Published online Feb 27, 2025. doi: 10.4240/wjgs.v17.i2.98585
Revised: October 12, 2024
Accepted: November 22, 2024
Published online: February 27, 2025
Processing time: 206 Days and 5.2 Hours
The efficacy of various bariatric surgeries varies in reducing blood glucose levels. Given the distinct mechanisms and anatomical alterations associated with each procedure, it is crucial to compare their glycemic control outcomes. We hypothe
To compare the effectiveness of PSIB, RYGB, and JIB in lowering blood glucose.
Rats with streptozotocin-induced diabetes were randomly divided into PSIB, RYGB, JIB, and sham-operated groups. Body weight, food intake, fasting blood glucose level, oral glucose tolerance test, insulin tolerance test, liver enzymes, and blood lipids were measured.
Postoperatively, only the JIB group had a lower body weight compared to the sham group. The food intake of the rats in all three surgical groups was signi
PSIB demonstrated excellent hypoglycemic effects in the early postoperative period, and had better efficacy than RYGB and JIB.
Core Tip: Proximal small intestinal bypass (PSIB, approximately 60%) significantly improves glucose metabolism in streptozotocin-induced nonobese diabetic rats but does not reduce body weight. The glucose-lowering effect of PSIB may be independent of weight loss. PSIB has better glucose-lowering efficacy than classic Roux-en-Y gastric bypass and conventional jejunoileal bypass. Additionally, PSIB reduced blood glucose levels without causing abnormal liver enzyme levels or lipid profiles in the rats.
- Citation: Xu CY, Tan C, Luo X, Yang K, Wu RR, Lin L, Liu GL, Duan JY. Proximal small intestinal bypass outperforms Roux-en-Y and jejunoileal bypass in glucose regulation in streptozotocin induced diabetic rats. World J Gastrointest Surg 2025; 17(2): 98585
- URL: https://www.wjgnet.com/1948-9366/full/v17/i2/98585.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i2.98585
The global incidence of type 2 diabetes mellitus, a chronic disease with substantial health concerns, has been gradually increasing, with the number of patients with diabetes quadrupling in the past three decades[1]. The estimated global prevalence is 8.8%, which is anticipated to increase to 9.9% (approximately 693 million adults) by 2040[2]. Diabetes is associated with fatal complications such as metabolic disorders, neuropathy, infections, cardiovascular disease, and retinopathy. Compared to conservative treatments, such as medications, metabolic surgery (MS) provides better control of blood glucose levels and diabetic complications[3-5].
Given its favorable outcomes in weight loss and hypoglycemic effects, jejunoileal bypass (JIB) was extensively used as a primary treatment for morbidly obese patients in the late 1960s and early 1970s. However, due to its adverse postoperative complications (including severe diarrhea, severe malnutrition, hepatic fibrosis, and blind ring syndrome), JIB was discontinued. With continued research and advancements, Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG) are currently the most commonly employed procedures in MS[6]. In addition to substantial weight loss, RYGB alleviates and reverses diabetes without the serious complications associated with JIB. However, in at least some regions, the proportion of SG among bariatric surgical procedures has been increasing, whereas the implementation of RYGB has remained relatively stable or even shown a decreasing trend[7,8]. Moreover, RYGB considerably alters the original physiological structure of the gastrointestinal tract, making it impossible to perform gastroscopy on the bypass portion, which may lead to missed diagnosis of certain gastric and duodenal diseases. Although there is an increasing trend for SG in clinical practice, its main role is weight loss, and its efficacy in treating diabetes is not as good as that of RYGB[9].
An extremely high percentage of absent intestines (up to 85%) could be attributed to the serious postoperative complications following conventional JIB. A modified approach to small intestinal bypass, which includes removing a smaller percentage of the proximal small intestines (approximately 60%) and preserving more functional small intestines, may contribute to effective glucose reduction and improve glucose tolerance. To our knowledge, systematic comparisons of the weight loss and hypoglycemic effects of proximal small intestinal bypass (PSIB), RYGB, and JIB in non-obese diabetic rats are lacking. In this study, PSIB, RYGB, and JIB surgeries were performed and compared their efficacies in terms of glucose reduction and weight loss. We chose to use the streptozotocin (STZ) non-obese diabetic rat model primarily to exclude the confounding effects of weight changes on glucose regulation, allowing us to more clearly observe the impact of different surgical procedures on glycemic control. The primary objective was to investigate whether bypassing a smaller percentage of the small intestine (approximately 60%) could achieve weight loss and hypoglycemic effect comparable to that of RYGB or conventional JIB.
A total of 60 male 7-week-old Sprague-Dawley rats weighing 278.0 ± 16 g were purchased from Shanghai Slaughter Laboratory Animals Ltd (Shanghai, China). All animals were housed at 18-25 °C under 12-hour light and dark cycles and had ad libitum access to food and water. The rats were acclimatized in a single cage for one week after intraperitoneal injection of 60 mg/kg STZ (Sigma, St. Louis, MO, United States). Seventy-two hours after the injection of STZ, rats with random blood glucose ≥ 16.7 mmol/L were selected for further study. The rats were randomly divided into PSIB group, RYGB group, and sham operation group. Body weight, food intake and fasting blood glucose (FBG) were recorded weekly after surgery. Oral glucose tolerance test (OGTT) and insulin tolerance test (ITT) were recorded at 2 and 6 weeks postoperatively, respectively. Animal experiments were conducted in accordance with the standards of the guidelines for animal experiments of Nanchang University and approved by the Animal Ethics Committee of Nanchang University. Standard animal care and laboratory guidelines were followed according to the ARRIVE guidelines.
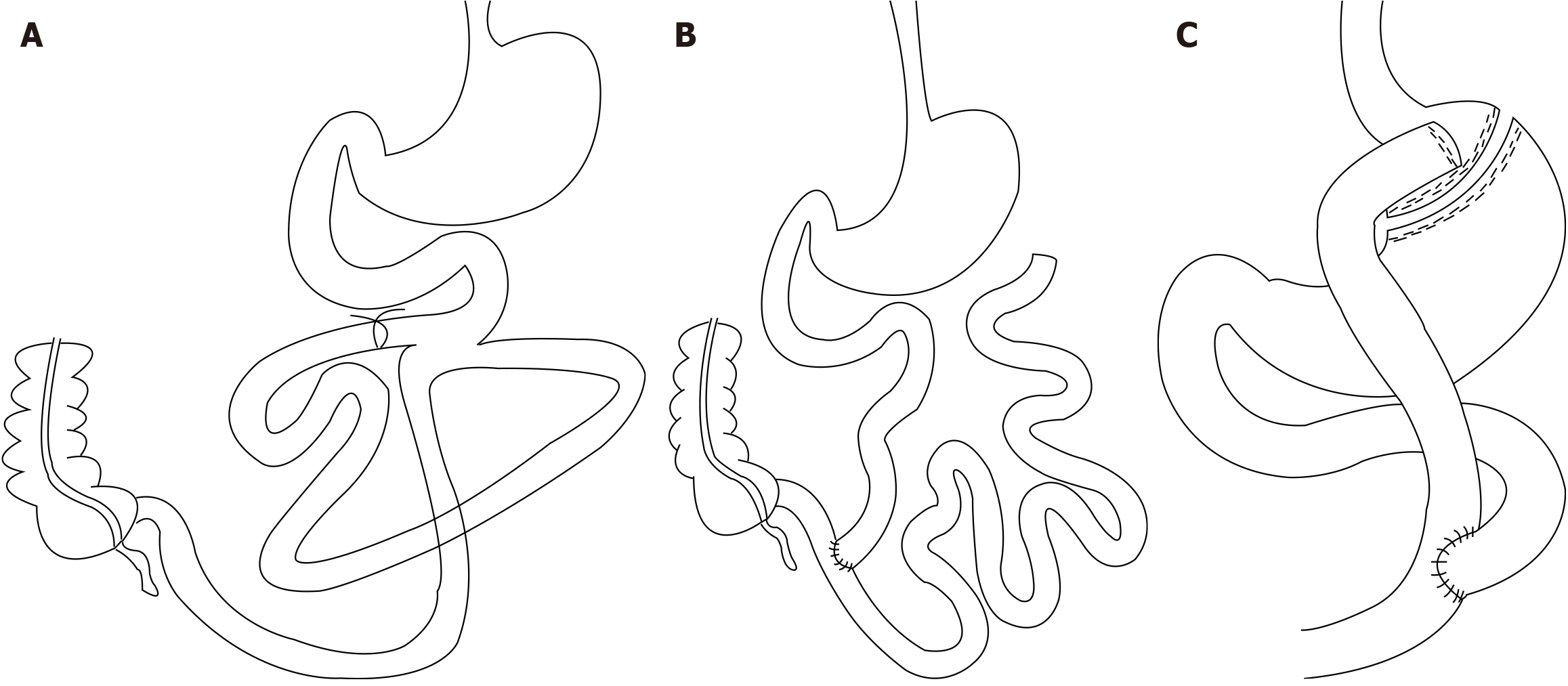
The rats were allowed to fast for 14 hours before surgery. Following anesthesia (isoflurane, 4% for induction and 2% for maintenance), the abdomen of the rats was carefully shaved, and a 4-cm-long incision was made in the middle of the abdomen. A side-to-side anastomosis was performed between the proximal jejunum and ileum at a distance of 10 cm distal to the ligament of Treitz and 30 cm proximal to the ileocecal valve as a point of reference (approximately 60% of the proximal small bowel was bypassed, and approximately 40% of the functional segment of the small bowel was preserved) to maintain parasympathetic activity in the small bowel and intestinal continuity. A single interrupted suture with a 6-0 nylon thread (Hangzhou Huawei Medical Treatment Articles Co., Ltd.) was placed. The length of the anastomosis was approximately 4 mm, and the intestinal collaterals were ligated with a No. 0 silk thread (ETHICON SA84G, Johnson & Johnson) near the small bowel anastomosis in the bypass. Finally, the abdomen was sutured using 3-0 silk thread (Figure 1A).
For JIB, the jejunum was dissected 10 cm distal to the ligament of Treitz, and the proximal small bowel was anastomosed end-to-side with the ileum. Anastomosis was performed approximately 5 cm from the ileum (bypassing approximately 85% of the small bowel). The blind ends of the jejunum and jejunum-ileum anastomosis were closed with a single interrupted layer of 6-0 nylon thread, and the anastomosis was approximately 4 mm long. Finally, the abdomen was sutured using a 3-0 silk thread (Figure 1B).
For RYGB, a gastric pouch of approximately 20% of the gastric volume was first created, and the pouch and distal stomach were closed using a single layer of interrupted 6-0 silk sutures. The jejunum was transected 10 cm distal to the ligament of Treitz, and the severed end of the jejunum was closed with a single layer of interrupted 6-0 silk suture, with the distal jejunum anastomosed side-to-side with the gastric pouch. At 15 cm from the gastro-jejunal anastomosis, the jejunal stump was anastomosed end-to-side with the distal jejunum. All anastomoses, measuring approximately 4 mm long, were closed with a 6-0 nylon thread in a single interrupted layer. Finally, the abdomen was sutured with a 3-0 silk thread (Figure 1C).
In the sham-operated group, the bowel was exposed, as previously described, without any other manipulation, and the duration of the operation was prolonged to produce an anesthetic stress comparable to that in the other two groups.
Glucose levels in the tail vein blood of conscious rats were measured using an electronic glucometer (Accu-Chek Performa®, Roche Diagnostics, Switzerland). FBG levels were measured at 9:00 am (after 14 hours of fasting) preoperatively and at 1, 2, 3, 4, 5, and 6 weeks postoperatively.
OGTT was performed at postoperative weeks 2 and 6, respectively. Rats were fasted overnight, and blood glucose was measured before and 15, 30, 60, 90, 120, and 180 minutes after gavage of 20% glucose (1 g/kg glucose), and the area under the glucose tolerance curve was calculated. ITT was performed on awake rats to assess insulin sensitivity by measuring glucose levels before and 15, 30, 45, and 60 minutes after intraperitoneal injection of 0.5 IU/kg human insulin (Wanbang Biopharmaceuticals, Jiangsu, China), and the area under the insulin tolerance curve was calculated.
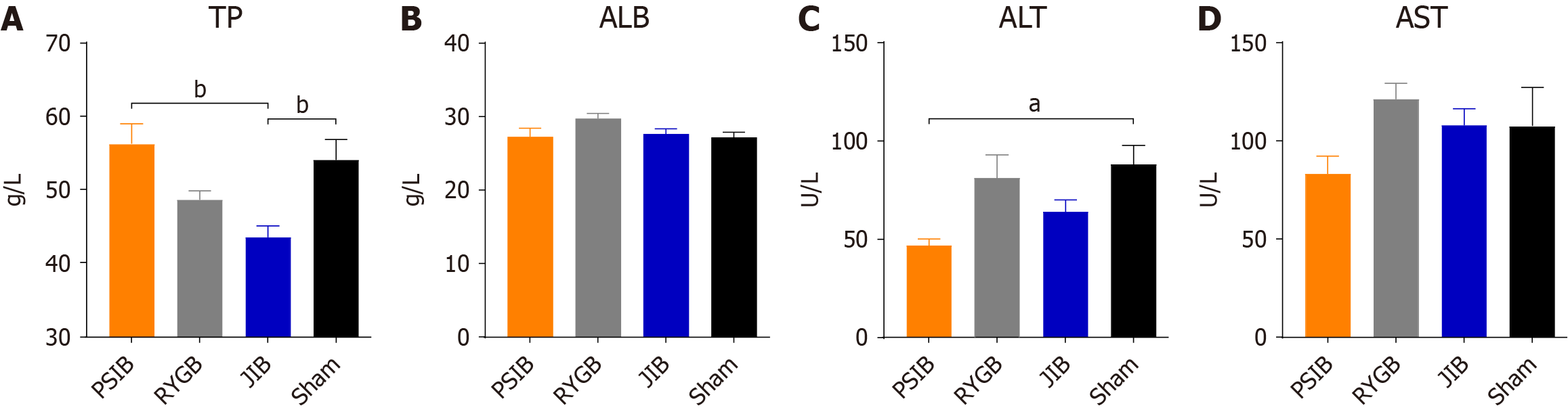
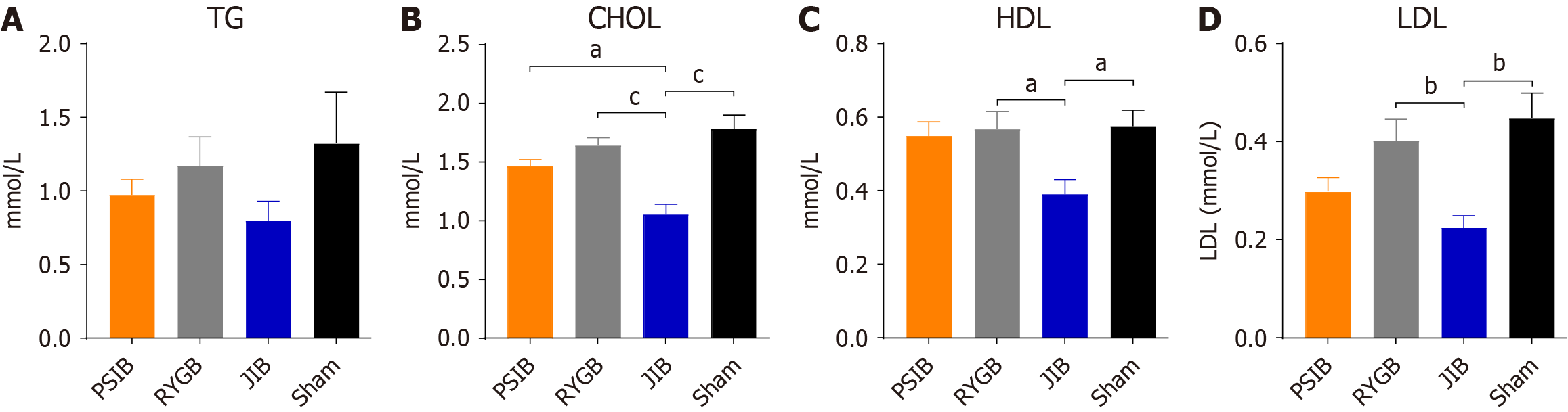
At 6 weeks postoperatively, the rats were euthanized after a 10-hour fast, and portal blood was collected and placed in biochemical test tubes containing coagulants. The blood was centrifuged at 3000 rpm for 15 minutes at 4 °C, then immediately transferred to new tubes and stored at -80 °C until further analysis. A fully automated biochemical analyzer was used to determine serum total cholesterol (TC), triglycerides, high-density lipoprotein (HDL), low-density lipoprotein (LDL), alanine aminotransferase, aspartate aminotransferase, total protein (TP), and albumin levels. All the assays and analyses were performed in a biochemical laboratory.
All data are expressed as the mean ± SE. All statistical analyses were performed using GraphPad Prism version 9.5.0 (La Jolla, CA, United States), and P < 0.05 indicated statistical significance. The area under the curve was calculated using the trapezoidal integration method. Two-way analysis of variance was used to analyze changes in body weight, food intake, FBG, OGTT, and ITT. One-way analysis of variance was used to analyze liver enzymes and lipid levels.
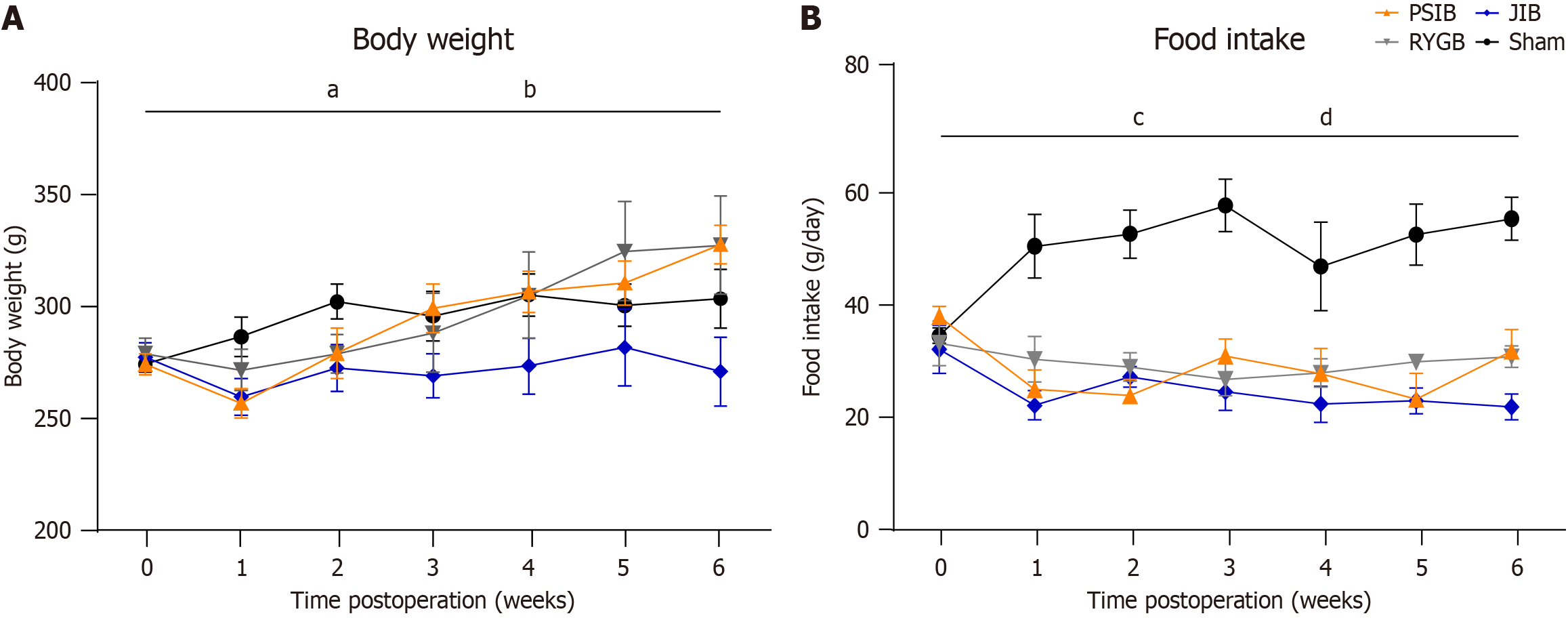
From postoperative weeks 2 to 6, all rats showed a slight increase in body weight. However, the body weights of rats in the JIB group were lower than those in the sham group (P < 0.05). Moreover, among the surgical groups, the weight of the JIB group was lower than that of the RYGB and PSIB groups (Figure 2A).
The global food intake of rats in all the surgical groups was significantly lower than that of rats in the sham group (all P < 0.01). Among the three surgical groups, rats in the JIB group had a lower food intake than those in the RYGB group (Figure 2B).
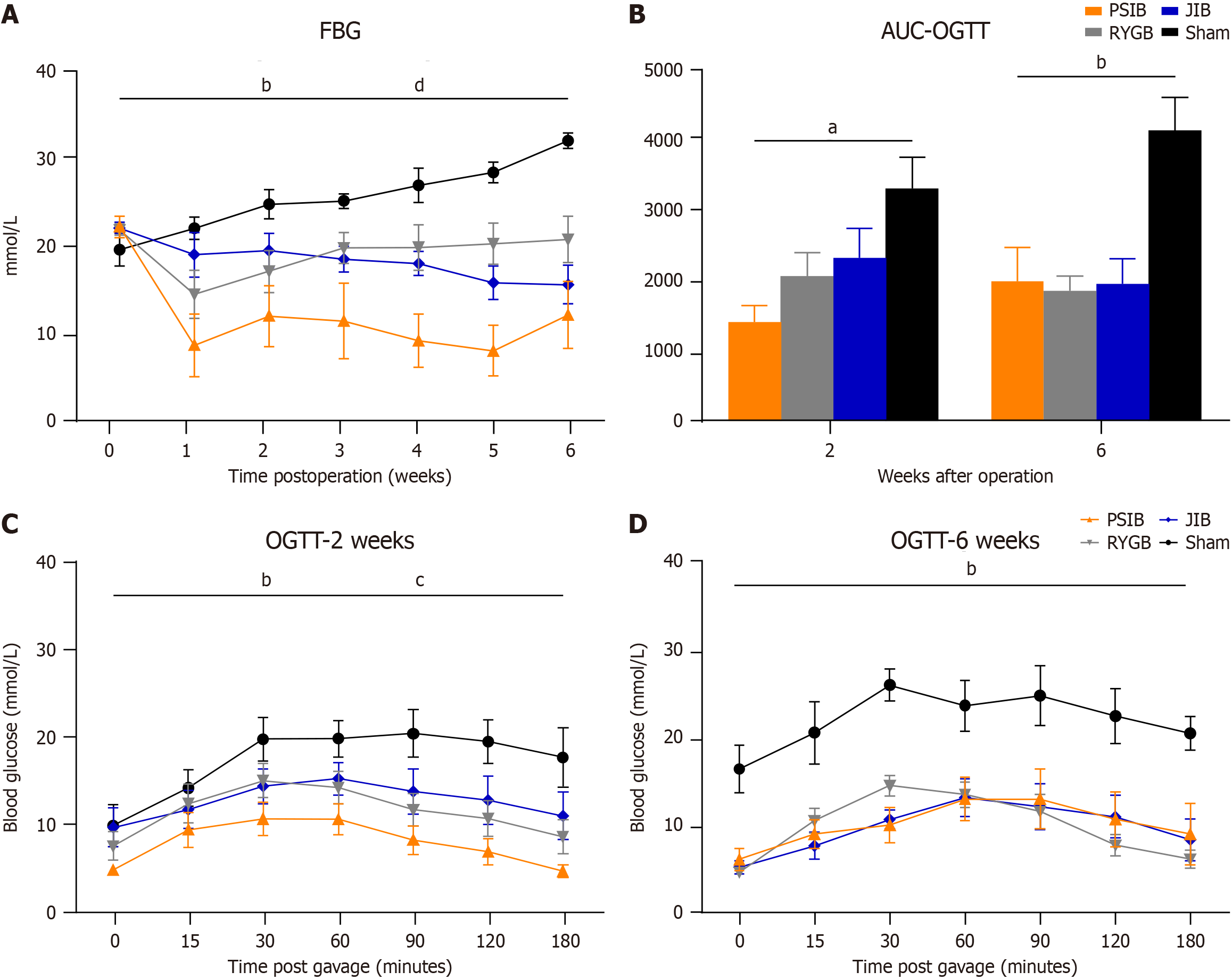
The postoperative FBG levels of the rats in the surgical and sham groups is shown (Figure 3A). No difference in preoperative FBG levels was observed among the rats. However, the FBG levels of rats in the PSIB, RYGB, and JIB groups were significantly lower than those in the sham group (all P < 0.01) between weeks 1 and 6 postoperatively. Moreover, among the operated groups, FBG levels in the PSIB group were lower than those in the RYGB and JIB groups (both P < 0.01) (Figure 3A).
Significant improvement in OGTT was observed among the rats in the PSIB, RYGB, and JIB groups at postoperative week 2 (all P < 0.01). However, rats in the PSIB group exhibited better OGTT than those in the RYGB and JIB groups (P < 0.05 and P < 0.01, respectively) (Figure 3B and C). By postoperative week 6, compared with rats in the sham group, rats in the surgical groups continued to exhibit improvement in OGTT (all P < 0.01). However, the difference was not statistically significant (Figure 3D).
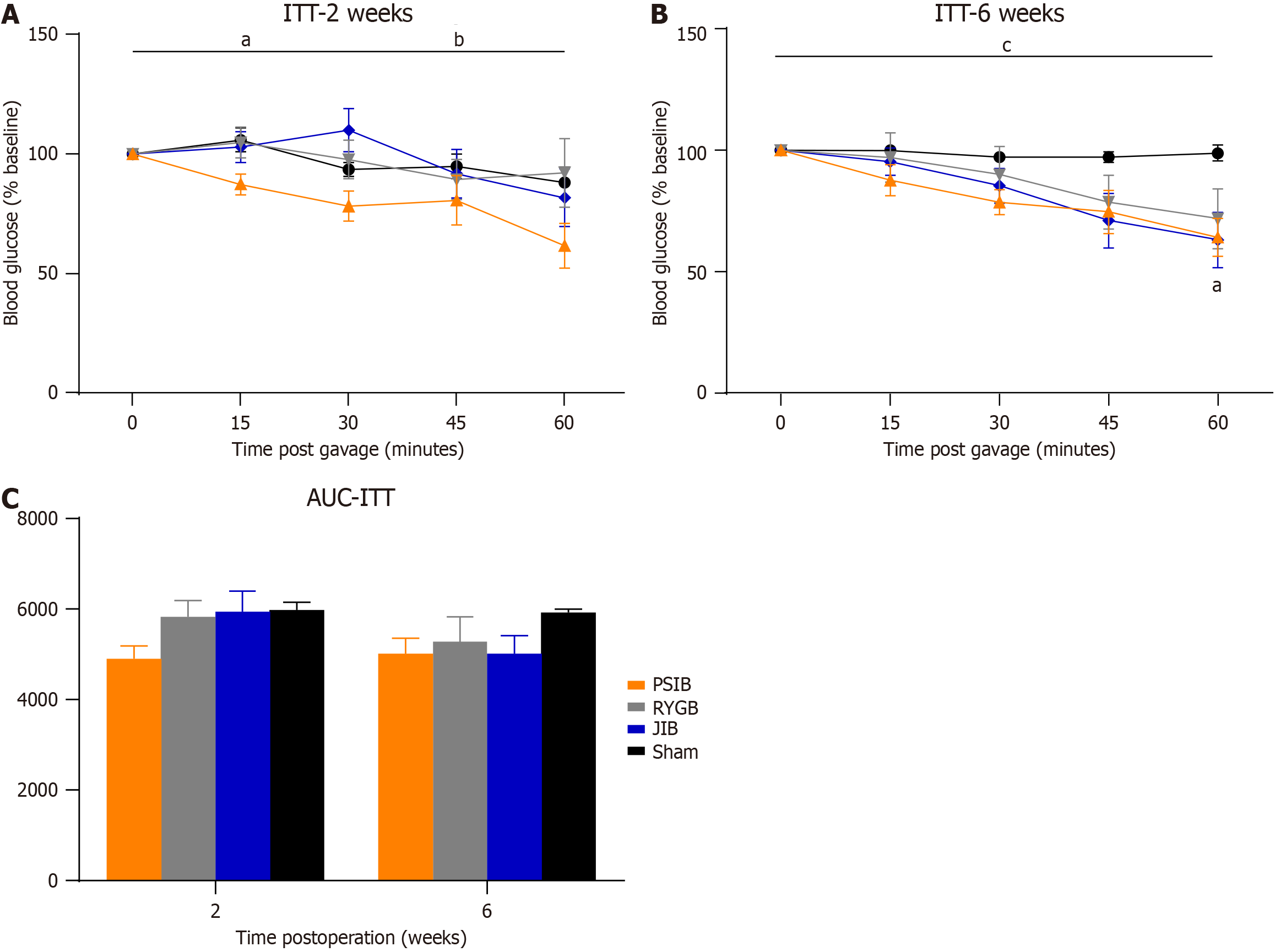
Figure 4 shows the ITT results of the rats in the surgical and sham-operated groups. At postoperative week 2, only rats in the PSIB group exhibited an improvement in insulin sensitivity (P < 0.05), with a statistically significant difference in insulin sensitivity between the PSIB group and the RYGB and JIB groups (both P < 0.05). At postoperative week 6, insulin sensitivity improved in both the PSIB and JIB groups (both P < 0.05), indicated by a faster rate of glycemic decline at 60 minutes of ITT. However, they did not demonstrate a lower area under the insulin tolerance curve.
At 6 weeks postoperatively, only the TP level in the JIB group was lower than that in the sham group (P < 0.01), whereas the TB level in the PSIB group was higher than that in the JIB group (P < 0.01), with no statistically significant difference in the albumin level between the groups (Figure 5A and B). In addition, only the alanine aminotransferase level in the PSIB group was lower than that in the sham group (P < 0.05), whereas no statistically significant difference in the aspartate aminotransferase levels was observed between the groups (Figure 5C and D). In terms of lipid metabolism, the levels of TC, HDL, and HDL in the JIB group were lower than those in the sham group (all P < 0.05). Moreover, the levels of TC in the JIB group were lower than those in the PSIB and RYGB groups, and the levels of HDL and LDL in the JIB group were lower than those in the RYGB group (Figure 6).
This is the first systematic study to compare the efficacy of PSIB with RYGB and JIB for weight loss and glucose reduction using the STZ-induced non-obese diabetic rat model. Our findings elucidated that: (1) All three surgical groups had reduced blood glucose levels and improved glucose tolerance, and the efficacy of PSIB was better than that of RYGB and JIB; (2) Although the rats in all three surgical groups exhibited reduced appetite, only JIB reduced body weight in STZ-DM rats; and (3) At least in the short term, PSIB did not exhibit any abnormalities in liver enzyme levels or lipid profiles.
Classical JIB requires transection of the jejunum and end-to-end anastomosis of the jejunum to the distal ileum, which separates the jejunal branch from the autologous pacemaker and disrupts the electrophysiology of the small bowel, producing an ectopic pacemaker[10]. Ectopic pacemakers are generated at a very low frequency in the middle of the bypassed small bowel segment and can also pace in a proximal direction. This indicates that the JIB does not actually produce 85% of the bypass because some of the food arriving at the anastomosis refluxes into the bypassed small-bowel segment to be digested and absorbed, thereby increasing blood glucose levels. In contrast, PSIB did not transect the jejunum, but only made a simple side-to-side anastomosis of the small bowel, which maintained the normal electrophysiology of the small bowel, enabling a certain amount of antegrade peristalsis in the bypassed small bowel segments, with a much smaller amount of food regurgitation than that of the end-to-side anastomosis of JIB. Conversely, the bypass small bowel segment of PSIB plays a minimal role in digestion and absorption. Therefore, we hypothesized that the superior efficacy of PSIB over JIB in lowering blood glucose levels and improving glucose tolerance could be attributed to the side-to-side anastomosis, which eliminates the retrograde peristalsis of the bypassed small bowel segment, thereby reducing glucose absorption. However, a limitation of this study is that the electrophysiological status of the small intestine was not assessed. In addition, Melissas et al[11] reported that simple side-to-side jejunoileal anastomosis to transfer food and biliopancreatic secretions to the distal small bowel normalized FBG levels and OGTT in Goto-Kakizaki rats. Subsequently, side-to-side jejunoileal anastomosis was performed in patients with diabetes with a body mass index of 28-32 kg/m2, and three of the six patients had complete remission of diabetes from the early postoperative period to 3 years postoperatively[12].
Compared to the proximal small intestine, the distal small intestine exhibits a more pronounced advantage in glucose regulation, which may be a crucial factor underlying the significant improvement in glycemic control observed with PSIB. Previous studies have demonstrated that, at least in humans, the rate of glucose absorption in the proximal small intestine is markedly higher than that in the distal segment[13]. Consequently, by bypassing the proximal small intestine and directly delivering nutrients to the distal portion, the velocity of glucose absorption can be slowed, thereby contributing to the mitigation of rapid increases in blood glucose levels. Furthermore, the response of the proximal small intestine to glucose stimulation primarily involves the release of hormones such as glucose-dependent insulinotropic polypeptide and cholecystokinin, whereas the distal small intestine predominantly facilitates the secretion of glucagon-like peptide-1 (GLP-1)[13]. The “hindgut hypothesis” posits that the accelerated transit of food into the distal small intestine following MS activates L-cells, promoting the production of GLP-1 and protein YY, thus exerting hypoglycemic effects[14]. Transcriptomic analyses have unveiled multiple adaptive changes occurring in the terminal ileum region post-RYGB, and there is evidence suggesting that the increased GLP-1 secretion post-RYGB might be attributed to carbohydrate absorption in the distal rather than the proximal small intestine[15]. These findings collectively suggest that hormones secreted by the distal small intestine demonstrate greater efficacy in modulating glucose metabolism compared to those released from the proximal part[16]. Such insights provide robust support for understanding the pivotal role of the distal small intestine in PSIB intervention.
The proximal end of the blind collaterals was ligated with silk threads, which prevented chyme ingress into the bypassed small bowel segment and reduced the digestive and absorptive area of the gastrointestinal tract, thereby reducing glucose absorption, and achieving a synergistic effect with the side-to-side anastomosis to ensure antegrade peristalsis of the small bowel. Ligation of the proximal end of the intestinal collaterals demonstrated better hypoglycemic and glucose tolerance efficacy than without ligation[17]. Furthermore, food cannot pass through after ligation of the proximal end of the blind collaterals. However, a small amount of bile and other digestive fluids are still allowed to pass through the intestinal collaterals, resulting in substantial changes in the intestinal flora and bile acid metabolism, which reduces blood glucose levels and improves glucose tolerance[18,19], highlighting the superior efficacy of PSIB over JIB in lowering blood glucose and improving glucose tolerance. However, further studies comparing PSIB and JIB in postoperative intestinal motility, changes in intestinal bacteria, and bile acid metabolism are warranted to validate this finding.
Our results showed that in a non-obese diabetic rat model, the efficacy of PSIB in lowering blood glucose levels and improving glucose tolerance was better than that of RYGB, with comparable weight loss. RYGB can reduce blood glucose levels and improve glucose tolerance after surgery by regulating gastrointestinal hormones, bile acids, β-cell function, and intestinal flora[20]. A study by Patel et al[21] revealed that gastric bypass plays a dominant role in regulating body weight, whereas small intestinal bypass affects glucose homeostasis mainly through body weight, insulin, and enteric proinsulin-independent mechanisms. This partially explains the effectiveness of MS involving small intestine bypass compared with that of MS of the open stomach for diabetes mellitus. Angelini et al[22] further revealed that the jejunum plays an important role in controlling insulin sensitivity. These two studies may partially explain the better effectiveness of PSIB vs RYGB in achieving hypoglycemia and improving glucose tolerance. However, additional in-depth studies are needed to investigate the specific mechanistic differences between the two procedures. In the model used in this study, no difference in weight control was observed between the two groups. However, further studies are needed to determine whether RYGB is more effective than PSIB in reducing weight in other models, such as the obese rat model. Given that an STZ-induced non-obese diabetic rat model rather than an obese rat model was used in this study, weight loss in non-obese rats did not differ significantly compared with that of the control group. However, the reason may be that PSIB and RYGB alleviated diabetes to a certain extent, which mitigated continued weight loss in diabetic rats. However, the alleviation of diabetes by JIB was not sufficient to compensate for weight loss due to maldigestion and malabsorption of nutrients. Compared with RYGB, PSIB enhances the hypoglycemic effect and improves glucose tolerance without substantial weight loss, which may be beneficial for non-obese or mildly obese patients with diabetes. This study also provides a theoretical basis for the clinical application of PSIB. However, further clinical studies are required.
The overall incidence of hypoalbuminemia after MS is 3%-18%[23]. In the present study, TP levels were significantly decreased in the JIB group, but not in the PSIB or RYGB groups. This aligns with those of previous findings, which demonstrates that excessive bypassing of the small intestine results in malnutrition and consequent liver impairment, a potential reason for avoiding JIB. In addition, HDL and LDL levels at 6 weeks were lower in the JIB group than in the sham group. Our data indicate that various MS may exert differential effects on the lipid profile. We hypothesize that such variations might be associated with alterations in the transit time and pathway of food through the intestine, which could influence the absorption and metabolism of lipids. Moreover, investigators have observed differences in cholesterol metabolism between patients undergoing biliopancreatic diversion and bilio-intestinal bypass and laparoscopic adjustable gastric banding[24]. Although both groups exhibited similar decreases in homeostatic model assessment of insulin resistance and triglyceride levels postoperatively, only the bilio-intestinal bypass group demonstrated reductions in serum TC, LDL, and non-HDL cholesterol, whereas no significant changes were noted in the laparoscopic adjustable gastric banding cohort[24]. However, the underlying mechanisms responsible for these outcomes remain unclear and warrant further investigation into the specific alterations in lipid profiles following different MS procedures.
Our study had certain limitations. First, this study included a small sample size and was conducted over a short period of time. Second, because non-obese rats were selected, significant differences in the efficacy of weight loss could not be demonstrated. Third, alterations in gastrointestinal hormones, pancreatic islet cells, and intestinal bacteria in the surgical group could not be measured or compared, warranting further investigations.
In conclusion, PSIB reduces blood glucose levels and improves glucose tolerance and insulin sensitivity in the early postoperative period, and its efficacy is superior to that of classical RYGB and conventional JIB. Because the non-obese diabetic rat model used in this study did not exhibit significant weight loss after PSIB, this may be beneficial for non-obese diabetic or mildly obese patients with diabetes to achieve good glycemic control without weight loss. Further clinical studies are required to validate these findings.
| 1. | Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2249] [Cited by in RCA: 3385] [Article Influence: 483.6] [Reference Citation Analysis (0)] |
| 2. | Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2306] [Cited by in RCA: 2503] [Article Influence: 312.9] [Reference Citation Analysis (0)] |
| 3. | Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, Kashyap SR; STAMPEDE Investigators. Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. N Engl J Med. 2017;376:641-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1626] [Cited by in RCA: 1888] [Article Influence: 236.0] [Reference Citation Analysis (0)] |
| 4. | Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Capristo E, Chamseddine G, Bornstein SR, Rubino F. Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2021;397:293-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 324] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 5. | Courcoulas AP, Patti ME, Hu B, Arterburn DE, Simonson DC, Gourash WF, Jakicic JM, Vernon AH, Beck GJ, Schauer PR, Kashyap SR, Aminian A, Cummings DE, Kirwan JP. Long-Term Outcomes of Medical Management vs Bariatric Surgery in Type 2 Diabetes. JAMA. 2024;331:654-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 93] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 6. | Fink J, Seifert G, Blüher M, Fichtner-Feigl S, Marjanovic G. Obesity Surgery. Dtsch Arztebl Int. 2022;119:70-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Alalwan AA, Friedman J, Park H, Segal R, Brumback BA, Hartzema AG. US national trends in bariatric surgery: A decade of study. Surgery. 2021;170:13-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 113] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 8. | Clapp B, Ponce J, Corbett J, Ghanem OM, Kurian M, Rogers AM, Peterson RM, LaMasters T, English WJ. American Society for Metabolic and Bariatric Surgery 2022 estimate of metabolic and bariatric procedures performed in the United States. Surg Obes Relat Dis. 2024;20:425-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 9. | Castellana M, Procino F, Biacchi E, Zupo R, Lampignano L, Castellana F, Sardone R, Palermo A, Cesareo R, Trimboli P, Giannelli G. Roux-en-Y Gastric Bypass vs Sleeve Gastrectomy for Remission of Type 2 Diabetes. J Clin Endocrinol Metab. 2021;106:922-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Mahmood A, Mahmood N, Robinson RB. Small Bowel Intussusception: A Dangerous Sequela of Bariatric Surgery. Radiol Case Rep. 2007;2:10-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Melissas J, Peirasmakis D, Lamprou V, Papadakis J. Is a Simple Food-Diverting Operation the Solution for Type 2 Diabetes Treatment? Experimental Study in a Non-Obese Rat Model. Obes Surg. 2016;26:1010-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Melissas J, ErenTaskin H, Peirasmakis D, Dimitriadis E, Papadakis M, Zengin SU, Yumuk V, Taskin M. A Simple Food-Diverting Operation for Type 2 Diabetes Treatment. Preliminary Results in Humans with BMI 28-32 kg/m2. Obes Surg. 2017;27:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Zhang X, Young RL, Bound M, Hu S, Jones KL, Horowitz M, Rayner CK, Wu T. Comparative Effects of Proximal and Distal Small Intestinal Glucose Exposure on Glycemia, Incretin Hormone Secretion, and the Incretin Effect in Health and Type 2 Diabetes. Diabetes Care. 2019;42:520-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Knop FK. Resolution of type 2 diabetes following gastric bypass surgery: involvement of gut-derived glucagon and glucagonotropic signalling? Diabetologia. 2009;52:2270-2276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Martinussen C, Bojsen-Møller KN, Dirksen C, Svane MS, Kristiansen VB, Hartmann B, Holst JJ, Madsbad S. Augmented GLP-1 Secretion as Seen After Gastric Bypass May Be Obtained by Delaying Carbohydrate Digestion. J Clin Endocrinol Metab. 2019;104:3233-3244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Xie C, Jones KL, Rayner CK, Wu T. Enteroendocrine Hormone Secretion and Metabolic Control: Importance of the Region of the Gut Stimulation. Pharmaceutics. 2020;12:790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Ren Q, Duan J, Cao J. Rapid Improvement in Diabetes After Simple Side-to-side Jejunoileal Bypass Surgery: Does It Need a Ligation or Not? Obes Surg. 2018;28:1974-1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Flynn CR, Albaugh VL, Abumrad NN. Metabolic Effects of Bile Acids: Potential Role in Bariatric Surgery. Cell Mol Gastroenterol Hepatol. 2019;8:235-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Anhê FF, Zlitni S, Zhang SY, Choi BS, Chen CY, Foley KP, Barra NG, Surette MG, Biertho L, Richard D, Tchernof A, Lam TKT, Marette A, Schertzer J. Human gut microbiota after bariatric surgery alters intestinal morphology and glucose absorption in mice independently of obesity. Gut. 2023;72:460-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 40] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 20. | Pucci A, Batterham RL. Mechanisms underlying the weight loss effects of RYGB and SG: similar, yet different. J Endocrinol Invest. 2019;42:117-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 21. | Patel RT, Shukla AP, Ahn SM, Moreira M, Rubino F. Surgical control of obesity and diabetes: the role of intestinal vs. gastric mechanisms in the regulation of body weight and glucose homeostasis. Obesity (Silver Spring). 2014;22:159-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Angelini G, Salinari S, Castagneto-Gissey L, Bertuzzi A, Casella-Mariolo J, Ahlin S, Boskoski I, Gaggini M, Raffaelli M, Costamagna G, Casella G, Marini PL, Gastaldelli A, Bornstein S, Mingrone G. Small intestinal metabolism is central to whole-body insulin resistance. Gut. 2021;70:1098-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Stein J, Stier C, Raab H, Weiner R. Review article: The nutritional and pharmacological consequences of obesity surgery. Aliment Pharmacol Ther. 2014;40:582-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 155] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 24. | Benetti A, Del Puppo M, Crosignani A, Veronelli A, Masci E, Frigè F, Micheletto G, Panizzo V, Pontiroli AE. Cholesterol metabolism after bariatric surgery in grade 3 obesity: differences between malabsorptive and restrictive procedures. Diabetes Care. 2013;36:1443-1447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |