello F, Frasson A, Tessari E, Bartolotta P, Gregori D, Sarzo G. Outcomes of Laparoscopic Surgery in Very Elderly Patients with Colorectal Cancer: A Survival Analysis and Comparative Study. J Clin Med. 2023;12:7122. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Hajibandeh S, Barghash M, Khan RMA, Milgrom D, Ali S, Ali S, Ali B. Predictive Significance of Tumour Size in Patients Undergoing Curative Surgery for Colorectal Cancer: A Retrospective Cohort Study. Cureus. 2022;14:e26656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Yin W, Zhang M, Ji Z, Li X, Zhang S, Liu G. Impact of tumor size on overall survival and cancer-specific survival of early-onset colon and rectal cancer: a retrospective cohort study. Int J Colorectal Dis. 2024;39:69. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Dai W, Li Y, Meng X, Cai S, Li Q, Cai G. Does tumor size have its prognostic role in colorectal cancer? Re-evaluating its value in colorectal adenocarcinoma with different macroscopic growth pattern. Int J Surg. 2017;45:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Aryal MP, Lee C, Hawkins PG, Chapman C, Eisbruch A, Mierzwa M, Cao Y. Real-Time Quantitative Assessment of Accuracy and Precision of Blood Volume Derived from DCE-MRI in Individual Patients During a Clinical Trial. Tomography. 2019;5:61-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Yang X, Xiao X, Lu B, Chen Y, Wen Z, Yu S. Perfusion-sensitive parameters of intravoxel incoherent motion MRI in rectal cancer: evaluation of reproducibility and correlation with dynamic contrast-enhanced MRI. Acta Radiol. 2019;60:569-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Gity M, Parviz S, Saligheh Rad H, Fathi Kazerooni A, Shirali E, Shakiba M, Baikpour M. Differentiation of Benign from Malignant Adnexal Masses by Dynamic Contrast-Enhanced MRI (DCE-MRI): Quantitative and Semi-quantitative analysis at 3-Tesla MRI. Asian Pac J Cancer Prev. 2019;20:1073-1079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Jackson A, Pathak R, deSouza NM, Liu Y, Jacobs BKM, Litiere S, Urbanowicz-Nijaki M, Julie C, Chiti A, Theysohn J, Ayuso JR, Stroobants S, Waterton JC. MRI Apparent Diffusion Coefficient (ADC) as a Biomarker of Tumour Response: Imaging-Pathology Correlation in Patients with Hepatic Metastases from Colorectal Cancer (EORTC 1423). Cancers (Basel). 2023;15:3580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 24. | Otto PO, Loft MK, Rafaelsen SR, Pedersen MRV. Diffusion-Weighted MRI as a Quantitative Imaging Biomarker in Colon Tumors. Cancers (Basel). 2023;16:144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 25. | Bodalal Z, Bogveradze N, Ter Beek LC, van den Berg JG, Sanders J, Hofland I, Trebeschi S, Groot Lipman KBW, Storck K, Hong EK, Lebedyeva N, Maas M, Beets-Tan RGH, Gomez FM, Kurilova I. Radiomic signatures from T2W and DWI MRI are predictive of tumour hypoxia in colorectal liver metastases. Insights Imaging. 2023;14:133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |