Published online Mar 27, 2025. doi: 10.4240/wjgs.v17.i3.102428
Revised: December 1, 2024
Accepted: January 16, 2025
Published online: March 27, 2025
Processing time: 116 Days and 1 Hours
Middle pancreatectomy (MP) is a surgical procedure that removes non-invasive lesions in the pancreatic neck and body, allowing for the preservation of pan
To compare perioperative and postoperative outcomes in patients who underwent IMPD-J bridge drainage and those underwent traditional duct-to-mucosa pancreatojejunostomy.
Patients who underwent MP in our hospital between October 1, 2011 and July 31, 2023 were enrolled in this study. Patients were divided into two groups based on their pancreatojejunostomy technique: IMPD-J bridge drainage group and duct-to-mucosa pancreatojejunostomy group. Demographic data (age, gender, body mass index, hypertension, diabetes, etc.) and perioperative indicators [operation time, intraoperative bleeding, clinically relevant postoperative pancreatic fistula (CR-POPF), delayed gastric emptying, etc.] were recorded and analyzed statistically.
A total of 53 patients were enrolled in this study, including 23 in the IMPD-J Bridge Drainage group and 30 in the traditional duct-to-mucosa pancreatojejunostomy group. There were no significant differences in demographic or preope
Compared to traditional duct-to-mucosa pancreatojejunostomy, IMPD-J bridge drainage has the advantages of simplicity and fewer perioperative complications, with favorable long-term outcomes.
Core Tip: Interlocking main pancreatic duct-jejunal bridge drainage emerges as a promising technique for middle pancreatectomy, offering shorter operation time, reduced risk of clinically relevant postoperative pancreatic fistula and delayed gastric emptying, and favorable long-term safety. Compared to traditional duct-to-mucosa pancreatojejunostomy, interlocking main pancreatic duct-jejunal bridge drainage demonstrates simplicity and fewer perioperative complications, suggesting its potential as a preferred method in middle pancreatectomy procedures.
- Citation: Lu XY, Tan XD. Clinical outcomes of interlocking main pancreatic duct-jejunal internal bridge drainage in middle pancreatectomy: A comparative study. World J Gastrointest Surg 2025; 17(3): 102428
- URL: https://www.wjgnet.com/1948-9366/full/v17/i3/102428.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i3.102428
Middle Pancreatectomy (MP) is a surgical technique primarily used to treat non-invasive lesions in the neck and body of the pancreas. As a parenchyma-sparing operation, it was first applied in 1957 by Guillemin and Bessot[1] on a patient with chronic pancreatitis. This method is designed to preserve as much normal pancreatic tissue as possible, thereby reducing the risk of both exocrine and endocrine insufficiency post-surgery. The technique is particularly advantageous for younger patients[2], where the preservation of pancreatic function can have a more significant impact on long-term outcomes. Despite its benefits, MP has not been widely adopted in clinical practice. The main reason is that compared with conventional pancreatic surgeries such as pancreaticoduodenectomy and pancreatic distal resection, MP is associated with a higher risk of postoperative complications, including postoperative pancreatic fistula (POPF), post-pancreatectomy hemorrhage, and delayed gastric emptying (DGE). POPF, in particular, has a high occurrence rate, with studies indicating that its incidence can reach up to 80.8%[3]. This increased risk is often attributed to the creation of two pancreatic stumps during MP surgery, thereby increasing the risk of pancreatic anastomotic leakage. In response to this risk, clinicians have explored various techniques to manage the stumps, such as duct-to-mucosa pancreaticojejunostomy, double pancreaticojejunostomy[4], pancreatogastrostomy, and the use of biomaterial coverage. Although duct-to-mucosa pancreaticojejunostomy is commonly used[5], no single technique has been proven to be optimal. In addition, the complex and time-consuming nature of the MP procedure further contributes to its lower utilization in clinical settings. According to a meta-analysis study published in 2022[6], traditional anastomosis requires additional Roux-en-Y gastrointestinal reconstruction after resection of the pancreatic mass, which increases the difficulty of the operation. When using laparoscopic or robot-assisted surgery, this complexity is particularly evident due to the restricted operating space and the need for hand-eye coordination in a confined environment, which amplifies the challenges. These factors collectively limit the broader adoption of MP, emphasizing the need for continued research and the development of more effective and streamlined techniques to reduce the risks and complications associated with this surgical approach.
Interlocking main pancreatic duct-jejunal (IMPD-J) internal bridge drainage is a novel pancreaticojejunostomy technique pioneered by our hospital. This surgical method was initially performed on a patient with anastomotic dehiscence of pancreaticojejunostomy in 2006[7]. The patient developed a pancreaticojejunostomy fistula on the 6th day after surgery, accompanied by fever, abdominal pain, elevated amylase in the drainage fluid, and obvious peritoneal irritation symptoms. Abdominal ultrasonography revealed a large amount of fluid in the upper abdomen. After exploratory laparotomy, there was a severe anastomotic dehiscence, and the length of the dehiscence exceeding half the circumference of the anastomosis. In this situation, simple reoperation or local drainage was not feasible. Therefore, we inserted one end of a silicone drainage tube into the main pancreatic duct and fixed the other end with embedded sutures in the lumen of the jejunal loop, with the aim of draining pancreatic secretions into the distal jejunum. The postoperative recovery was successful, and the patient was discharged 34 days after surgery, without any signs of diabetes or pseudocyst formation during follow-up. This surgical method was later applied to two other patients with severe pancreaticojejunostomy fistulas, with similar favorable outcomes. Due to its lower incidence of POPF rate, simplicity of operation, and suitability for minimally invasive surgery in limited visual fields, this IMPD-J bridge drainage surgical method is widely adopted by our team. After years of application, this retrospective study aimed to explore whether IMPD-J bridge drainage was superior to traditional pancreatic duct-to-mucosa anastomosis, as well as to evaluate its long-term safety. The results of this study could guide surgeons in adopting more effective and safer methods for pancreaticojejunostomy, ultimately improving patient outcomes and reducing complications.
Demographic and surgical data of patients who underwent MP surgery in the First General Surgery Department of Shengjing Hospital Affiliated to China Medical University between October 1, 2011, and July 31, 2023, were collected for this study. The inclusion criteria included: (1) Patients who had MP surgery; and (2) Patients whose distal pancreatic procedures involved pancreaticojejunostomy. The exclusion criteria were: (1) Patients who had emergency surgery; (2) Patients with other significant illnesses; and (3) Patients with a history of abdominal surgery. This study was approval by the Ethics Committee of Shengjing Hospital (approval No. 2024PS903K).
Preoperative, intraoperative and postoperative data were collected to compare the effectiveness and safety outcomes. Preoperative data included gender, age, body mass index (BMI), history of hypertension, history of diabetes, smoking history, alcohol consumption history, and preoperative laboratory indicators [white blood cells (WBCs), neutrophils, red blood cells, platelets, total protein, albumin, prealbumin, and total bilirubin]. Intraoperative data included the maximum tumor diameter, the diameter of the main pancreatic duct, total operation time, intraoperative blood loss, and transfusion volume. Postoperative outcomes include the incidence of clinically relevant POPF (CR-POPF), duration of nasogastric tube retention, the incidence of DGE, incidence of peritoneal effusion, incidence of abdominal and pulmonary infections, retention time of abdominal drainage tube, postoperative bleeding incidence, second surgery rate, postoperative hospital stay, and postoperative mortality rate.
The retention time of the abdominal drainage tube is defined as the duration for which the longest-lasting drainage tube remains in place when multiple drainage tubes are used. The diagnosis of POPF is based on the definition of the International Study Group of Pancreatic Surgery (ISGPS)[8]. Grade A POPF is characterized by a drainage fluid amylase level exceeding three times the upper limit of serum amylase. Grade A POPF is also called “biochemical leak” (BL), which does not require treatment. Grade B POPF is defined as the occurrence of severe complications on the basis of BL, requiring a change in the postoperative management (abdominal drainage tube retention time > 3 weeks). Grade C POPF refers to the failure of one or several organs that require reoperation and/or death due to surgery-related complications. The diagnosis of DGE is based on the ISGPS guidelines released in 2007[9], which is defined as patients who need nasogastric tube retention for more than 7 days postoperatively, or require long-term enteral nutrition.
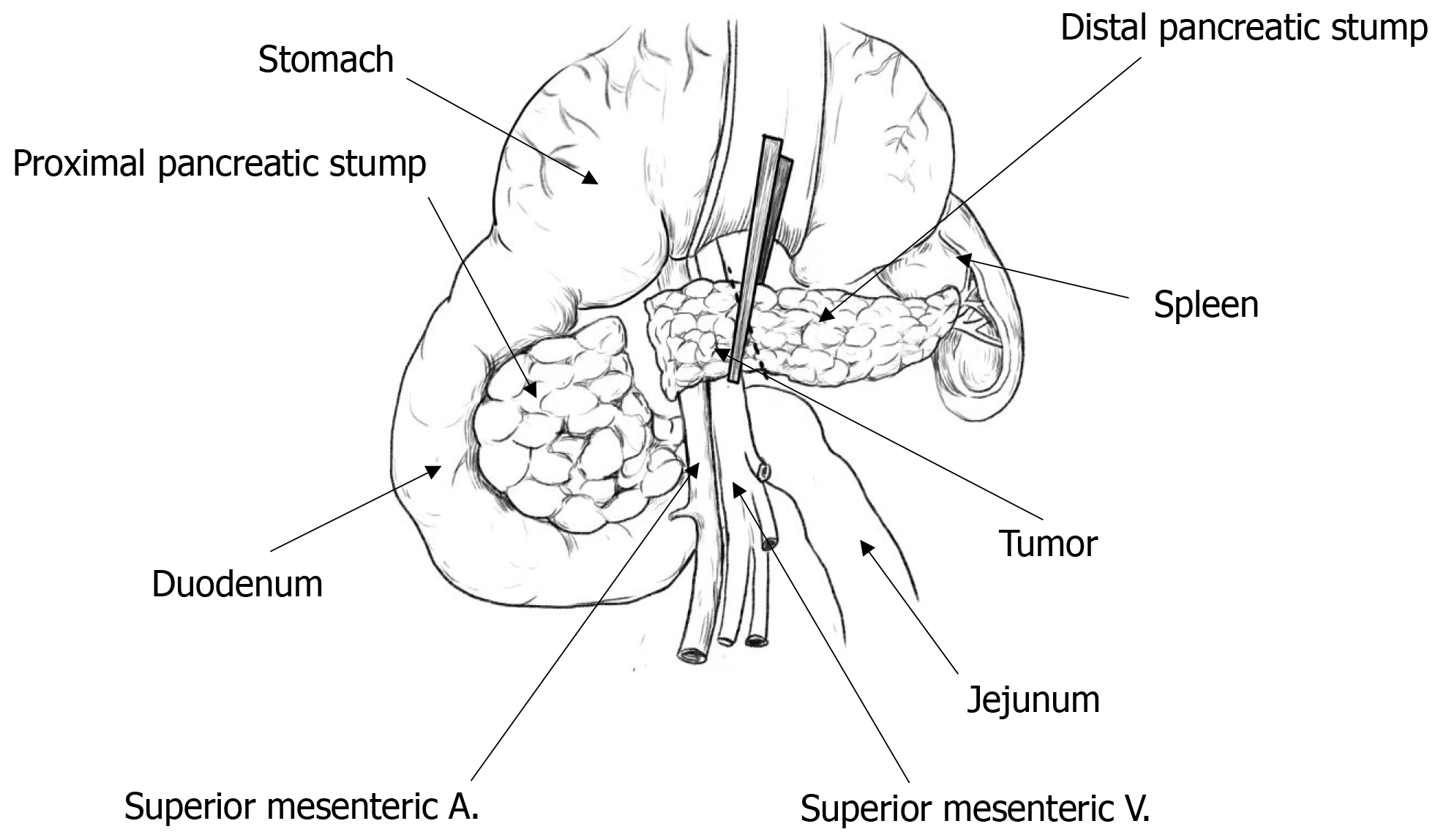
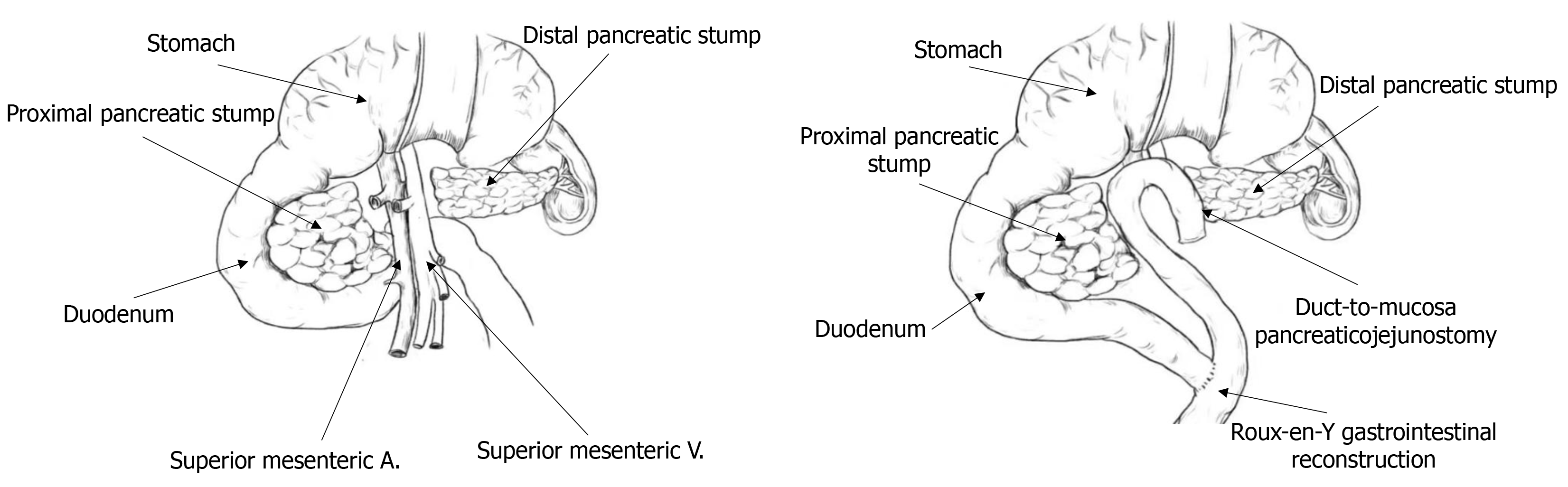
Before performing the MP surgery, all patients underwent laboratory tests and imaging examinations. MP surgery was performed only if experienced physicians judged that there was a high likelihood of a non-invasive lesion. Anesthesia was administered using a combination of intravenous and inhalation anesthesia. Once surgery began, the gastrocolic ligament at the upper edge of the transverse colon mesentery was incised with an ultrasonic scalpel, and the adhesions were carefully separated to examine the pancreatic neck and pancreatic body where the mass was located. To better expose the superior mesenteric vein, a combination of frontal and lateral approaches was used. The space between the pancreatic neck and the portal vein was carefully separated, followed by the upper and lower margins of the pancreas. In the area behind the left side of the mass, the superior mesenteric vein and the splenic vein were isolated and exposed. The pancreas was cut approximately 1 cm from the left side of the mass using an ultrasonic scalpel, and the main pancreatic duct was examined. The right pancreatic stump was lifted and then separated along the surface of the superior mesenteric vein and the portal vein towards the right side. The right branch of the portal vein was closed with a Hem-o-lok clip and then cut. Around 1 cm from the right side of the mass, the pancreatic head tissue was bundle-clipped and severed, allowing the mass and part of the pancreatic tissue to be removed entirely. If intraoperative pathology report revealed a non-invasive lesion, a pancreatojejunostomy was performed. However, if it indicated malignancy, distal pancreatectomy and abdominal lymph node dissection were performed. Figure 1 shows a schematic diagram of pancreatic mass resection.
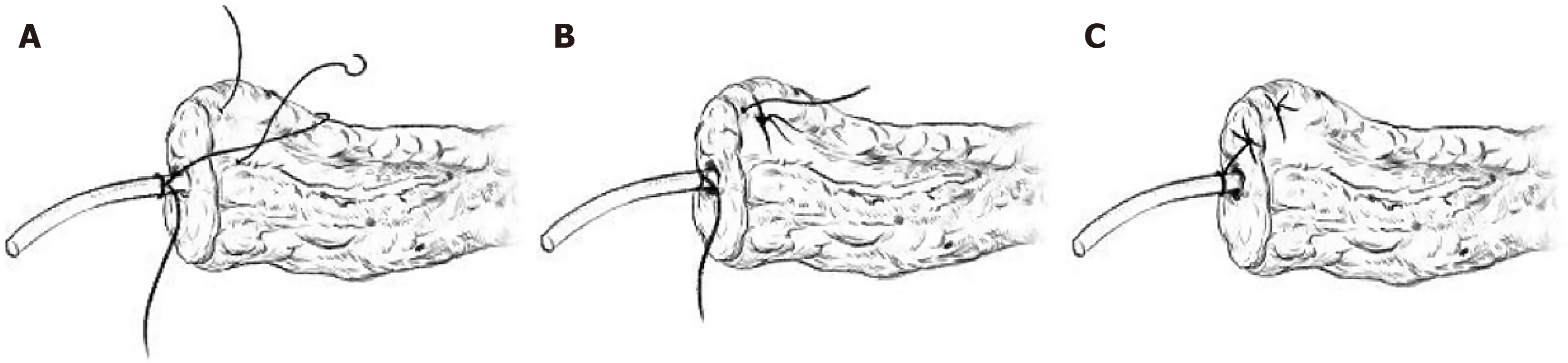
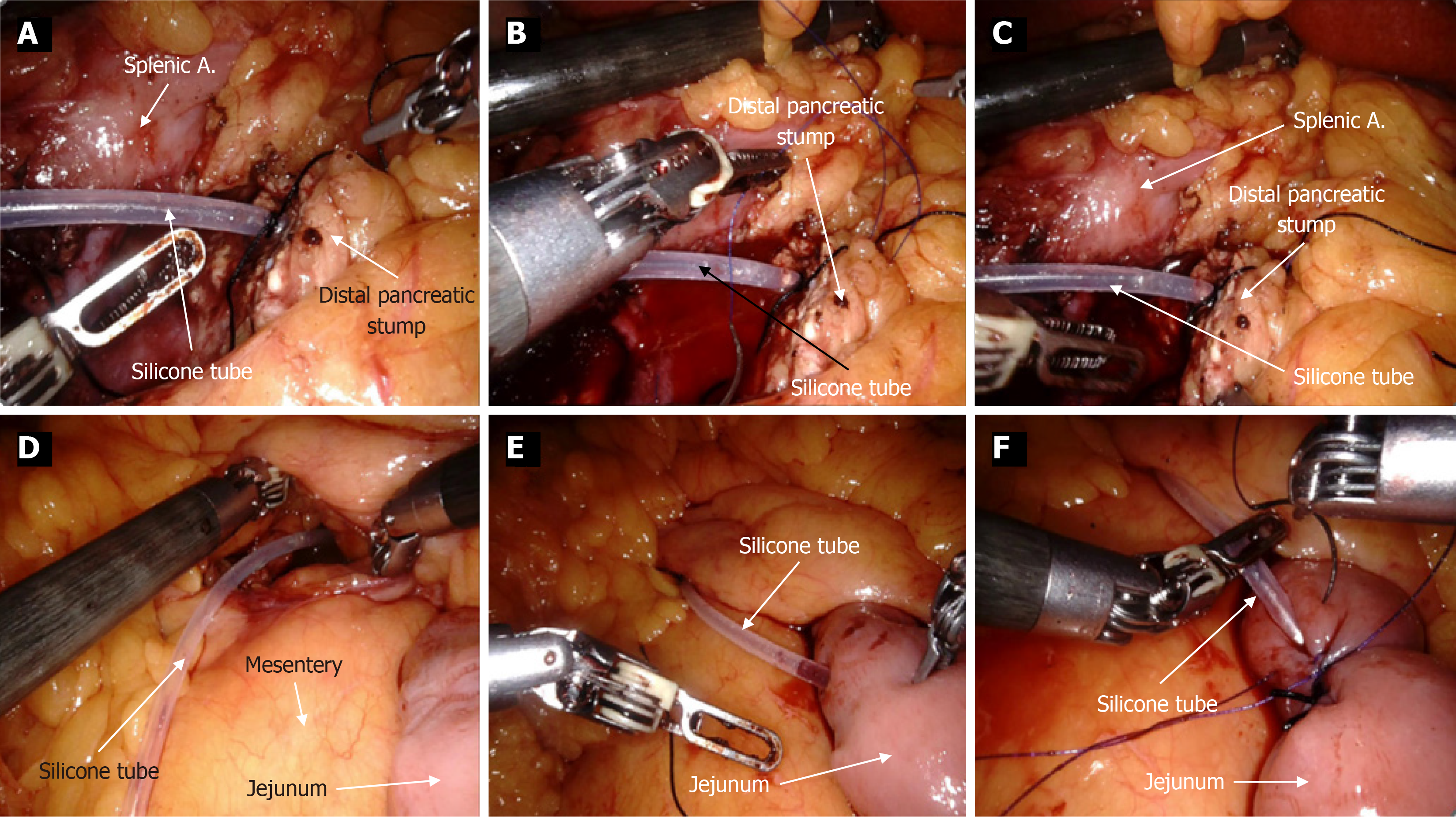
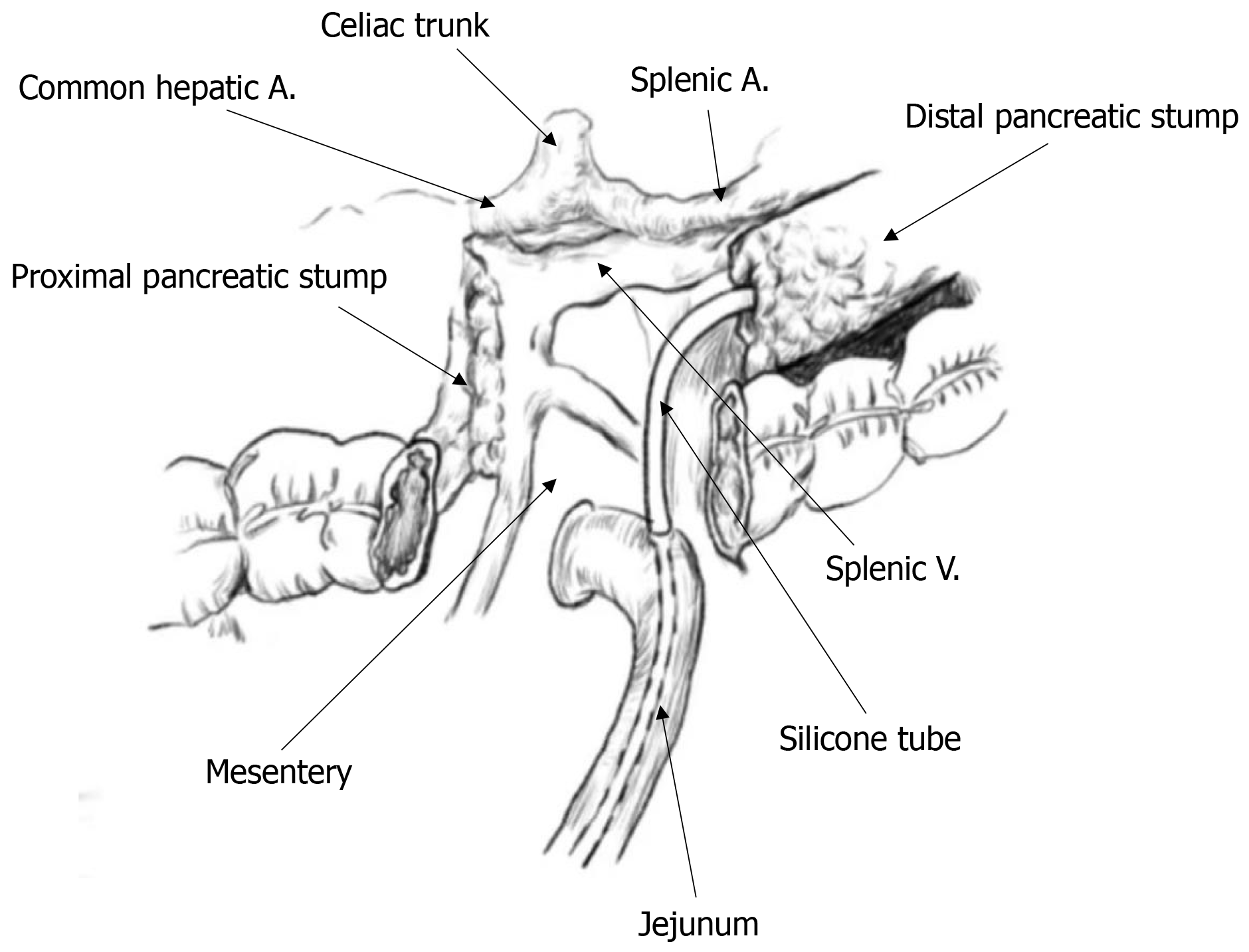
For the IMPD-J bridge drainage procedure, the steps are as follows: First, a silicone drainage tube was selected according to the diameter of the main pancreatic duct (approximately 20-30 cm in length). A No. 4 suture was tied 2-3 cm from one end of the drainage tube. As shown in Figure 2, this knotted end was then inserted into the main pancreatic duct. A U-shaped suture was then placed on the pancreatic stump using 4-0 polypropylene non-absorbable suture (PROLENE® Polypropylene Suture). Before tightening the knot, the suture tied to the pancreatic duct was interlocked with the U-shaped suture, securing the drainage tube to the upper and lower edges of the pancreas. The other end of the drainage tube was then passed through the transverse mesocolon and into the jejunum approximately 10-15 cm below the ligament of Treitz. After ensuring that there is no tension at the anastomosis, suture and embed to secure the suture on the intestinal side to the drainage tube. Barbed suture was used to create a submucosal tunnel within the intestinal wall to embed the drainage tube, ensuring both the pancreatic end and the intestinal end of the tube are securely fixed. Figure 3 shows clinical photographs that depict the step-by-step process of the bridge drainage procedure. Figure 4 demonstrates the application of IMPD-J bridge drainage for MP.
The surgical procedure for duct-to-mucosa pancreaticojejunostomy is as follows: First, the main pancreatic duct was located, and a suitable silicone drainage tube was chosen based on the duct’s diameter and carefully inserted into the duct. Next, in the lower section of the colon, the jejunal mesentery was incised, and the jejunum was cut about 15 cm away from the ligament of Treitz. The distal end was closed with size 0 sutures, and the stump was sutured. The proximal end was prepared for anastomosis with the pancreas. An incision was then made in the avascular region of the transverse mesocolon, and the distal jejunal loop was pulled into the upper section of the colon. Subsequently, a small incision, approximately 3 mm in diameter, was made in the distal jejunal loop using an electrocautery hook to expose the mucosal layer. The main pancreatic duct was mobilized by 1 to 2 cm. Starting from the posterior wall, 4-0 polydioxanone absorbable sutures were used to anastomose the main pancreatic duct to the jejunal mucosa (6-8 stitches). This suturing process ensured a tight, tension-free, and leak-proof connection. The final step involved creating an end-to-side anastomosis between the proximal and distal jejunal loops, about 20 cm away from the pancreaticojejunal anastomosis. The diameter of the anastomosis was approximately 2 cm, ensuring it remains open and without leakage. Figure 5 shows the schematic diagram of traditional duct-to-mucosa pancreaticojejunostomy used in MP. Following the completion of the anastomosis, the abdominal cavity was irrigated with warm saline to remove any residual debris and ensure a clean environment. After confirming that there was no bleeding, a flushable drainage tube was placed near the pancreatic section, with Venturi foramens to aid in drainage and reduce the risk of fluid accumulation. Once the drainage tube was in place, the abdomen was closed securely to complete the procedure.
For postoperative care following pancreatic surgery, routine treatments included hemostasis, pain relief, fluid replacement, anti-inflammatory medication, acid suppression, nebulization, and parenteral nutrition. After removing the nasogastric tube, patients were allowed to eat orally. Amylase levels in the drainage fluid were measured daily, and the required volume of saline for flushing was adjusted based on measured levels. If the amylase levels in the drainage fluid was below 100 U/L for two consecutive tests, the abdominal drainage tube could be removed. If DGE, abdominal fluid accumulation, intra-abdominal or thoracic infections, post-pancreatectomy hemorrhage, or other serious complications were suspected, further examinations were performed, such as computed tomography or ultrasound examinations. If necessary, endoscopic procedures, interventional treatments, or a second surgery could be performed.
All statistical analyses were performed using SPSS version 26.0. Statistical significance is defined as a two-sided P < 0.05. All data underwent the single-sample Kolmogorov-Smirnov (K-S) test to determine whether they conformed to a normal distribution. Continuous data with normal distribution are presented as mean ± SD, and differences between groups are examined using t-tests. Continuous data without normal distribution are presented with median and interquartile range, and differences between groups are examined using Mann-Whitney U test. Categorical data are presented as number and percentage, and differences between groups are tested using χ2-tests or Fisher’s exact test. Binary logistic regression analysis was employed to identify risk factors associated with CR-POPF. Risk factors with a P value < 0.1 in univariate analysis were included in the binary logistic regression model for multivariate analysis. Factors with a P value < 0.05 were considered as independent risk factor for CR-POPF.
A total of 54 patients were enrolled in this study, including 23 patients in the IMPD-J bridge drainage group and 30 patients in the traditional duct-to-mucosa pancreaticojejunostomy group. Table 1 shows the demographics and patient characteristics of the two groups. There were no statistical differences between the two groups in terms of gender, age, BMI, smoking history, alcohol consumption history, hypertension, diabetes, and preoperative laboratory parameters (P > 0.05).
| IMPD-J bridge drainage (n = 23) | Duct-to-mucosa pancreaticojejunostomy (n = 30) | χ²/t/z | P value | |
| Age, years, n (%) | 57.0 (52.0, 64.0) | 61.0 (51.0, 67.0) | 0.944 | 0.345 |
| Male, n (%) | 12 (52.2) | 15 (50.0) | 0.025 | 0.875 |
| Female, n (%) | 11 (47.8) | 15 (50.0) | ||
| BMI, kg/m2 | 23.2 (20.4, 24.6) | 22.2 (19.6, 23.4) | -1.212 | 0.225 |
| Smoking, n (%) | 7 (30.4) | 15 (50.0) | 2.053 | 0.152 |
| Alcohol consumption, n (%) | 7 (30.4) | 4 (13.3) | 2.315 | 0.128 |
| Hypertension, n (%) | 5 (21.7) | 8 (26.6) | 0.171 | 0.679 |
| Diabetes, n (%) | 10 (43.5) | 7 (23.3) | 2.425 | 0.119 |
| WBC, 109/L | 5.3 (4.7, 5.8) | 5.0 (4.9, 6.1) | 0.117 | 0.907 |
| Neutrophils, 109/L | 2.8 (2.5, 3.1) | 3.0 (2.6, 3.6) | 0.808 | 0.419 |
| Platelet, 109/L | 224.7 ± 63.1 | 198.3 ± 55.3 | 1.619 | 0.112 |
| Albumin, g/L | 42.6 ± 3.9 | 41.9 ± 3.8 | 0.480 | 0.633 |
| Prealbumin, mg/dL | 20 ± 10 | 20 ± 10 | 0.03 | 0.977 |
| Total bilirubin, μmol/L | 11.4 (6.7, 15.9) | 11.4 (10.1, 14.5) | 0.188 | 0.851 |
Table 2 shows the intraoperative data of patients in the two groups. There were no significant differences between the two groups in terms of intraoperative blood loss red blood cells, intraoperative plasma transfusion, tumor diameters, and pancreatic duct diameters (P > 0.05). However, IMPD-J bridge drainage group had a significant shorter operation time than traditional duct-to-mucosa pancreaticojejunostomy group (4.3 ± 1.3 hours vs 5.8 ± 1.8 hours, P = 0.002). Regarding to the postoperative outcomes (Table 3), IMPD-J bridge drainage group had a significantly shorter nasogastric tube retention days than traditional duct-to-mucosa pancreaticojejunostomy group (5.3 ± 1.7 days vs 6.5 ± 2.0 days, P = 0.031), lower incidence of DGE (8.7% vs 36.7%, P = 0.019), and lower incidence of CR-POPF (39.1% vs 70.0%, P = 0.025). No differences were observed between groups with regards to postoperative body temperature (> 38.5 °C), blood-borne infection, drainage tube infection, peritoneal effusion, postoperative bleeding, interventional/endoscopic treatment, intubation time, and length of hospital stay (P > 0.05).
| IMPD-J bridge drainage (n = 23) | Duct-to-mucosa pancreaticojejunostomy (n = 30) | χ²/t/z | P value | |
| Operation time, hours | 4.3 ± 1.3 | 5.8 ± 1.8 | -3.233 | 0.002 |
| Blood loss, mL | 100.0 (50.0, 200.0) | 100.0 (87.5, 300.0) | -1.894 | 0.058 |
| RBC transfusion, u | 0 (0, 0) | 0 (0, 0) | -0.197 | 0.843 |
| Plasma transfusion, mL | 0 (0, 0) | 0 (0, 75.0) | -0.507 | 0.612 |
| Tumor diameter, cm | 4.1 ± 2.3 | 3.9 ± 1.9 | 0.303 | 0.763 |
| Pancreatic duct diameter, mm | 3.0 (3.0, 5.0) | 3.0 (2.4, 6.0) | -2.420 | 0.809 |
| IMPD-J bridge drainage (n = 23) | Duct-to-mucosa pancreaticojejunostomy (n = 30) | χ²/t/z | P value | |
| Temperature > 38.5 °C, n (%) | 5 (21.7) | 5 (16.7) | 0.013 | 0.910 |
| Blood culture (positive), n (%) | 0 (0) | 2 (6.7) | NA | 0.499 |
| Drainage tube infection, n (%) | 4 (17.4) | 4 (13.3) | NA | 0.715 |
| Nasogastric tube retention, days | 5.3 ± 1.7 | 6.5 ± 2.0 | -2.218 | 0.031 |
| DGE, n (%) | 2 (8.7) | 11 (36.7) | 5.502 | 0.019 |
| Peritoneal effusion, n (%) | 1 (4.4) | 6 (20.0) | 2.782 | 0.095 |
| Intervention/endoscopic treatment, n (%) | 0 (0) | 1 (3.3) | NA | 1.000 |
| Postoperative bleeding, n (%) | 1 (4.4) | 1 (3.3) | NA | 1.000 |
| Length of hospital stay, days | 24.0 (14.0, 29.0) | 29 (9.8, 40.3) | -0.844 | 0.399 |
| Drainage tube retention time, days | 20.0 (13.0, 36.0) | 27 (17.8, 40.0) | -1.096 | 0.273 |
| POPF 0, n (%) | 6 (26.1) | 5 (16.7) | NA | NA |
| A, n (%) | 8 (34.8) | 4 (13.3) | NA | NA |
| B, n (%) | 8 (34.8) | 21 (70.0) | NA | NA |
| C, n (%) | 1 (4.4) | 0 (0) | NA | NA |
| CR-POPF, n (%) | 9 (39.1) | 21 (70.0) | 5.051 | 0.025 |
Univariable binary logistic regression analysis was used to identify the unique characteristics associated with the CR-POPF. As shown in Table 4, type of pancreaticojejunostomy and plasma prealbumin content were potential influencing factors for the occurrence of CR-POPF. Multivariate logistic regression analysis further confirmed that type of pancreaticojejunostomy (odds ratio = 4.219, 95% confidence interval = 1.238-14.379, P = 0.021) and plasma prealbumin content (odds ratio = 1.132, 95% confidence interval = 1.001-1.281, P = 0.049) were independent risk factor for the occurrence of CR-POPF (Table 5). This result suggests that the risk of CR-POPF in MP patients who underwent traditional duct-to-mucosa pancreaticojejunostomy is 4.219 times higher than those who underwent IMPD-J bridge drainage.
| OR | 95%CI | P value | |
| Age, year | 0.971 | (0.925, 1.019) | 0.229 |
| Gender (ref: Male) | 2.034 | (0.673, 6.146) | 0.208 |
| BMI, kg/m2 | 1.508 | (0.891, 1.257) | 0.517 |
| Smoking | 0.632 | (0.209, 1.907) | 0.415 |
| Alcohol consumption | 0.567 | (0.149, 2.158) | 0.405 |
| Diabetes | 0.396 | (0.121, 1.289) | 0.124 |
| Hypertension | 0.862 | (0.245, 3.003) | 0.817 |
| WBC | 0.719 | (0.439, 1.715) | 0.188 |
| Platelet | 0.999 | (0.990, 1.009) | 0.912 |
| Albumin | 1.115 | (0.956, 1.301) | 0.166 |
| Prealbumin | 1.111 | (0.994, 1.242) | 0.063 |
| Total bilirubin | 0.911 | (0.801, 1.307) | 0.158 |
| Operation type (ref: IMPD-J bridge drainage) | 3.630 | (1.155, 11.406) | 0.027 |
| Tumor diameter | 0.908 | (0.697, 1.183) | 0.475 |
| Pancreatic duct diameter | 0.912 | (0.707, 1.176) | 0.477 |
| Bleeding | 1.002 | (0.999, 1.006) | 0.218 |
| Blood transfusion | 1.102 | (0.665, 1.827) | 0.706 |
| Plasma transfusion | 1.000 | (0.998, 1.002) | 0.943 |
| OR | 95%CI | P value | |
| Operation type (ref: IMPD-J bridge drainage) | 4.219 | (1.238, 14.379) | 0.021 |
| Prealbumin | 1.132 | (1.001, 1.281) | 0.049 |
Patients were followed up until July 31, 2023. In the IMPD-J bridge drainage group, 18 patients were follow-up, with durations ranging from 4 to 65 months. Among these patients, 13 did not experience recurrent abdominal pain, back pain, steatorrhea, fever, pancreatitis, pseudocysts, or significant weight loss after discharge. Two patients occasionally experienced mild discomfort in the upper abdomen and back, but laboratory indicators and imaging examinations showed that there was no pancreatitis or pseudocyst. One patient experienced recurrence of pancreatitis (no pseudocyst). In addition, the patient reported decreased tolerance to alcohol, and was prone to experience upper abdominal and back pain after drinking. A 64-year-old patient with atypical adenomatous hyperplasia was discharged on the 9th postoperative day, but was readmitted 4 months later due to jaundice. Image examinations revealed local recurrence with extensive peritoneal lymph node metastasis. Carbohydrate antigen 19-9 levels increased to 47.6 U/mL (normal: 0-37 U/mL). This patient was discharged after completing one round of chemotherapy and was subsequently lost to follow-up. Out of the 18 patients, only one experienced recurrent pancreatitis accompanied by steatorrhea and lost 30 kg in body weight within 5 months, without developing diabetes. Subsequent endoscopic ultrasound revealed significant dilation at the distal end of the main pancreatic duct, where a silicone tube was inserted. It is speculated that the silicone tube was too large, causing overexpansion and damage to the duct, eventually leading to recurrent pancreatitis. This patient underwent a second surgery 6 months after the operation. After the silicone tube was removed, the symptoms of pancreatitis disappeared without recurrence.
Since the first case of MP was performed in 1957, its application has expanded from chronic pancreatitis to non-invasive tumors in the neck and body of the pancreas, with intraductal papillary mucinous neoplasms and pancreatic neuroendocrine tumors being the most commonly treated tumors. Currently, most researchers believe that compared to traditional Whipple surgery and distal pancreatectomy, MP, while preserving pancreatic function, is more likely to cause one or more perioperative complications. Klotz et al[10] found that patients undergoing MP were more likely to experience abdominal bleeding, although it did not lead to more severe outcomes such as CR-POPF and an extended hospital stay. Studies by Regmi et al[11] and Asano et al[12] suggested that the incidence of CR-POPF is higher in MP compared to traditional pancreatic surgeries. This observation is not surprising, as MP leaves two pancreatic stumps after surgery, increasing the likelihood of pancreatic fluid leakage into the abdominal cavity[13]. Clinically, the proximal pancreatic stump can be closed directly using a right-angle cutter or sutured manually after ultrasonic dissection. However, managing the distal pancreatic stump is challenging. If it is closed off like the proximal pancreatic stump, pancreatic fluid won’t be able to escape, increasing the risk of pancreatitis and reducing the preservation of pancreatic function. Therefore, pancreatojejunostomy and pancreatogastrostomy are two common surgical methods for the management of distal pancreatic stump. Although there’s no clear consensus on which anastomotic method is preferable[14], a long-term analysis of residual pancreatic function indicated that pancreatogastrostomy may impair long-term exocrine pancreatic function due to excessive growth of gastric mucosa[15]. In the treatment experience of our team, we tend to use pancreaticojejunostomy, and IMPD-J bridge drainage is also an improvement based on the traditional duct-to-mucosa pancreaticojejunostomy.
IMPD-J bridge drainage is a relatively novel anastomotic technique originally developed to manage a pancreaticojejunostomy leak in a patient after Whipple surgery. The severe dehiscence of the original anastomosis, coupled with significant edema, rendered re-anastomosis unfeasible. Additionally, the patient’s advanced age and severe infection further reduced the likelihood of a successful re-anastomosis. Under these challenging circumstances, our surgical team resorted to this innovative approach. Remarkably, the patient’s recovery was unexpectedly smooth, leading to discharge 34 days after re-surgery. During the subsequent 27 months of follow-up, no postoperative complications such as diabetes or pseudocysts were observed.
The results from this study further confirm the advantages of applying IMPD-J bridge drainage in MP, with a mean operating time of 4.3 ± 1.3 hours, which significantly shorter than the 5.8 ± 1.8 hours of the traditional duct-to-mucosa pancreaticojejunostomy (P < 0.05). This notable difference in operating time could offer greater flexibility for the surgical team and benefit the patients. In addition, compared to the conventional duct-to-mucosa anastomosis, the key feature of IMPD-J bridge drainage is that it doesn’t require direct anastomosis between the main pancreatic duct and the intestinal mucosa. Instead, it uses a silicone tube as a “bridge” to transport pancreatic fluid. This design reduces the risk of leakage that might occur with traditional anastomosis, thereby decreasing the likelihood of postoperative complications. Of note, the digestive tract does not require Roux-en-Y reconstruction. These findings suggest that IMPD-J bridge drainage not only shortens operating time by simplifying the surgical steps, but also effectively lowers the risk of postoperative complications. For robotic or laparoscopic surgery, the complexity of robotic arm operations and the separation between hand and eye movements further highlight the clinical applicability of this method that simplify surgical procedures.
The study found that compared to patients who underwent traditional duct-to-mucosa pancreaticojejunostomy, patients who underwent IMPD-J bridge drainage had significantly lower nasogastric tube retention time (5.3 ± 1.7 days vs 6.5 ± 2.0 days, P < 0.05) and lower risk of DGE (8.7% vs 36.7%, P < 0.05), which could be attributed to the fact that IMPD-J bridge drainage does not require Roux-en-Y digestive tract reconstruction. Although Roux-en-Y reconstruction is generally considered safe[16,17], it still disrupts the physiological integrity of the digestive tract. In contrast, the operation of IMPD-J bridge drainage only requires a small incision in the jejunum, embedding and fixing a silicone drainage tube using barbed sutures, thereby maintaining the integrity of the digestive tract to a certain extent. Only two patients had to continue using the nasogastric tube due to nausea and gastroesophageal reflux after removal of the tube. However, both patients also had successful nasogastric tube removal on the eighth postoperative day and were able to eat orally. By contrast, among patients who underwent traditional duct-to-mucosa pancreaticojejunostomy, 11 cases of DGE occurred, with three requiring nasogastric tubes for up to 10 days. These findings clearly show that IMPD-J bridge drainage surgery has advantages in terms of shorter nasogastric tube retention time and lower DGE incidence.
Regarding POPF, this study found that IMPD-J bridge drainage had significantly lower incidence of CR-POPF compared to duct-to-mucosa pancreaticojejunostomy (39.1% vs 70.0%, P < 0.05). According to the latest 2016 ISGPS criteria for POPF, 17 patients in the IMPD-J bridge drainage group developed POPF. Among them, 8 had grade A POPF, manifested by elevated amylase levels in the drainage fluid on the third day after operation (no further management required). Two patients experienced stress-induced hyperthermia, with the highest recorded temperature being 38.6 °C - one on the first day and the other on the ninth day. Blood and drainage fluid cultures taken during the fever episodes were negative for bacterial growth. The patient with fever on the first day after surgery had an increase in WBCs to 16 × 109/L, which may be related to the surgery. For the patient who had a fever on the ninth day after operation, the number of WBCs did not increase. Both patients’ temperatures returned to normal within 24 hours without any change in the type or dosage of antibiotics, relying solely on physical cooling. Therefore, these two people were not classified as CR-POPF. A total of 9 patients developed CR-POPF, 8 of whom were grade B POPF, mainly manifested by elevated amylase levels in the drainage fluid, and the mean drainage tube retention time was more than 21 days (the longest retention time was 106 days). Among these 8 patients, 3 experienced chills and high fever, with 2 having positive bacterial cultures from abdominal drainage fluid, while the third had unknown infection sources (both abdominal drainage fluid and blood cultures testing negative). Nonetheless, the source of the infection was deduced to be from the drainage tube, because the patient’s abdominal computed tomography scan on the day of fever suggested increased fluid leakage in the surgical area. In addition, 2 of the 8 patients with grade B POPF did not develop symptoms such as chills and high fever, but the bacterial culture of the drainage fluid was positive on the 14th and 13th days postoperative days, and they were accompanied by a rapid increase in WBCs. Only one patient was classified as grade C POPF because of abdominal bleeding on the second postoperative day and the bleeding was immediately stopped during laparotomy. It was found that a local hematoma had formed, with a volume of approximately 300 mL. The bleeding site was located at the incision surface of the proximal residual pancreas, but not at the pancreaticojejunostomy, distal remnant pancreas, and splenic artery and vein. The cause of the bleeding is suspected to be either a slipped suture or the rupture of an electrocautery hemostasis point. However, since the amylase level had reached 3200 U/L at the time of the bleeding, the possibility of bleeding caused by local blood vessel erosion due to pancreatic fluid leakage cannot be completely ruled out.
In comparison, duct-to-mucosa pancreaticojejunostomy had a higher incidence of POPF. Out of 30 patients, 25 developed POPF, with only 4 having grade A POPF, while the remaining 21 were classified as grade B POPF due to complications such as disease progression, intra-abdominal bleeding, interventional treatment, or long-term drainage. The longest drainage time was 96 days, experienced by a 33-year-old male who underwent a MP for a solid pseudopapillary tumor. This patient had two abdominal drains placed postoperatively, one at the Winslow’s foramen and one at the pancreatic transection site. The drainage tube placed at Winslow’s foramen was kept in place for up to 17 days because of elevated amylase levels in the drainage fluid near the transection site, which caused by persistent pancreatic fluid leakage. Even with daily irrigation with saline, white flocculent material continued to form. In addition, prolonged pancreatic fluid leakage resulted in pancreatic fluid collection at the transection site. The patient underwent ultrasound-guided thoracentesis and abdominal puncture 48 days after the operation and then discharged from the hospital with a drainage tube in place 78 days after the operation. The tube was removed during a follow-up visit on the 96th day. This patient’s prolonged drainage may be attributable to severe obesity, as the patient had a preoperative BMI of 37.04, although there was no history of diabetes or alcohol consumption. Among the 21 patients with grade B POPF, 5 experienced chills and high fever, requiring an escalation in antibiotic treatment. The bacterial culture of abdominal drainage fluid of 3 people was positive. The other 2 had unknown sources of infection, with negative results for both drainage fluid and blood cultures, and no evidence of pancreatic fluid collection on imaging. They only showed clinical symptoms and increased WBC counts. Among the patients with normal body temperature after surgery, one patient’s drainage fluid bacterial culture was positive, but the WBC count did not increase significantly. Since this patient had no clinical symptoms, no treatment was administered; instead, the situation was closely monitored. The patient's abdominal drainage tube was removed 40 days after operation, without any evidence of abdominal effusion.
Traditional duct-to-mucosa pancreatojejunostomy involves creating an anastomosis between the main pancreatic duct and the jejunal mucosa. If using too few stitches during suturing, gaps may form between the pancreatic duct and the jejunal mucosa, potentially leading to leakage. Although too many stitches can ensure a tight enough anastomosis, it will also increase the risk of damage to the pancreatic duct and the intestinal mucosa. In particular, the use of multiple stitches is more likely to aggravate the damage to capillaries at the anastomotic site, greatly increasing the risk of POPF. Studies have shown that inadequate blood supply at the anastomotic site is more likely to cause delayed healing or even ischemic necrosis[18,19]. The emergence of IMPD-J bridge drainage offers a solution to this problem. While the silicone tube acting as a bridge is fixed to the main pancreatic duct, the suture needle only needs to penetrate the pancreatic parenchyma. During the subsequent anastomosis, there is no need to insert a needle from the main pancreatic duct, which reduced the risk of damage to the main pancreatic duct. On the other hand, enterokinase secreted by the small intestine can activate trypsinogen. If the main pancreatic duct is directly anastomosed to the jejunum, pancreatic juice will be quickly activated by enterokinase as soon as it enters the jejunum, which can lead to erosion at the anastomosis site. The advantage of the IMPD-J bridge drainage is demonstrated here. It can drain pancreatic juice to a location further away from the anastomosis site. This means that even if trypsinogen is activated, it won’t directly contact the anastomosis site, lowering the risk of POPF.
When IMPD-J bridge drainage was initially applied in three patients with anastomotic dehiscence, several steel beads were placed inside the silicone tube, and absorbable sutures were used to secure the silicone tube to the pancreas. After a few months, the steel beads’ weight would cause the silicone tube to fall off, enter the intestinal tract, and be expelled from the body. During this period, pancreatic duct epithelial cells would grow along the silicone tube to the anastomotic site, wrap around the omentum, and subsequently form sinus tracts locally. This prevented pancreatic fluid leakage even after the silicone tube had detached. The three patients expelled the silicone tube at 67, 180, and 92 days after operation, respectively. During subsequent follow-ups, ranging from 5 to 27 months, no pancreatitis, pancreatic fluid leakage, or pseudocysts were observed. The original intention of this approach is to consider that was based on the fact that silicone is a foreign material. Although it is highly resistant to corrosion, long-term retention could potentially lead to rejection, recurrent inflammation, repeated infections, organ adhesions, and pseudocyst formation. However, with advances in medical devices and improved healthcare practices, silicone is now considered a relatively safe material and is widely used in prosthetics and patches. Therefore, in later IMPD-J bridge drainage procedures, the placement of steel beads to ensure silicone tube detachment was deemed unnecessary. The focus shifted to securing the silicone tube between the pancreas and the jejunum to reduce perioperative pancreatic fluid leakage and the risk of POPF. For this reason, non-absorbable sutures were used to fix the silicone tube to the pancreas.
Our IMPD-J bridge drainage procedure has some similarities to the modified MP procedure developed by Liu and Zhao[20] in 2018. Here’s a brief introduction to the procedure. According to Liu and Zhao[20], when the main pancreatic duct resection is less than 5 cm, the reconstruction involves a bridging repair of the main pancreatic duct plus an end-to-end pancreatic anastomosis. In this approach, a silicone tube is inserted into the main pancreatic duct and fixed with absorbable sutures. The two ends of the pancreatic remnant are then brought together and sutured with non-absorbable stitches. However, if the resection is more than 5 cm, bridging repair of the main pancreatic duct plus pancreatic stent exclusion are used, where the silicone tube is left in the main duct to facilitate pancreatic fluid drainage. Compared to our IMPD-J bridge drainage approach, the key differences are: (1) This method reconstructs the main pancreatic duct between the proximal and distal residual pancreas without performing pancreaticojejunostomy; and (2) The silicone tube is always fixed to the pancreas using absorbable sutures, allowing for eventual expulsion after a few months. In this approach, 13 patients developed POPF, mostly grade BL and grade B, with drainage times ranging from 13 days to 10 months, and no formation of pancreatic-cutaneous fistulas. In follow-up over 1 to 16 months, three patients did not expel the silicone tube. However, this report did not discuss the long-term effects of having an externally implanted silicone tube in the pancreatic duct. According to the results from our follow-up, most tolerated the intra-abdominally placed silicone tube well. Only one patient experienced recurrent pancreatitis due to the large diameter of the silicone tube and had it removed six months after surgery. The silicone tubes in the remaining patients were well-secured. Therefore, from a long-term perspective, our IMPD-J bridge drainage approach appears to be a feasible approach.
This study has a few limitations. First, it is a single-center retrospective study with a limited sample size. Second, the time period between cases was too long, and some patients were lost to follow-up. Third, although IMPD-J bridge drainage could shorten the operation time, there is a lack of data on pancreaticojejunostomy time in the medical records, making further analysis not possible. Therefore, in subsequent studies, it would be important to separately record the overall surgical time and the time taken for pancreatojejunostomy. Lastly, this study only compared IMPD-J bridge drainage with traditional duct-to-mucosa pancreatojejunostomy. To further validate its safety and simplicity, comparisons with other surgical approaches would be helpful.
IMPD-J bridge drainage is easy to perform, with the advantages of shorter operation time, lower risk of CR-POPF and DGE, and long-term safety. It is a suitable technique for MP procedures.
| 1. | Guillemin P, Bessot M. [Chronic calcifying pancreatitis in renal tuberculosis: pancreatojejunostomy using an original technic]. Mem Acad Chir (Paris). 1957;83:869-871. [PubMed] |
| 2. | Goudard Y, Gaujoux S, Dokmak S, Cros J, Couvelard A, Palazzo M, Ronot M, Vullierme MP, Ruszniewski P, Belghiti J, Sauvanet A. Reappraisal of central pancreatectomy a 12-year single-center experience. JAMA Surg. 2014;149:356-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 3. | Wolk S, Distler M, Kersting S, Weitz J, Saeger HD, Grützmann R. Evaluation of central pancreatectomy and pancreatic enucleation as pancreatic resections--A comparison. Int J Surg. 2015;22:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Sho M, Akahori T, Nagai M, Satoi S, Yanagimoto H, Kinoshita S, Yamamoto T, Ikeda N, Kwon AH, Nakajima Y. Central Pancreatectomy with Double Pancreaticojejunostomy. J Am Coll Surg. 2015;221:e15-e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Yang F, Jin C, Di Y, He H, Hao S, Yao L, Li J, Fu D. Central pancreatectomy with external drainage of monolayer pancreaticojejunostomy for prevention of postoperative pancreatic fistula: A retrospective cohort study. Int J Surg. 2018;51:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Farrarons SS, van Bodegraven EA, Sauvanet A, Hilal MA, Besselink MG, Dokmak S. Minimally invasive versus open central pancreatectomy: Systematic review and meta-analysis. Surgery. 2022;172:1490-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 7. | Bu X, Xu J, Dai X. A novel technique for the management of pancreaticojejunal anastomosis dehiscence following pancreaticoduodenectomy. Dig Surg. 2010;27:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CM, Wolfgang CL, Yeo CJ, Salvia R, Buchler M; International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3041] [Cited by in RCA: 2945] [Article Influence: 368.1] [Reference Citation Analysis (35)] |
| 9. | Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Traverso LW, Yeo CJ, Büchler MW. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 2321] [Article Influence: 128.9] [Reference Citation Analysis (0)] |
| 10. | Klotz R, Schilling C, Kuner C, Hinz U, Klaiber U, Holze M, Tjaden C, Loos M, Büchler MW, Hackert T. Central pancreatectomy prevents postoperative diabetes. J Hepatobiliary Pancreat Sci. 2023;30:951-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Regmi P, Yang Q, Hu HJ, Liu F, Karn HR, Ma WJ, Ran CD, Li FY. Overall Postoperative Morbidity and Pancreatic Fistula Are Relatively Higher after Central Pancreatectomy than Distal Pancreatic Resection: A Systematic Review and Meta-Analysis. Biomed Res Int. 2020;2020:7038907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Asano Y, Kato H, Arakawa S, Ito M, Nagakawa T, Nakao A, Ohta T, Yamaue H, Yamamoto M, Satoi S, Kodera Y, Takeyama Y, Ohtsuka M, Endo I, Takada T, Horiguchi A. Clinical outcomes of organ-preserving pancreatectomy for benign or low-grade malignant pancreatic tumors: A multicenter nationwide survey in Japan. J Hepatobiliary Pancreat Sci. 2022;29:898-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 13. | Iacono C, Verlato G, Ruzzenente A, Campagnaro T, Bacchelli C, Valdegamberi A, Bortolasi L, Guglielmi A. Systematic review of central pancreatectomy and meta-analysis of central versus distal pancreatectomy. Br J Surg. 2013;100:873-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 14. | Borel F, Ouaissi M, Merdrignac A, Venara A, De Franco V, Sulpice L, Hamy A, Regenet N. Pancreatico-jejunostomy decreases post-operative pancreatic fistula incidence and severity after central pancreatectomy. ANZ J Surg. 2018;88:77-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Benini L, Gabbrielli A, Cristofori C, Amodio A, Butturini G, Cardobi N, Sozzi C, Frulloni L, Mucelli RP, Crinò S, Bassi C, Marchegiani G, Andrianello S, Malleo G, Salvia R. Residual pancreatic function after pancreaticoduodenectomy is better preserved with pancreaticojejunostomy than pancreaticogastrostomy: A long-term analysis. Pancreatology. 2019;19:595-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Rotellar F, Pardo F, Montiel C, Benito A, Regueira FM, Poveda I, Martí-Cruchaga P, Cienfuegos JA. Totally laparoscopic Roux-en-Y duct-to-mucosa pancreaticojejunostomy after middle pancreatectomy: a consecutive nine-case series at a single institution. Ann Surg. 2008;247:938-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Xiao Z, Luo G, Liu Z, Jin K, Xu J, Liu C, Liu L, Ni Q, Long J, Yu X. Roux-en-Y pancreaticojejunostomy reconstruction after deep enucleation of benign or borderline pancreatic lesions: a single-institution experience. HPB (Oxford). 2016;18:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Zheng M, Liu A, Li J, Liang X, Peng J, Chen D, Shi L, Fu Z, Ji M, Yang G, Yang T, Tang L, Shao C. Comparison of early postoperative outcomes between omega-like duct-to-mucosa pancreatojejunostomy and conventional duct-to-mucosa pancreatojejunostomy after pancreaticoduodenectomy. HPB (Oxford). 2022;24:606-615. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Zhou Y, Yang J, Wei L, Lin Q, Zheng S, Liu G, Zhou Q, Tan X, Chen R. A novel anastomosis technique facilitates pancreaticojejunostomy in total laparoscopic pancreaticoduodenectomy (with video). Langenbecks Arch Surg. 2021;406:2891-2897. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Liu R, Zhao GD. [Promotion of innovative theory of bridge closure in changing traditional surgical approaches for benign pancreatic diseases]. Zhongguo Shiyong Waike Zazhi. 2018;27:263-268. [DOI] [Full Text] |