Published online Feb 27, 2025. doi: 10.4240/wjgs.v17.i2.99510
Revised: November 21, 2024
Accepted: December 9, 2024
Published online: February 27, 2025
Processing time: 103 Days and 1.4 Hours
Although substantial evidence supports the advantages of cold snare polypec
To compare the efficacy-cost of EMR and CSP in the treatment of intestinal polyps.
A total of 100 patients with intestinal polyps were included in the retrospective data of our hospital from April 2022 to May 2023. According to the treatment methods, they were divided into EMR (n = 46) group and CSP (n = 54) group. The baseline data of the two groups were balanced by 1:1 propensity score matching (PSM), and the cost-effectiveness analysis was performed on the two groups after matching. The recurrence rate of the two groups of patients was followed up for 1 year, and they were divided into recurrence group and non-recurrence group according to whether they recurred. Multivariate logistic regression analysis was used to screen out the influencing factors affecting the recurrence of intestinal polyps after endoscopic resection.
Significant disparities were observed in the number of polyps and smoking background between the two groups before PSM (P < 0.05). Following PSM, the number of polyps and smoking history were well balanced between the EMR and CSP groups. The direct cost incurred by the CSP group was markedly higher than that incurred by the EMR group. Concurrently, the cost-effectiveness ratio in the CSP group was substantially reduced when juxtaposed with that in the EMR group (P < 0.05). Upon completion of the 1-year follow-up, the rate of recurrence after endoscopic intestinal polypectomy was 38.00%. Multivariate methods revealed that age ≥ 60 years, male sex, number of polyps ≥ 3, and pathological type of adenoma were risk factors for recurrence after endoscopic intestinal polypectomy (all P < 0.05).
CSP was more cost-effective for the treatment of intestinal polyps. An age ≥ 60 years, male sex, having a number of polyps ≥ 3, and pathological type of adenoma are independent influencing factors for recurrence.
Core Tip: This study utilizes propensity score matching to evaluate the efficacy and cost-effectiveness of cold snare polypectomy (CSP) vs endoscopic mucosal resection in colorectal polyp treatment. CSP shows a higher rate of complete resection and a more favorable cost-to-effectiveness ratio. The research also identifies an age over 60 years, male sex, presence of three or more polyps, and adenomatous histology as key predictors of polyp recurrence, advocating for CSP's adoption and personalized patient follow-up strategies.
- Citation: Zhang SY, Wang YC, Liu LL, Wang ZH, Guan XM. Efficacy-cost analysis of endoscopic mucosal resection and cold snare polypectomy: A propensity score matching analysis. World J Gastrointest Surg 2025; 17(2): 99510
- URL: https://www.wjgnet.com/1948-9366/full/v17/i2/99510.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i2.99510
Intestinal polyps, a common digestive tract disease, refer to vegetation on the surface of the colon and rectal mucosa protruding into the intestinal cavity[1]. Clinically, to distinguish the possibility of cancer, intestinal polyps are divided into adenomatous polyps and non-adenomatous polyps, in which adenomatous polyps are closely related to intestinal cancer[2]. Therefore, it is recommended that colorectal polyps be clinically removed. In recent years, endoscopic mucosal resection (EMR) has frequently been used to resect colon polyps in clinical practice. In addition, argon knife resection, high-frequency electrocoagulation electro-resection, hot snare polypectomy, endoscopic mucosal dissection, and other techniques have been used for the EMR of colon polyps. EMR is characterized by low cost and fast recovery; therefore, it is preferred by clinicians and patients[3,4]. However, there are complications related to EMR, such as bleeding, perfora
Cold snare polypectomy (CSP) is a suitable endoscopic resection technique for small polyps and is recommended by several national endoscopic society guidelines[6]. Moreover, CSP does not result in bleeding due to electrocoagulation, which can reduce damage to the surrounding blood vessels and intestinal walls; therefore, CSP is associated with low risk of bleeding[7,8].
EMR and CSP have their own advantages and disadvantages, which have been disputed by many scholars. Although there is much evidence indicating the advantages of CSP in terms of the efficacy of polypectomy and postoperative adverse events, few studies have explored the difference in treatment costs between CSP and traditional EMR. Therefore, this study aimed to analyze the cost-effectiveness of the two surgical methods for the treatment of intestinal polyps based on the propensity score matching (PSM) method through an in-depth study of CSP and EMR. Furthermore, we evaluated the health economics to provide patients with the best treatment options.
The cancer rate of intestinal polyps is high, and adenomatous polyps are considered precancerous lesions in colorectal cancer. Previous studies have shown that 60%-80% of colon cancers progress from intestinal polyps[9]. Therefore, emphasis should be placed on early diagnosis of the disease, and targeted treatment should be provided based on a clear diagnosis to alleviate symptoms and delay disease progression. Endoscopic procedures represent the primary therapeutic approach for the management of intestinal polyps and effectively lower the incidence and mortality rates of colorectal cancer[10]. However, the postoperative recurrence rate is high, and invasive colorectal cancer can develop in severe cases. Therefore, this study aimed to explore the factors associated with the postoperative recurrence of intestinal polyps, providing a reference for the formulation and implementation of prevention and treatment measures.
One hundred patients with intestinal polyps who were treated at the Ezhou Central Hospital between April 2022 and May 2023 were selected as research participants. The inclusion criteria were as follows: (1) Colonoscopy and histopathological examination in line with the clinical diagnostic criteria for intestinal polyps[11]; (2) No previous history of colon surgery; (3) Complete clinical data; and (4) Not taking antiplatelet or anticoagulant drugs. The exclusion criteria were as follows: (1) Patients with mental disorders, cognitive dysfunction, or autoimmune diseases; (2) Patients with abnormal coagulation function, intestinal malignant disease, or intestinal tuberculosis; (3) Patients with surgical contraindications, severe disturbance of consciousness, or pregnancy or lactation; and (4) Patients with serious diseases of the heart, liver, kidney, or other organs. Furthermore, the exclusion shedding criteria were as follows: (1) After inclusion, cases that did not meet the inclusion criteria, did not use drugs according to the test plan, or cases with incomplete data were excluded; (2) Patients who withdrew or did not complete the whole course of treatment and affected the efficacy or safety judgment were excluded; and (3) Patients who required specialist treatment or transfer because of other diseases were removed from the study. The study was reviewed and approved by the Institutional Review Board of Ezhou Central Hospital.
The EMR group underwent the following surgical protocol: (1) First, patients underwent bowel preparation, including emptying the bowel and fasting, to ensure that the operation was performed smoothly; (2) The endoscope was inserted through the anus and entered into the colon. The clinician observed the polyps in the intestine through an endoscope and used special instruments to clamp and fix the polyps; (3) Next, the doctor passed the resection device through the endoscopic catheter, clipped the polyp tissue, and removed it. Clinicians carefully observed the endoscopic operation to ensure that the resection range was correct and that the blood vessels were not damaged; (4) After the operation was complete, it was necessary to perform hemostatic treatment at the resection site, check whether the resection range was sufficient, and assess whether there were any other abnormalities. The entire operational process requires extensive operational skill and rich experience. Clinicians strictly followed the operational processes and norms to ensure the safety and effectiveness of the operation; and (5) After surgery, patients underwent a recovery period and avoided overconsumption or eating irritating food. Simultaneously, the wound was closely monitored to determine whether bleeding or infection occurred; if so, timely medical treatment was sought.
The CSP group underwent the following surgical protocol: (1) A routine colonoscopy was performed, and the appro
R language software (R 4.1.2, Lucent Technologies, Molly Mountain, NJ, United States) was used for PSM. When the caliper value was set to 0.2, the EMR and CSP groups were matched at a ratio of 1:1 for variables with differences in the baseline data analysis before matching, such as smoking and number of polyps. The standardized mean difference (SMD) was used to test the balance between the groups after PSM, and a SMD < 0.1 was considered to be a good matching effect[12].
Efficacy-cost analysis was performed according to the following guidelines: (1) Cost estimation refers to the determination of the resource value consumed by a specific treatment plan or drug treatment that is concerned or needed, including hidden, direct, and indirect costs. Various types of direct costs are involved. The income cost of all patients in this study was calculated according to the charging standards set by the local price bureau. The direct costs for the CSP and EMR groups were anesthesia fee + examination and laboratory fee + operation fee + registration fee + drug fee + bed care fee; (2) The effect index (efficacy) of this study was replaced with the histological complete resection rate. If the resected polyp specimens and the bottom margin biopsy specimens were all negative, it was a histologically complete resection; and if there was ≥ 1 lesion tissue, it was incomplete resection; and (3) Efficacy-cost = cost (C)/efficacy (E).
Patients were followed-up with for 1 year. Subsequently, the patients were classified into recurrence and non-recurrence groups based on the presence or absence of recurrence. Recurrence rates were assessed and documented for both groups, with subsequent examination and comparison of the clinical profiles between patients with recurrent episodes and those who remained free from recurrence. To analyze the factors influencing recurrence, various items were substituted into the logistic regression equation.
Statistical software SPSS (SPSS 20.0, IBM, United States) was used to analyze the data. The measurement data that were normally distributed were reported as the mean ± SD, and the t test was used for comparison between groups. The measurement data with a skewed distribution were reported as the median and quartiles [M (P25, P75)], and the Mann-Whitney U test was used for comparisons. The tabulated count data were articulated as fractions, n (%), and a comparative analysis between groups was executed using the χ2 test for R × C contingency tables. Subsequently, logistic regression analysis was conducted to discern the determinants affecting the recurrence of intestinal polyps post-surgery. Statistical significance was set at a P value < 0.05.
Of the 100 patients enrolled in the study, 46 underwent EMR and 54 underwent CSP. As detailed in Table 1, notable disparities were observed in smoking habits and the number of polyps between the two groups (P < 0.05).
| Items | EMR group (n = 46) | CSP group (n = 54) | χ2/t | P value |
| Age | 1.771 | 0.183 | ||
| ≥ 60 years | 22 (47.83) | 33 (61.11) | ||
| < 60 years | 24 (52.17) | 21 (38.89) | ||
| Sex | 0.100 | 0.752 | ||
| Male | 27 (58.70) | 30 (55.56) | ||
| Female | 19 (41.30) | 24 (44.44) | ||
| BMI (kg/m2) | 22.48 ± 1.43 | 22.19 ± 2.68 | 0.678 | 0.499 |
| Smoking | 3.904 | 0.048 | ||
| Yes | 27 (58.70) | 21 (38.89) | ||
| No | 19 (41.30) | 33 (61.11) | ||
| Diabetes mellitus | 0.549 | 0.459 | ||
| Yes | 23 (50.00) | 23 (42.59) | ||
| No | 23 (50.00) | 31 (57.41) | ||
| Combined hypertension | 0.343 | 0.558 | ||
| Yes | 24 (52.17) | 25 (46.30) | ||
| No | 22 (47.83) | 29 (53.70) | ||
| Polyp diameter | 0.244 | 0.621 | ||
| ≥ 10 mm | 21 (45.65) | 22 (40.74) | ||
| < 10 mm | 25 (54.35) | 32 (59.26) | ||
| Number of polyps | 3.904 | 0.048 | ||
| ≥ 3 | 27 (58.70) | 21 (38.89) | ||
| < 3 | 19 (41.30) | 33 (61.11) | ||
| Polyp location | 0.362 | 0.547 | ||
| Colon | 28 (60.87) | 36 (66.67) | ||
| Rectum | 18 (39.13) | 18 (33.33) | ||
| Pathological type | 2.347 | 0.126 | ||
| Inflammatory hyperplasia | 30 (65.22) | 27 (50.00) | ||
| Adenomatous | 16 (34.78) | 27 (50.00) |
Following 1:1 PSM, a cohort of 80 patients across both groups was successfully paired. After matching, the baseline data, such as smoking history and polyp number, of the two groups were balanced (Table 2).
| Items | EMR group (n = 40) | CSP group (n = 40) | χ2/t | P value |
| Age, | 1.818 | 0.178 | ||
| ≥ 60 years | 25 (62.50) | 19 (47.50) | ||
| < 60 years | 15 (37.50) | 21 (52.50) | ||
| Sex, | 0.051 | 0.822 | ||
| Male | 22 (55.00) | 23 (57.50) | ||
| Female | 18 (45.00) | 17 (42.50) | ||
| BMI (kg/m2) | 22.55 ± 1.45 | 21.97 ± 2.68 | 1.209 | 0.230 |
| Smoking | 0.000 | 1.000 | ||
| Yes | 21 (52.50) | 21 (52.50) | ||
| No | 19 (47.50) | 19 (47.50) | ||
| Diabetes mellitus | 0.202 | 0.653 | ||
| Yes | 19 (47.50) | 17 (42.50) | ||
| No | 21 (52.50) | 23 (57.50) | ||
| Combined hypertension | 0.000 | 1.000 | ||
| Yes | 19 (47.50) | 19 (47.50) | ||
| No | 21 (52.50) | 21 (52.50) | ||
| Polyp diameter | 0.052 | 0.820 | ||
| ≥ 10 mm | 16 (40.00) | 17 (42.50) | ||
| < 10 mm | 24 (60.00) | 23 (57.50) | ||
| Number of polyps | 0.000 | 1.000 | ||
| ≥ 3 | 21 (52.50) | 21 (52.50) | ||
| < 3 | 19 (47.50) | 19 (47.50) | ||
| Polyp location | 0.220 | 0.639 | ||
| Colon | 25 (62.50) | 13 (32.50) | ||
| Rectum | 15 (37.50) | 27 (67.50) | ||
| Pathological type | 2.489 | 0.115 | ||
| Inflammatory hyperplasia | 26 (65.00) | 19 (47.50) | ||
| Adenomatous | 14 (35.00) | 21 (52.50) |
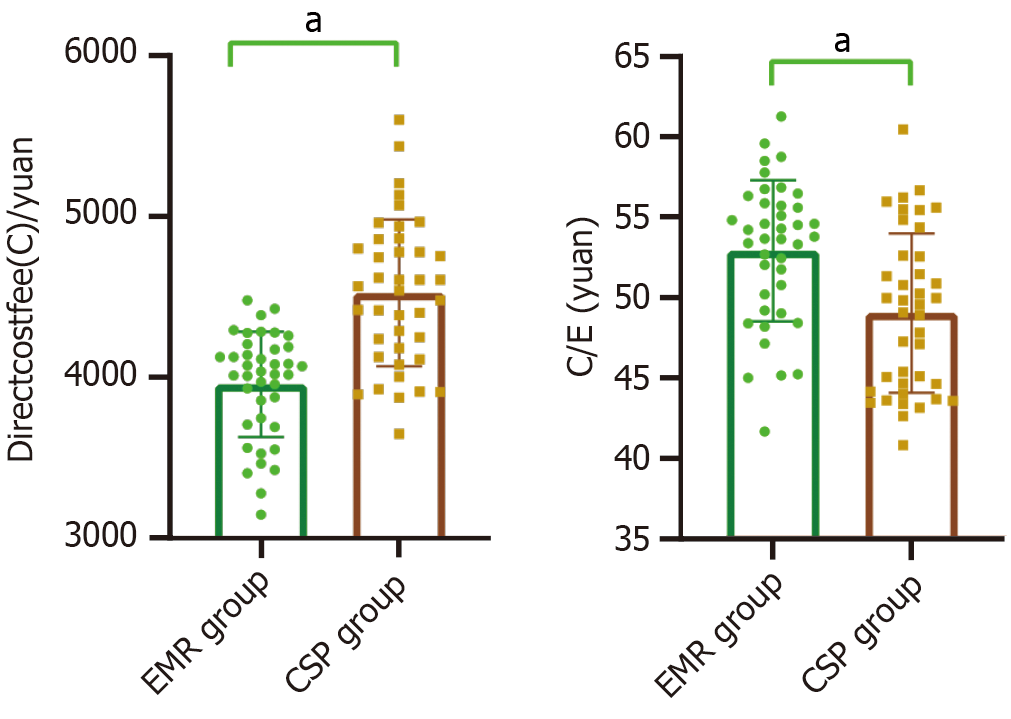
The direct cost (C) of the CSP group was 4528.23 ± 457.19 yuan, which was higher than that of the EMR group (3961.56 ± 328.47 yuan; t = 6.366, P < 0.001). The histological complete resection rate in the CSP group was 92.50% (37/40), which was higher than the 75.00% (32/48) in the EMR group (χ2 = 4.501, P = 0.034). The cost-efficacy (C/E) value of the CSP group (48.95 ± 4.94) was significantly lower than that of the EMR group (52.82 ± 4.38) (t = 3.707, P < 0.001), as shown in Figure 1.
After 1 year of follow-up, among the 100 patients with intestinal polyps included, 38 instances (38.00%) of recurrence were observed following endoscopic intestinal polypectomy, categorizing these patients into the recurrence group. Conversely, 62 cases remained recurrence-free and were allocated to the non-recurrence group. Divergent trends were noted when comparing the two groups in terms of age, sex, the diameter of the polyps, polyp count, and histological classification (P < 0.05), with specifics provided in Table 3.
| Items | Recurrence group (n = 38) | Non-recurrence group (n = 62) | χ2/t | P value |
| Age | 4.461 | 0.035 | ||
| ≥ 60 years | 26 (68.42) | 29 (46.77) | ||
| < 60 years | 12 (31.58) | 33 (53.23) | ||
| Sex | 4.938 | 0.026 | ||
| Male | 27 (71.05) | 30 (48.39) | ||
| Female | 11 (28.95) | 32 (51.61) | ||
| BMI (kg/m2) | 21.96 ± 2.03 | 22.57 ± 2.26 | -1.360 | 0.177 |
| Smoking | 0.261 | 0.609 | ||
| Yes | 17 (44.74) | 31 (50.00) | ||
| No | 21 (55.26) | 31 (50.00) | ||
| Diabetes mellitus | 0.395 | 0.530 | ||
| Yes | 19 (50.00) | 27 (43.55) | ||
| No | 19 (50.00) | 35 (56.45) | ||
| Combined hypertension | 2.226 | 0.136 | ||
| Yes | 15 (39.47) | 34 (54.84) | ||
| No | 23 (60.53) | 28 (45.16) | ||
| Polyp diameter | 5.548 | 0.019 | ||
| ≥ 10 mm | 22 (57.89) | 21 (33.87) | ||
| < 10 mm | 16 (42.11) | 41 (66.13) | ||
| Number of polyps | 10.240 | 0.001 | ||
| ≥ 3 | 26 (68.42) | 22 (35.48) | ||
| < 3 | 12 (31.58) | 40 (64.52) | ||
| Polyp location | 3.438 | 0.064 | ||
| Colon | 20 (52.63) | 44 (70.97) | ||
| Rectum | 18 (47.37) | 18 (29.03) | ||
| Pathological type | ||||
| Inflammatory hyperplasia | 13 (34.21) | 44 (70.97) | 12.987 | < 0.001 |
| Adenomatous | 25 (65.79) | 18 (29.03) | ||
| Surgical approach | 3.491 | 0.062 | ||
| EMR | 22 (57.89) | 24 (38.71) | ||
| CSP | 16 (42.11) | 38 (61.29) |
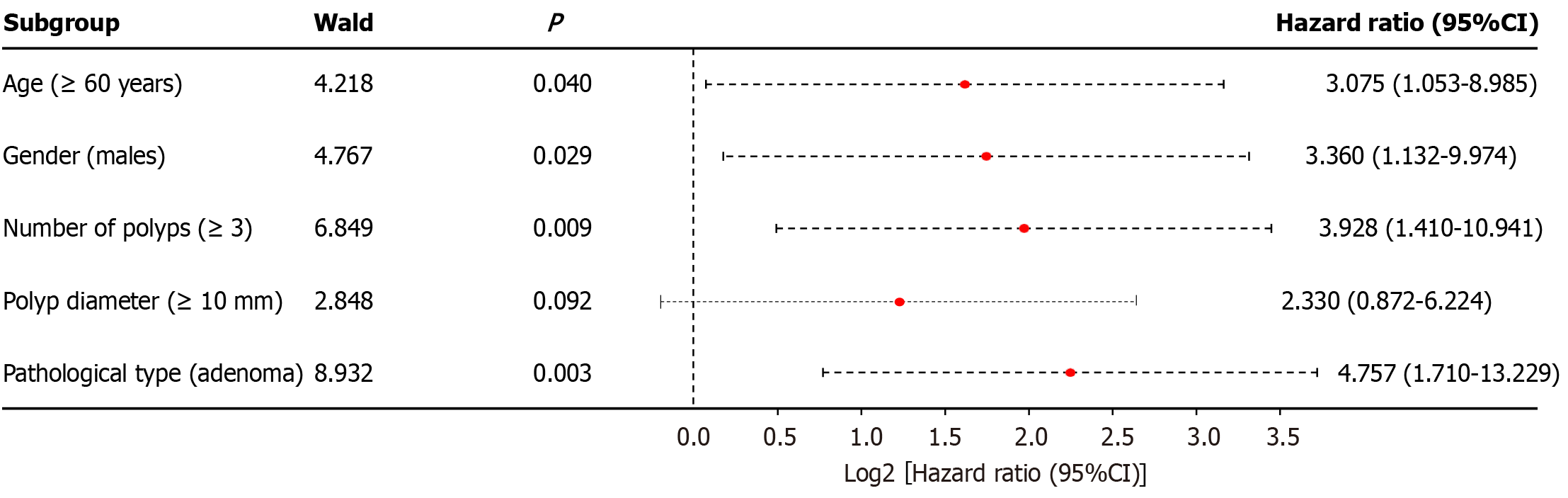
Recurrence after surgery was the dependent variable (recurrence = 1, no recurrence = 0). The statistically significant variables included as independent variables in the multivariate analysis, and the assignment of each variable, are shown in Table 4. The multivariate analysis showed that age ≥ 60 years, male sex, number of polyps ≥ 3, and pathological type of adenoma were risk factors for recurrence after endoscopic intestinal polypectomy (P < 0.05), as shown in Figure 2.
| Variable | Assignment |
| Age (≥ 60 years) | Yes = 1, No = 0 |
| Sex (male) | Yes = 1, No = 0 |
| Number of polyps (≥ 3) | Yes = 1, No = 0 |
| Polyp diameter (≥ 10 mm) | Yes = 1, No = 0 |
| Pathological type (adenoma) | Yes = 1, No = 0 |
Intestinal polyps refer to the abnormal growth of tissues protruding from the surface of the intestinal mucosa. Colonic and rectal polyps are the most common, whereas small intestinal polyps are less common. Clinical manifestations include diarrhea, lower gastrointestinal bleeding, and other symptoms, and there is a risk of cancerization[13]. Approximately two-thirds of all colonic polyps are adenomas[14], and 60%-70% of colon cancers develop from adenomas[15]. With continuous improvements in living standards, changes in eating habits have led to various intestinal problems. The incidence and mortality of colorectal cancer have maintained a significant upward trend[16]. Approximately 800000 people worldwide die of colorectal cancer each year, accounting for the top five causes of cancer-related deaths world
Endoscopic EMR and CSP are common minimally invasive treatment methods for colonic polyps. The complete histopathological resection rate is an important index for evaluating the effect of polypectomy procedures. In this study, the complete resection rate was higher in the CSP group than in the EMR group, indicating a greater effect of CSP surgery. Bleeding during CSP is rare; therefore, the endoscopist does not need to flush out the bleeding, the field of vision is clear, and the tissue can be quickly found for resection to ensure a high complete histological resection rate. Incomplete resection of polyps will increase the risk of polyp recurrence. Repeated invasive procedures not only increase the economic pressure of patients, but also increase the psychological burden of patients.
With an increase in the number of patients with intestinal polyps and the diversification of methods for treating the disease, researchers have paid increasing attention to the economics of colonic polyps. The DISCARD3 study[19] showed that the overall medical cost of patients with colorectal polyps was about USD 88000. A cost-benefit analysis of CSP[20] pointed out that the medical cost of treating giant sessile colorectal polyps was about USD 5213, and the number of days of lost productivity due to hospitalization was about 6.2 days. From the perspective of treatment, an analysis was carried out by comparing different treatment methods, and the optimal scheme was selected[21]. Based on PSM, this study conducted a cost-effectiveness analysis of the two methods in patients with intestinal polyps. The findings indicated that the C/E ratio for the CSP group was inferior to that of the EMR group, indicating that CSP surgery reduced the medical and economic costs for patients. CSP has certain economic advantages and helps reduce the medical costs of patients with colorectal polyps. Postoperative adverse events and lesion recurrence may lead to prolonged hospital stay and increased costs. Compared with EMR, CSP may cause fewer complications, reducing the possibility of additional treatment or hospitalization due to complications. Although the direct cost of CSP is higher, the C/E value is lower, indicating that CSP is more cost-effective in obtaining the same effect. Regardless of whether the cost or treatment results are ignored, both are indispensable. Therefore, balancing the best treatment effects with the lowest cost has become a hot topic in medical economics.
Previous studies[22,23] have shown that the recurrence rate of intestinal polyps is 25.8%-48.3%. In most cases, the recurrence of colorectal polyps is related to continuous mechanical and inflammatory stimulation. Therefore, colonoscopy findings should be regularly reviewed after polypectomy. In this study, the recurrence rate of intestinal polyps was 38.00%, which is similar to the results reported in the literature. This finding suggests that more attention should be paid to the clinical and active measures to reduce the risk of recurrence. In addition, after endoscopic treatment, it is particularly important to understand the risk factors for the recurrence of colonic polyps, select a reasonable follow-up time, and prevent the recurrence of colonic polyps. Logistic regression analysis showed that male sex, age ≥ 60 years, number of intestinal polyps ≥ 3, Polyp diameter ≥ 10 mm, and histopathological type of adenoma were risk factors for postoperative recurrence of intestinal polyps, which was similar to the results of previous studies[23]. A possible reason for the sex difference is that the estrogen/progesterone levels in female patients are higher than those in male patients, which can reduce the recurrence rate of colon polyps[24]. In addition, there were significant differences in smoking and drinking habits between men and women, which may also be one of the reasons for the high recurrence rate in men. Previous studies on the risk factors for recurrence of colon polyps in the general population have also confirmed the role of age ≥ 60 years in the prevalence and recurrence of colon polyps. With the increasing age of the patients, the physio
The concept of a greater risk of recurrence after multiple polyps has been recognized by many researchers. The increase in the number of intestinal polyps provides conditions for further polyp growth. Studies have shown that the risk of cancer in patients with intestinal polyps increases with the number of polyps[27]. This study also confirmed that an independent risk factor for postoperative recurrence was having three or more intestinal polyps, consistent with the results in the literature[28]. The size of intestinal polyps remains a controversial risk factor for recurrence of intestinal polyps. Other studies used the size, number, and tissue type of colonic polyps as determinants of the monitoring interval for colonic polyps[11]. However, this study did not find that the size of the intestinal polyps was related to their recurrence after surgery. This discrepancy may be due to differences in race, diet, or other factors, as different countries and regions use different monitoring guidelines.
This study found a correlation between the recurrence of intestinal polyps and the pathological types, especially adenomatous intestinal polyps, which have a high recurrence rate and a high possibility of cancer. This may be because adenomatous intestinal polyps have a faster division growth rate than normal tissues, which is more likely to induce the recurrence and carcinogenesis of intestinal polyps.
This study has some limitations, such as a small sample size, limited analysis of direct costs, and lack of assessment of the influence of other factors, such as differences in diet, exercise, living habits, use of non-steroidal anti-inflammatory drugs, and inconsistent review time of intestinal polyps, which will be analyzed in a follow-up study.
In summary, this study suggests that CSP is more economical than EMR for patients with intestinal polyps. There are many schemes for the endoscopic resection of intestinal polyps. In future studies, further health economic evaluations of more endoscopic schemes can provide a basis for clinical decision-making and the rational allocation of medical resources, which has both clinical and public health significance. In addition, understanding the factors influencing the recurrence of colonic polyps, formulating individualized follow-up plans, and rationally using medical resources can help prevent their recurrence.
| 1. | Gao P, Zhou K, Su W, Yu J, Zhou P. Endoscopic management of colorectal polyps. Gastroenterol Rep (Oxf). 2023;11:goad027. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Moriichi K, Fujiya M. Cancer Research in Adenocarcinoma, Adenoma, Adenomatous Polyposis Coli, and Colitis-Associated Neoplasia: A Special Issue. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Ye B, Wu Y, Tang X. Risk factors of post-polypectomy bleeding and recurrence in children with colorectal polyps after endoscopic mucosal resection: a retrospective cohort study. Transl Pediatr. 2022;11:1823-1830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Draganov PV, Aihara H, Karasik MS, Ngamruengphong S, Aadam AA, Othman MO, Sharma N, Grimm IS, Rostom A, Elmunzer BJ, Jawaid SA, Westerveld D, Perbtani YB, Hoffman BJ, Schlachterman A, Siegel A, Coman RM, Wang AY, Yang D. Endoscopic Submucosal Dissection in North America: A Large Prospective Multicenter Study. Gastroenterology. 2021;160:2317-2327.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 5. | Yuan X, Gao H, Liu C, Cui H, Zhang Z, Xie J, Lu H, Xu L. Effectiveness and safety of the different endoscopic resection methods for 10- to 20-mm nonpedunculated colorectal polyps: A systematic review and pooled analysis. Saudi J Gastroenterol. 2021;27:331-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Uraoka T, Takizawa K, Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano HO, Saito S, Hisabe T, Yao T, Watanabe M, Yoshida M, Saitoh Y, Tsuruta O, Igarashi M, Toyonaga T, Ajioka Y, Fujimoto K, Inoue H. Guidelines for Colorectal Cold Polypectomy (supplement to "Guidelines for Colorectal Endoscopic Submucosal Dissection/Endoscopic Mucosal Resection"). Dig Endosc. 2022;34:668-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 7. | Shimodate Y, Itakura J, Mizuno M, Takezawa R, Kobayashi M, Yamazaki T, Doi A, Nishimura N, Mouri H, Matsueda K, Yamamoto H. Factors Associated with possibly Inappropriate Histological Evaluation of Excised Specimens in Cold-snare Polypectomy for Small Colorectal Polyps. J Gastrointestin Liver Dis. 2018;27:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Kamal F, Khan MA, Lee-Smith W, Sharma S, Acharya A, Farooq U, Agarwal A, Aziz M, Chuang J, Kumar A, Schlachterman A, Loren D, Kowalski T, Adler D. Cold snare versus cold forceps polypectomy for endoscopic resection of diminutive polyps: meta-analysis of randomized controlled trials. Gastrointest Endosc. 2023;98:7-18.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Chakradhar S. Colorectal cancer: 5 big questions. Nature. 2015;521:S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Wei MT, Zhou MJ, Li AA, Ofosu A, Hwang JH, Friedland S. Multicenter evaluation of recurrence in endoscopic submucosal dissection and endoscopic mucosal resection in the colon: A Western perspective. World J Gastrointest Endosc. 2023;15:458-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Abu-Freha N, Katz LH, Kariv R, Vainer E, Laish I, Gluck N, Half EE, Levi Z. Post-polypectomy surveillance colonoscopy: Comparison of the updated guidelines. United European Gastroenterol J. 2021;9:681-687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Benedetto U, Head SJ, Angelini GD, Blackstone EH. Statistical primer: propensity score matching and its alternatives. Eur J Cardiothorac Surg. 2018;53:1112-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 189] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 13. | Shin HS, Cho YJ. Insulin levels are associated with risk of colon adenoma and not nonadenomatous polyps: A retrospective, hospital-based study. Medicine (Baltimore). 2022;101:e30200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Shah J, Shahidullah A. Spontaneous Expulsion per Rectum of a Colorectal Polyp: A Rare and Unusual Case. Gastroenterology Res. 2018;11:329-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Simon K. Colorectal cancer development and advances in screening. Clin Interv Aging. 2016;11:967-976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 427] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 16. | Liang Y, Zhang N, Wang M, Liu Y, Ma L, Wang Q, Yang Q, Liu X, Zhou F, Wei Y. Distributions and Trends of the Global Burden of Colorectal Cancer Attributable to Dietary Risk Factors over the Past 30 Years. Nutrients. 2023;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 17. | McFerran E, Boeri M, Kee F. Patient Preferences in Surveillance: Findings From a Discrete Choice Experiment in the "My Follow-Up" Study. Value Health. 2020;23:1373-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Hosotani K, Yabuuchi Y, Yamashita D, Inokuma T. Detecting remnant sessile serrated lesion after piecemeal cold snare polypectomy using acetic acid with narrow-band imaging. Endosc Int Open. 2022;10:E1595-E1596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 19. | Orlovic M, Ahmad A, Saunders BP. Economic impact of implementing optical diagnosis with a "resect and discard" strategy within the English Bowel Cancer Screening Programme: findings from the DISCARD3 study. Gastrointest Endosc. 2023;98:73-81.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 20. | Mehta D, Loutfy AH, Kushnir VM, Faulx AL, Smith ZL. Cold versus hot endoscopic mucosal resection for large sessile colon polyps: a cost-effectiveness analysis. Endoscopy. 2022;54:367-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Zilberberg MD, Shorr AF. Understanding cost-effectiveness. Clin Microbiol Infect. 2010;16:1707-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Hao Y, Wang Y, Qi M, He X, Zhu Y, Hong J. Risk Factors for Recurrent Colorectal Polyps. Gut Liver. 2020;14:399-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Choi WS, Han DS, Eun CS, Park DI, Byeon JS, Yang DH, Jung SA, Lee SK, Hong SP, Park CH, Lee SH, Ji JS, Shin SJ, Keum B, Kim HS, Choi JH, Jung SH. Three-year colonoscopy surveillance after polypectomy in Korea: a Korean Association for the Study of Intestinal Diseases (KASID) multicenter prospective study. Intest Res. 2018;16:126-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Štor Z, Blagus R, Tropea A, Biondi A. Net survival of patients with colorectal cancer: a comparison of two periods. Updates Surg. 2019;71:687-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Pommergaard HC, Burcharth J, Rosenberg J, Raskov H. Advanced age is a risk factor for proximal adenoma recurrence following colonoscopy and polypectomy. Br J Surg. 2016;103:e100-e105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Iqbal U, Nawaz A, Ahmed Z, Kamal F, Lee-Smith W, Khan MA, Alastal Y, Confer BD, Khara HS. Safety of endoscopic mucosal resection of large colonic polyps in elderly patients: a systematic review and meta-analysis. Ann Gastroenterol. 2022;35:420-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 27. | Kay M, Eng K, Wyllie R. Colonic polyps and polyposis syndromes in pediatric patients. Curr Opin Pediatr. 2015;27:634-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1443] [Article Influence: 111.0] [Reference Citation Analysis (0)] |