Published online Feb 27, 2025. doi: 10.4240/wjgs.v17.i2.102342
Revised: December 7, 2024
Accepted: December 26, 2024
Published online: February 27, 2025
Processing time: 76 Days and 2 Hours
Ulcerative colitis (UC) is a complex inflammatory bowel disease, and its etiology and pathogenesis remain incompletely elucidated.
To analyze the effects of Saccharomyces boulardii in combination with sulfasalazine on intestinal microbiota and intestinal barrier function in patients with UC.
A retrospective analysis of clinical data from 127 UC patients admitted to our hospital between January 2021 and January 2023 was conducted. All patients met complete inclusion and exclusion criteria. Based on the treatment interventions received, they were divided into a control group (n = 63) and an observation group (n = 64). Both groups of patients received routine treatment upon admission. The control group received sulfasalazine in addition to routine interventions, while the observation group received a combination of Saccharomyces boulardii on the basis of the control group’s treatment. The clinical efficacy, improvement in symptoms, modified Baron endoscopic scores, quality of life “inflammatory bowel disease questionnaire (IBDQ)”, levels of intestinal microbial indicators (such as Lactobacillus, Bifidobacterium, Enterococcus, and Escherichia coli), intestinal mucosal barrier function indicators [diamine oxidase (DAO), lipopolysaccharide (LPS), D-lactic acid (D-LA)], and adverse reaction occurrences were compared between the two groups.
(1) Clinical efficacy: The total effective rate in the control group was 79.37%, while in the observation group, it was 93.75%, significantly higher than that of the control group (P < 0.05); (2) Improvement in symptoms: The observation group showed significantly lower relief time for abdominal pain, diarrhea, rectal bleeding, fever symptoms, and mucosal healing time compared to the control group (P < 0.05); (3) Baron endoscopic scores and IBDQ scores: Before treatment, there was no significant difference in Baron endoscopic scores and IBDQ scores between the two groups (P > 0.05). However, after treatment, the observation group showed significantly lower Baron endoscopic scores and higher IBDQ scores compared to the control group (P < 0.05); (4) Levels of intestinal microbial indicators: Before treatment, there was no significant difference in the levels of Lactobacillus, Bifidobacterium, Enterococcus, and Escherichia coli between the two groups (P > 0.05). After treatment, the levels of Lactobacillus and Bifidobacterium in the observation group were significantly higher than those in the control group, while the levels of Enterococcus and Escherichia coli were significantly lower than those in the control group (P < 0.05); (5) Levels of intestinal mucosal barrier function indicators: Before treatment, there was no significant difference in the levels of DAO, LPS, and D-LA between the two groups (P > 0.05). However, after treatment, the levels of DAO, LPS, and D-LA in the observation group were significantly lower than those in the control group (P < 0.05); and (6) Occurrence of adverse reactions: The incidence of adverse reactions in the control group was 9.52%, while in the observation group, it was 10.94%. There was no significant difference in the occurrence of adverse reactions between the two groups (P > 0.05).
The application of Saccharomyces boulardii in combination with sulfasalazine in UC patients demonstrates significant effectiveness. Compared to sole sulfasalazine intervention, the combined application of Saccharomyces boulardii further promotes the relief of relevant symptoms in patients, alleviates intestinal mucosal inflammation, and improves the quality of life. Its action may be related to rectifying the imbalance in intestinal microbiota and improving intestinal mucosal barrier function. Moreover, the combined use of Saccharomyces boulardii does not increase the risk of adverse reactions in patients, indicating a higher level of medication safety and advocating for its clinical promotion and application.
Core Tip: The application of Saccharomyces boulardii in combination with sulfasalazine in ulcerative colitis patients demonstrates significant effectiveness. Compared to sole sulfasalazine intervention, the combined application of Saccharomyces boulardii further promotes the relief of relevant symptoms in patients, alleviates intestinal mucosal inflammation, and improves the quality of life. Its action may be related to rectifying the imbalance in intestinal microbiota and improving intestinal mucosal barrier function. Moreover, the combined use of Saccharomyces boulardii does not increase the risk of adverse reactions in patients, indicating a higher level of medication safety and advocating for its clinical promotion and application.
- Citation: Yang CC, Zhang S, Zhang R, Zhao YN, Yang DW, Yang MY, Huang LJ. Application of Saccharomyces boulardii in combination with sulfasalazine in ulcerative colitis patients demonstrates significant effectiveness. World J Gastrointest Surg 2025; 17(2): 102342
- URL: https://www.wjgnet.com/1948-9366/full/v17/i2/102342.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i2.102342
Ulcerative colitis (UC) is a complex inflammatory bowel disease, and its etiology and pathogenesis remain incompletely elucidated. Although the pathogenic factors for UC are diverse, studies have indicated the significant roles played by abnormal immune system function, genetic susceptibility, and environmental factors in its development[1,2]. This chronic condition is often accompanied by symptoms such as abdominal pain, diarrhea, rectal bleeding, severely impacting patients’ quality of life, and potentially leading to severe damage and functional impairment of the intestinal mucosa[3]. Over the past few decades, research into UC treatment has advanced, and sulfasalazine has been widely used in its treatment. However, resistance or adverse reactions to sulfasalazine exist in some patients, limiting its clinical effectiveness[4]. Therefore, finding new therapeutic strategies to enhance efficacy and reduce adverse reactions is one of the current research focuses. In recent years, growing evidence has highlighted the critical role of gut microbiota and intestinal barrier dysfunction in UC pathogenesis[5,6]. The intestinal mucosa, as a crucial barrier between the intestine and the external environment, malfunction of which might directly lead to pathological deterioration. Hence, modulating the gut microbiota structure and enhancing intestinal barrier function has become one of the crucial approaches to improve UC treatment outcomes.
Saccharomyces boulardii, as a probiotic, holds the potential to regulate gut microbiota and enhance intestinal barrier function[7]. The combined application of sulfasalazine and Saccharomyces boulardii is expected to ameliorate clinical symptoms in UC patients, alleviate intestinal mucosal inflammation, and enhance patients’ quality of life. However, the specific efficacy of this combined treatment and its impact on the composition of intestinal microbiota and barrier function in clinical practice require further systematic research and validation. Thus, this study aims to systematically assess the efficacy and safety of Saccharomyces boulardii in combination with sulfasalazine in treating UC, with a focus on its effects on symptom relief, regulation of gut microbiota, and intestinal mucosal barrier function in UC patients. Through an in-depth analysis of clinical data and biomarker changes, we anticipate providing more options for personalized UC treatment and offering scientific evidence for the development of therapeutic strategies involving the regulation of gut microbiota. The results of this study are expected to provide new clinical treatment ideas and strategies to enhance UC treatment outcomes and patients’ quality of life.
A retrospective analysis was conducted on clinical data from 127 UC patients admitted to our hospital between January 2021 and January 2023. Based on the treatment interventions received, patients were divided into a control group (n = 63) and an observation group (n = 64). All patients received routine treatments upon admission, with the control group receiving sulfasalazine in addition to routine treatments, while the observation group received Saccharomyces boulardii in combination with the treatment given to the control group. To minimize confounding factors, the baseline characteristics (age, gender, body mass index, duration of UC, and disease severity) of the two groups were compared. No significant differences were found, suggesting comparability between the groups at baseline. Although patients were not formally matched, potential confounders such as diet, previous treatments, and comorbidities were assessed during data collection to ensure homogeneity of the sample.
Inclusion criteria: (1) Patients diagnosed with UC through clinically relevant tests; (2) Patients aged between 18 years and 70 years, regardless of gender; (3) Disease severity was assessed using the modified Mayo score, a standardized clinical scoring system for UC that includes stool frequency, rectal bleeding, endoscopic findings, and physician’s global assessment. Patients with scores of 3-6 were classified as having mild UC, while those with scores of 7-9 were considered to have moderate UC[8]; (4) Patients with no recent history of UC-related treatments; and (5) Patients with complete and reliable clinical data available for analysis.
Exclusion criteria: (1) Exclusion of patients with severe UC conditions; (2) Exclusion of individuals with severe organ dysfunction; (3) Exclusion of those with abnormalities in the immune system, coagulation function, etc.; (4) Exclusion of patients with a history of gastrointestinal surgery; (5) Exclusion of individuals with known allergies or contraindications to the drugs or methods used in this study; and (6) Exclusion of patients with concurrent cognitive or consciousness disorders.
Upon admission, both groups received routine symptomatic treatments including nutritional supplementation, antidiarrheal medications, anti-inflammatory drugs, and maintenance of electrolyte balance. Patients were advised to follow a bland diet and avoid spicy food during the treatment period. To ensure adherence to the prescribed regimen, all patients were regularly monitored by trained medical staff who conducted follow-up visits every two weeks. During these visits, patients were asked about their medication usage, and medication logs were provided to track the administration of sulfasalazine and Saccharomyces boulardii. Any discrepancies or non-compliance were addressed immediately by the clinical staff. In addition, no other concomitant treatments were allowed during the study period to avoid potential confounding effects.
Control group: Patients in the control group received sulfasalazine in addition to routine treatments. The medication was administered rectally, twice a day after morning and evening bowel movements. The patient assumed a left lateral position, the anus was exposed, and the sulfasalazine suppository (from Sichuan Aba Pharmaceutical Co., Ltd., National Medicine Standard H20058244) was inserted approximately 8-10 cm deep into the rectum, at a dose of 0.5 g each time, twice a day.
Observation group: Patients in the observation group received Saccharomyces boulardii in combination with the treatment received by the control group. The specifications, usage, and dosage of the Sulfasalazine suppository in the observation group were consistent with those in the control group. Additionally, Saccharomyces boulardii (from BIOCODEX, France, Approval No. S20150051) was administered orally at a dose of 0.5 g, twice a day. Both groups underwent continuous treatment for 2 months.
Observational indices: (1) Clinical efficacy: Complete remission, after treatment, symptoms like diarrhea, abdominal pain, bloody stools disappeared, bowel movements were ≤ 2 times/day, normal results were observed in routine stool examination and colonoscopy re-examination; (2) Effective: After treatment, symptoms significantly improved or almost disappeared, bowel movements were formed, occurring 2-4 times/day, routine examination indicated red blood cells and white blood cells ≤ 10 per high-power field, mild inflammation was observed in the intestinal mucosa, and some pseudopolyps formed; (3) Ineffective: After treatment, symptoms did not meet the above criteria or even worsened. The total effective rate of treatment = (number of complete remission cases + number of effective cases)/total number of cases × 100%; (4) Symptomatic improvement: The observed indicators for symptom improvement in this study included time for relief of abdominal pain, diarrhea, bloody stools, fever, and mucosal healing time. These indicators were uniformly recorded by relevant medical staff in our hospital; (5) Modified Baron endoscopic score[9]: Before and after treatment, the severity of intestinal mucosal inflammation in patients was evaluated using the modified Baron endoscopic score, which grades the degree of inflammation observed during endoscopy into 4 levels, ranging from 0-3 points, where higher scores indicate more severe mucosal inflammation; (6) Quality of life: Before and after treatment, the inflammatory bowel disease questionnaire (IBDQ) scale[10] was used to assess the patients’ quality of life. The IBDQ scale consists of 32 questions covering aspects related to intestinal symptoms, systemic symptoms, emotional function, and social function. Each question is scored from 1 point to 7 points, with a total score ranging from 32 points to 224 points. Higher scores indicate better quality of life; (7) Quantitative levels of intestinal flora indices: Before and after treatment, fresh fecal samples (0.5 g) were collected from each patient, serially diluted 9-fold to 10-fold, and cultured on selective media to isolate and culture intestinal flora (Bifidobacterium, Lactobacillus, Enterococcus, Escherichia coli). The results were quantified using an automated microbial identification system, expressed as the logarithm of colony-forming units per gram of wet feces (lgCFU/g); (8) Intestinal mucosal barrier function indices: Before and after treatment, 3 mL of fasting venous blood was collected from each patient in the morning. The plasma was obtained by routine centrifugation, and the levels of diamine oxidase (DAO), lipopolysaccharide (LPS), and D-lactic acid (D-LA) were measured using an enzyme-linked immunosorbent assay; and (9) Occurrence of adverse reactions: Adverse reactions observed in this study included nausea, vomiting, decreased appetite, indigestion, and gastric burning sensation. The occurrence of these adverse reactions was uniformly recorded by relevant medical staff in our hospital.
GraphPad Prism 8 was used for graphical representation, and SPSS 22.0 was employed for data analysis. For continuous data, mean and standard deviation were used to describe the distribution, and statistical analysis was performed using t-tests or analysis of variance. For categorical data, frequency and percentage were used for distribution, and statistical analysis was carried out using χ2 tests or Fisher’s exact tests. A significance level of P < 0.05 was considered statistically significant.
The baseline data of the two patient groups were comparable, showing no significant differences upon comparison (P > 0.05), as detailed in Table 1.
| Characteristic | Control (n = 63) | Observation (n = 64) | t/χ2 | P value |
| Gender | - | - | 0.065 | 0.797 |
| Male | 35 | 37 | - | - |
| Female | 28 | 27 | - | - |
| Age (years), mean ± SD | 44.27 ± 9.63 | 43.86 ± 9.87 | 0.236 | 0.813 |
| BMI (kg/m²) | 23.59 ± 1.86 | 24.11 ± 1.74 | 1.627 | 0.106 |
| Duration (years), mean ± SD | 2.65 ± 0.84 | 2.72 ± 0.81 | 0.478 | 0.633 |
| Severity | - | - | 0.411 | 0.521 |
| Mild | 25 | 29 | - | - |
| Moderate | 38 | 35 | - | - |
The total effective rate was 79.37% in the control group and 93.75% in the observation group, with the observation group exhibiting significantly higher effectiveness than the control group (P < 0.05). Refer to Table 2 for details.
| Group | n | Complete remission | Effective | Ineffective | Total effective rate (%) |
| Control | 63 | 20 | 30 | 13 | 79.37% |
| Observation | 64 | 27 | 33 | 4 | 93.75% |
| χ2 | - | - | - | - | 5.666 |
| P value | - | - | - | - | 0.017 |
The observation group exhibited significantly lower relief times for abdominal pain, diarrhea, rectal bleeding, fever symptoms, and mucosal healing compared to the control group (P < 0.05). Refer to Table 3 for details.
| Symptom | Control (n = 63) | Observation (n = 64) | t | P value |
| Abdominal pain | 11.47 ± 2.15 | 8.35 ± 1.96 | 8.548 | < 0.001 |
| Diarrhea | 11.04 ± 2.47 | 8.62 ± 1.85 | 6.255 | < 0.001 |
| Rectal bleeding | 8.93 ± 1.64 | 6.32 ± 1.07 | 10.638 | < 0.001 |
| Fever | 7.68 ± 1.42 | 5.43 ± 1.15 | 9.820 | < 0.001 |
| Mucosal healing | 9.63 ± 1.86 | 6.12 ± 1.29 | 12.373 | < 0.001 |
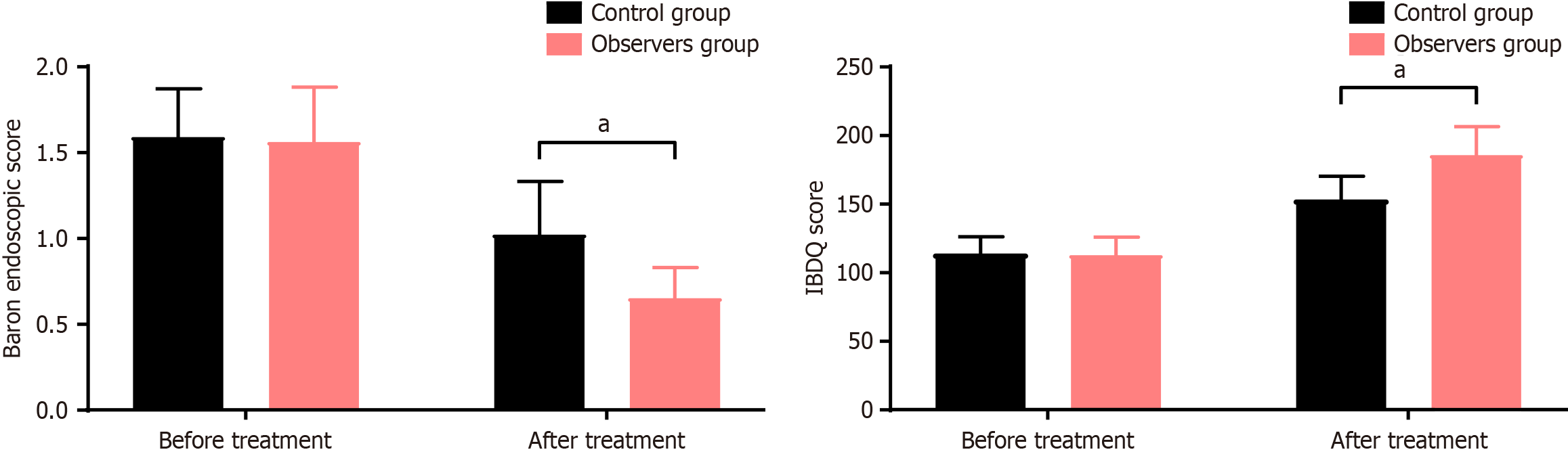
As shown in Figure 1, the Baron endoscopy scores before and after treatment in the control group were (1.59 ± 0.28, 1.02 ± 0.31), and the IBDQ scores were (114.89 ± 12.36, 154.17 ± 17.23). In the observation group, the Baron endoscopy scores before and after treatment were (1.56 ± 0.32, 0.65 ± 0.18), and the IBDQ scores were (113.74 ± 13.21, 186.75 ± 20.83). Prior to treatment, there was no significant difference in the Baron endoscopy scores and IBDQ scores between the two groups (P > 0.05). After treatment, the Baron endoscopy scores were significantly lower in the observation group compared to the control group, while the IBDQ scores were markedly higher in the observation group (P < 0.05).
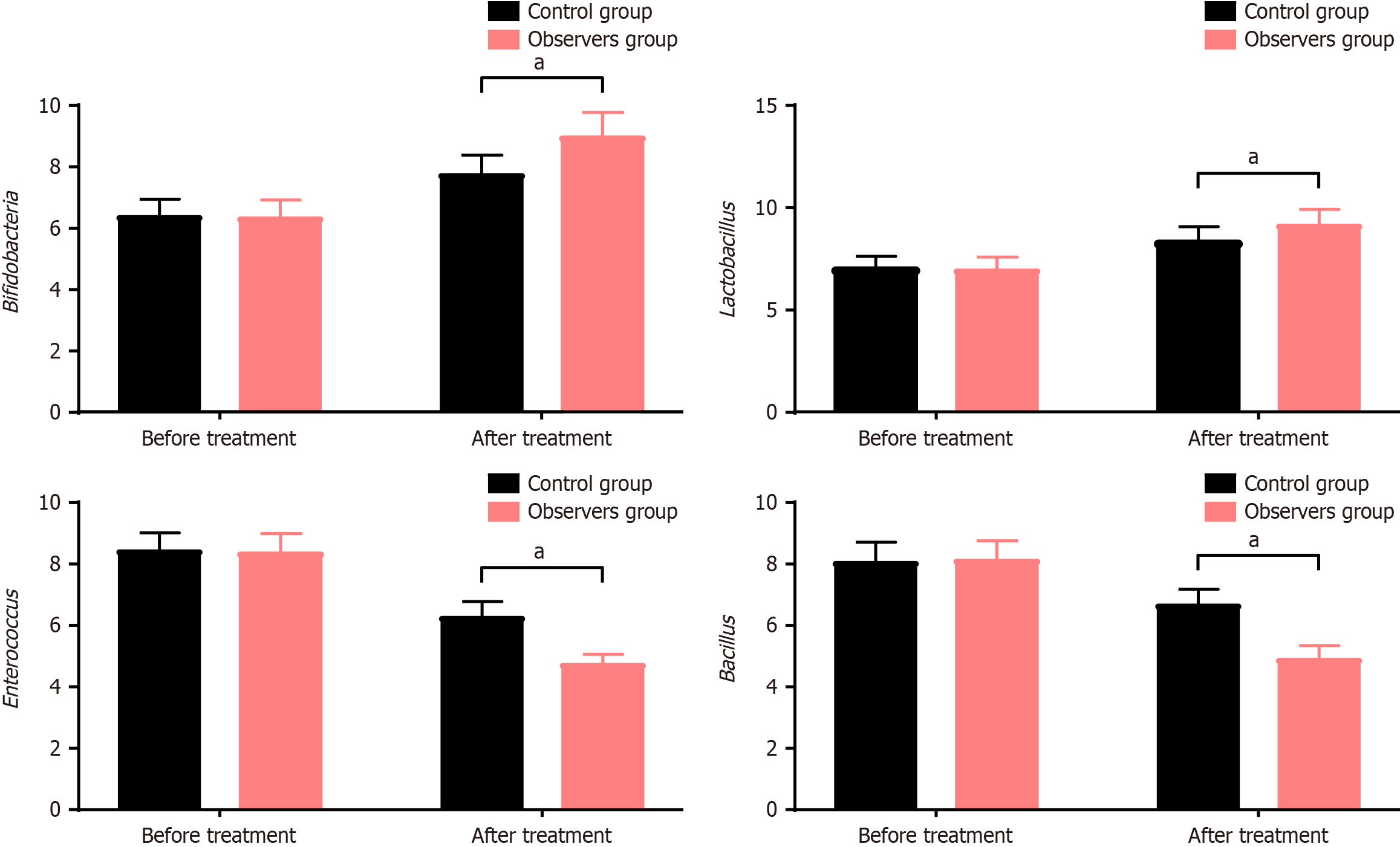
As depicted in Figure 2, the quantities of Bifidobacterium, Lactobacillus, Enterococcus, and Escherichia coli in the control group before and after treatment were (6.37 ± 0.56, 7.74 ± 0.63), (7.03 ± 0.59, 8.34 ± 0.72), (8.43 ± 0.59, 6.27 ± 0.51), (8.07 ± 0.65, 6.67 ± 0.52), respectively. In the observation group, the quantities before and after treatment were (6.32 ± 0.58, 8.97 ± 0.78), (6.97 ± 0.62, 9.16 ± 0.77), (8.36 ± 0.63, 4.74 ± 0.32), (8.12 ± 0.64, 4.91 ± 0.43), respectively. Before treatment, there were no significant differences in the levels of Bifidobacterium, Lactobacillus, Enterococcus, and Escherichia coli between the two groups (P > 0.05). After treatment, the quantities of Bifidobacterium and Lactobacillus were significantly higher, while Enterococcus and Escherichia coli were markedly lower in the observation group compared to the control group (P < 0.05).
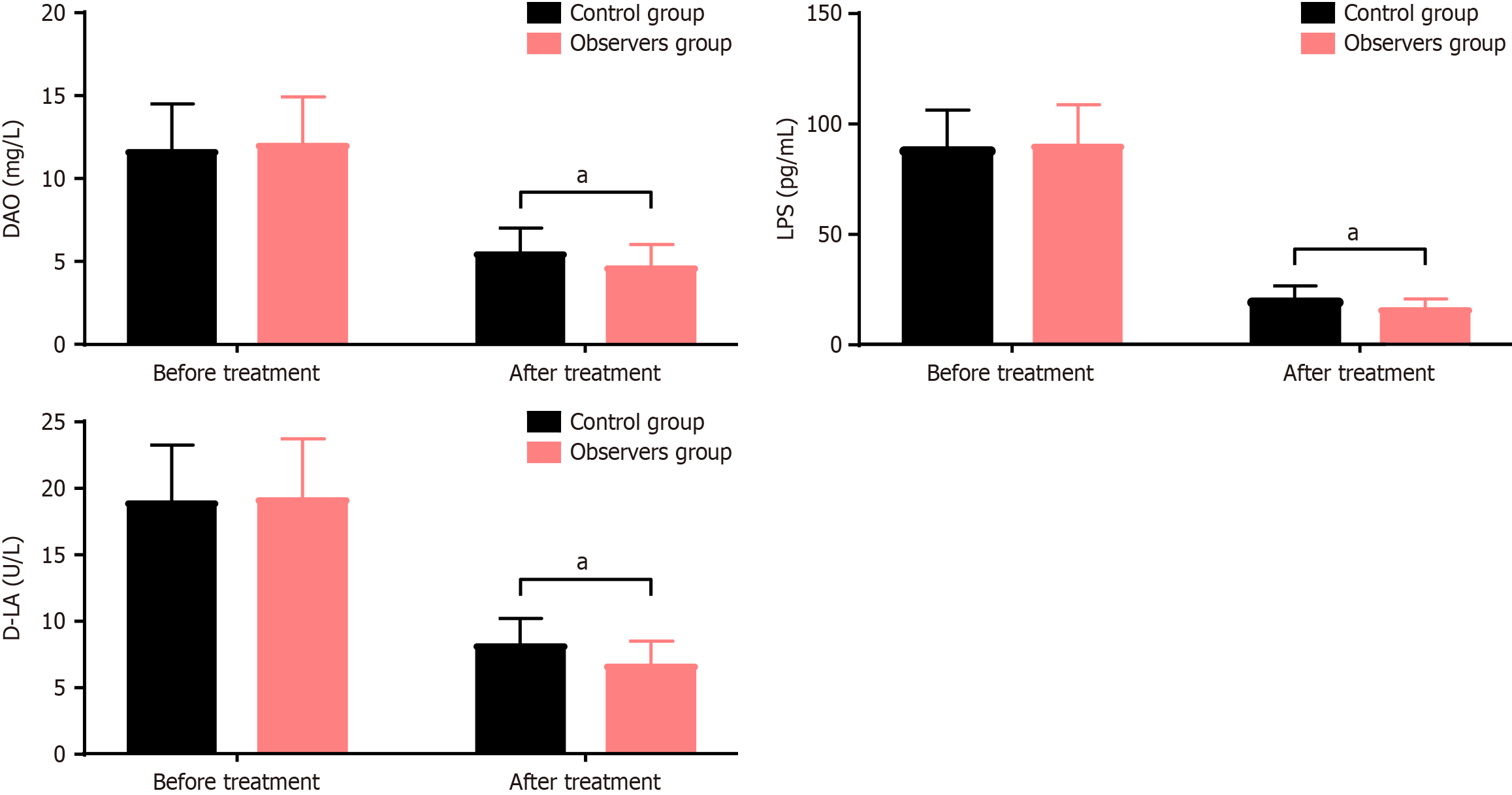
As shown in Figure 3, the levels of DAO, LPS, and D-LA in the control group before and after treatment were (11.67 ± 2.82, 5.53 ± 1.46), (88.95 ± 17.37, 20.53 ± 6.29), (18.94 ± 4.35, 8.19 ± 2.02), respectively. In the observation group, the levels before and after treatment were (12.07 ± 2.84, 4.65 ± 1.39), (90.51 ± 18.23, 16.27 ± 4.62), (19.21 ± 4.53, 6.67 ± 1.82), respectively. Before treatment, there were no significant differences in the levels of DAO, LPS, and D-LA between the two groups (P > 0.05). After treatment, the levels of DAO, LPS, and D-LA were significantly lower in the observation group compared to the control group (P < 0.05).
The occurrence rate of adverse reactions in the control group was 9.52%, and in the observation group, it was 10.94%. The comparison of adverse reaction occurrence rates between the two groups showed no significant difference (P > 0.05). Refer to Table 4 for details.
| Adverse reaction | Control (n = 63) | Observation (n = 64) | χ2 | P value |
| Nausea/vomiting | 2 | 2 | - | - |
| Decreased appetite | 2 | 2 | - | - |
| Indigestion | 1 | 1 | - | - |
| Gastric burning sensation | 1 | 2 | - | - |
| Total occurrence rate (%) | 9.52% | 10.94% | 0.069 | 0.792 |
UC is a prevalent and challenging gastrointestinal disease, with its incidence steadily rising each year. Additionally, the prevalence varies significantly among different regions and ethnic groups[11]. Research indicates that the lesions in UC primarily affect the mucosa and underlying tissues of the colon, often initiating as proctitis and potentially progressing to left-sided or extensive colitis[12]. While the exact etiology of UC remains unclear, modern medicine suggests its multifactorial nature involving the immune system, psychological state, genetic factors, microbiota, infections, environmental elements, and intestinal mucosal barrier function[13,14]. Among these factors, the mucosal immune response in the gut is considered one of the most critical. In UC patients, an overactivated intestinal immune system triggers a series of pathophysiological changes, including disrupting microbial balance, inciting pathological inflammation, altering the activity and functionality of other inflammatory cells, and mediating epithelial cell injury. These alterations play significant roles in the occurrence and progression of UC[15]. Presently, the primary clinical goals for treating UC are symptom relief and mucosal healing to reduce complications and enhance patients’ quality of life. Medication remains the primary approach, particularly aminosalicylates for mild-to-moderate cases. Among these drugs, sulfasalazine, a traditional medication, is an antimicrobial sulfa drug that exhibits a high affinity for connective tissue. Once in the intestinal wall, it breaks down into 5-aminosalicylic acid and sulfapyridine[16]. The active component, 5-aminosalicylic acid, exerts dual antimicrobial and anti-inflammatory actions by inhibiting the synthesis and release of inflammatory factors like prostaglandins and leukotrienes. It also modulates immunity, impacting the immune pathological damage in UC, thereby alleviating intestinal inflammation and mucosal injury. However, sulfapyridine, as a carrier molecule, might cause several adverse reactions that could influence the overall therapeutic efficacy. Rectal administration helps mitigate the occurrence of adverse effects to some extent[17].
Saccharomyces boulardii, as a fungal probiotic, possesses distinct characteristics compared to bacterial probiotics. It does not engage in bacterial gene transfer, demonstrating stronger antioxidant properties and acid resistance. Moreover, it can be used concomitantly with antibiotics[18]. In recent years, Saccharomyces boulardii has found widespread application in treating UC. Relevant studies indicate that it may exert anti-inflammatory effects on UC through various pathways, such as directly or indirectly eliminating pathogenic microbial toxins, suppressing harmful microbial growth, nourishing intestinal mucosal cells, maintaining microbial balance, regulating metabolic equilibrium, and enhancing intestinal immune function[19]. Saccharomyces boulardii regulates intestinal barrier function through multiple mechanisms, particularly by enhancing the expression of tight junction proteins (such as claudin, occludin, and zonulin) and restoring the integrity of the intestinal barrier. First, Saccharomyces boulardii modulates the toll-like receptor (TLR) signaling pathways, particularly the expression of TLR4 and TLR2, which weakens the activation of downstream nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase pathways, reducing the production of pro-inflammatory cytokines like tumor necrosis factor α and interleukin (IL)-6. This, in turn, reduces intestinal inflammation and promotes the synthesis of tight junction proteins. Secondly, Saccharomyces boulardii regulates the gene expression of intestinal epithelial cells, enhancing the synthesis of tight junction proteins, particularly by inhibiting inflammation via the NF-κB pathway, further repairing the intestinal barrier. Additionally, Saccharomyces boulardii’s anti-inflammatory effects help reduce the damage to tight junction proteins, thereby stabilizing the intestinal barrier. Its anti-inflammatory mechanisms also include increasing the production of anti-inflammatory cytokines like IL-10, alleviating inflammation and protecting tight junction proteins. Moreover, Saccharomyces boulardii helps restore gut microbiota balance by promoting beneficial bacteria (such as Lactobacillus and Bifidobacterium) and reducing harmful bacteria. This balance helps maintain intestinal epithelial health and protects tight junction proteins from damage. Furthermore, Saccharomyces boulardii promotes the repair and proliferation of intestinal epithelial cells, indirectly enhancing the synthesis of tight junction proteins and strengthening the intestinal barrier’s protective function. In summary, Saccharomyces boulardii improves intestinal barrier function, alleviates intestinal inflammation, and restores microbial balance through multiple pathways, thereby enhancing gut health. Simultaneously, research by Gu et al[20] and others suggests that Saccharomyces boulardii may achieve its goal of treating intestinal inflammation by inhibiting the activation of signaling pathways like TLR/myeloid differentiation factor 88/NF-κB/mitogen-activated protein kinase. The results of this study demonstrate that the treatment’s overall efficacy was 79.37% in the control group and notably higher at 93.75% in the observation group (P < 0.05). The observation group exhibited significantly shorter relief times for abdominal pain, diarrhea, rectal bleeding, fever symptoms, and mucosal healing compared to the control group (P < 0.05). Post-treatment, the observation group showed markedly lower Baron endoscopy scores and significantly higher IBDQ scores than the control group (P < 0.05). These findings indicate that the combined use of Saccharomyces boulardii contributes to enhancing the overall therapeutic effect of UC, alleviating symptoms, expediting mucosal healing, “alleviating intestinal mucosal inflammation, thereby improving patients” quality of life. Additionally, this study analyzed the safety of the combined regimen, revealing adverse reaction rates of 9.52% in the control group and 10.94% in the observation group, with no significant difference between the two (P > 0.05). All reported adverse reactions, including nausea, decreased appetite, and gastric burning sensation, were mild and self-limiting, requiring no discontinuation of therapy or additional treatment. Saccharomyces boulardii’s favorable safety profile can be attributed to its unique fungal probiotic nature, which is inherently resistant to gastric acids and lacks the capacity for horizontal gene transfer, thus minimizing risks associated with antibiotic resistance or gut microbiota disruption. These findings indicate that adjunctive Saccharomyces boulardii does not exacerbate adverse reaction risks when combined with sulfasalazine suppositories.
Research indicates that intestinal dysbiosis is a crucial pathological factor in the occurrence and development of UC[21]. Patients exhibit significantly reduced intestinal microbial biodiversity, characterized by an increase in harmful bacterial groups like Enterococcus and Escherichia, which gradually become dominant. Conversely, beneficial bacteria such as Lactobacillus and Bifidobacterium decrease notably. Imbalance in the gut microbiota participates in the pathophysiology of UC through various mechanisms, including mediating bacterial recognition defects, enhancing intestinal mucosal immune responses, and compromising mucosal barrier function[22]. Animal experiments demonstrate that Saccharomyces boulardii can restrain the decrease in beneficial gut flora under pathological conditions and decrease the quantity of harmful flora, thereby stabilizing the intestinal environment[23]. Conversely, due to a stable gut flora structure, reduced LPS release and diminished activity can occur, leading to reduced release of pro-inflammatory cytokines like IL-6 and tumor necrosis factor α, ultimately reducing the body’s inflammation level[24]. The results of this study reveal that post-treatment, the observation group exhibited significantly higher levels of Bifidobacterium and Lactobacillus compared to the control group, while Enterococcus and Escherichia levels were notably lower than the control group (P < 0.05). These findings suggest that adding Saccharomyces boulardii to sulfasalazine suppository therapy for UC further ameliorates the imbalance in the patients’ gut microbiota, enhancing the therapeutic effects. Intestinal mucosal barrier damage is a crucial pathological feature in UC patients, and increased intestinal permeability is a significant manifestation of mucosal barrier dysfunction[25]. D-LA is a product of microbial fermentation in the intestines, while DAO is an enzyme specifically expressed in intestinal mucosal epithelial cells. When the mucosal barrier is impaired, both are released into the blood in large quantities[26]. LPS is a major component of the cell walls of Gram-negative bacteria, and the intestines constitute the body’s largest reservoir of LPS. When the intestinal barrier function is compromised, LPS from the intestines can enter the bloodstream through damaged intestinal mucosa, and this can mediate intestinal mucosal inflammation and exacerbate intestinal barrier dysfunction[27]. The study results demonstrate that post-treatment, levels of DAO, LPS, and D-LA were significantly lower in the observation group than the control group (P < 0.05). These findings suggest that the combined use of Saccharomyces boulardii and sulfasalazine suppositories more effectively preserves the intestinal mucosal barrier function in UC patients, improving intestinal mucosal permeability and promoting recovery. The reasons might lie in Saccharomyces boulardii’s ability to regulate intestinal immune responses by rectifying gut microbiota imbalances, inhibiting abnormal expression of pro-inflammatory factors, correcting imbalances in pro-inflammatory or anti-inflammatory factor expression, and thereby facilitating the repair of mucosal mechanical barriers. Additionally, it competes with pathogenic bacteria for adherence to intestinal mucosal epithelial cells and stimulates the secretion of mucus, preventing the invasion of pathogenic microorganisms[28]. While the study assessed DAO, LPS, and D-LA levels as biomarkers for intestinal barrier function, it does not establish direct causal relationships between these biomarkers and clinical outcomes such as symptom relief, endoscopic healing, or overall disease remission. Although the decrease in these biomarkers suggests an improvement in intestinal barrier integrity, further research is needed to determine whether these biomarkers directly correlate with improvements in symptoms or mucosal healing. Other markers of barrier integrity, such as tight junction proteins (claudin, occludin), could also be considered to provide a more comprehensive understanding of the relationship between barrier function and clinical outcomes. Additionally, future studies should explore whether these biomarkers are predictive of long-term remission and whether their levels correlate with improvements in disease severity and quality of life.
In order to strengthen future research, several methodological improvements are necessary. The inclusion of molecular methods, such as 16S ribosomal RNA sequencing or metagenomic analysis, could provide deeper insights into changes in gut microbiota composition and diversity, shedding light on the mechanisms underlying treatment efficacy. Furthermore, multicenter studies with larger sample sizes and adjustments for dietary, genetic, and lifestyle factors are needed to enhance the generalizability and robustness of these findings.
The combined application of Saccharomyces boulardii and sulfasalazine in UC patients exhibits significant efficacy. Compared to sole sulfasalazine intervention, the combined use of Saccharomyces boulardii further promotes relief of related symptoms in patients, alleviates intestinal mucosal inflammation, and enhances the quality of life. Its effect might be associated with rectifying gut microbiota imbalance and improving intestinal mucosal barrier function. Moreover, the combined application of Saccharomyces boulardii does not increase the risk of adverse reactions in patients, demonstrating a relatively high level of safety in medication and worthy of clinical promotion. It is important to note that although this study has achieved some positive results in analyzing the impact of Saccharomyces boulardii combined with sulfasalazine on the gut microbiota and intestinal barrier function in UC patients, the study itself still has several limitations. These include: (1) Limited sample size: The sample size in this study is relatively small, including only 127 UC patients. A small sample size may affect the stability and generalizability of the results; (2) Restriction in study design: This study is a retrospective analysis, potentially introducing information bias and treatment selection preferences. Employing randomized controlled trial or prospective study designs would provide more robust and convincing evidence; (3) Limited study duration: The relatively short timeframe of this study limits a comprehensive observation of long-term treatment effects. Future studies with longer follow-up periods could better evaluate sustained effects and assess remission and relapse rates for a more comprehensive understanding of efficacy; and (4) Inadequate consideration of individual differences: There might be individual differences among the patient population in this study, including physiological conditions, age, gender, etc. Stratified analysis or subgroup analysis may better illustrate different groups’ responses to treatment. In summary, these limitations emphasize certain constraints on the results of this study. In future research, we aim to enhance and deepen this field of study by expanding sample size, employing randomized controlled trial designs, and conducting longer-term follow-ups.
| 1. | Kucharzik T, Koletzko S, Kannengiesser K, Dignass A. Ulcerative Colitis-Diagnostic and Therapeutic Algorithms. Dtsch Arztebl Int. 2020;117:564-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 2. | Du L, Ha C. Epidemiology and Pathogenesis of Ulcerative Colitis. Gastroenterol Clin North Am. 2020;49:643-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 354] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 3. | Le Berre C, Honap S, Peyrin-Biroulet L. Ulcerative colitis. Lancet. 2023;402:571-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 594] [Reference Citation Analysis (103)] |
| 4. | Sulfasalazine. In: Bethesda. Drugs and Lactation Database (LactMed®). New York: LactMed, 2006. |
| 5. | Porter RJ, Kalla R, Ho GT. Ulcerative colitis: Recent advances in the understanding of disease pathogenesis. F1000Res. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 145] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 6. | Feuerstein JD, Moss AC, Farraye FA. Ulcerative Colitis. Mayo Clin Proc. 2019;94:1357-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 271] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 7. | Pais P, Almeida V, Yılmaz M, Teixeira MC. Saccharomyces boulardii: What Makes It Tick as Successful Probiotic? J Fungi (Basel). 2020;6:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 138] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 8. | Singh S, Murad MH, Fumery M, Dulai PS, Sandborn WJ. First- and Second-Line Pharmacotherapies for Patients With Moderate to Severely Active Ulcerative Colitis: An Updated Network Meta-Analysis. Clin Gastroenterol Hepatol. 2020;18:2179-2191.e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 274] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 9. | Mohammed Vashist N, Samaan M, Mosli MH, Parker CE, MacDonald JK, Nelson SA, Zou GY, Feagan BG, Khanna R, Jairath V. Endoscopic scoring indices for evaluation of disease activity in ulcerative colitis. Cochrane Database Syst Rev. 2018;1:CD011450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 10. | Zavala-Solares MR, Salazar-Salas L, Yamamoto-Furusho JK. Validity and reliability of the health-related questionnaire IBDQ-32 in Mexican patients with inflammatory bowel disease. Gastroenterol Hepatol. 2021;44:711-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Nakase H, Sato N, Mizuno N, Ikawa Y. The influence of cytokines on the complex pathology of ulcerative colitis. Autoimmun Rev. 2022;21:103017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 222] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 12. | Burri E, Maillard MH, Schoepfer AM, Seibold F, Van Assche G, Rivière P, Laharie D, Manz M; Swiss IBDnet, an official working group of the Swiss Society of Gastroenterology. Treatment Algorithm for Mild and Moderate-to-Severe Ulcerative Colitis: An Update. Digestion. 2020;101 Suppl 1:2-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 13. | Yao D, Dai W, Dong M, Dai C, Wu S. MUC2 and related bacterial factors: Therapeutic targets for ulcerative colitis. EBioMedicine. 2021;74:103751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 140] [Article Influence: 35.0] [Reference Citation Analysis (1)] |
| 14. | Krugliak Cleveland N, Torres J, Rubin DT. What Does Disease Progression Look Like in Ulcerative Colitis, and How Might It Be Prevented? Gastroenterology. 2022;162:1396-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 96] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 15. | Pabla BS, Schwartz DA. Assessing Severity of Disease in Patients with Ulcerative Colitis. Gastroenterol Clin North Am. 2020;49:671-688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 16. | Choi J, Patel P, Fenando A. Sulfasalazine. 2024 Mar 21. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 17. | Ferro JM, Oliveira Santos M. Neurology of inflammatory bowel disease. J Neurol Sci. 2021;424:117426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 18. | Kaźmierczak-Siedlecka K, Ruszkowski J, Fic M, Folwarski M, Makarewicz W. Saccharomyces boulardii CNCM I-745: A Non-bacterial Microorganism Used as Probiotic Agent in Supporting Treatment of Selected Diseases. Curr Microbiol. 2020;77:1987-1996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 19. | Wang C, Li W, Wang H, Ma Y, Zhao X, Zhang X, Yang H, Qian J, Li J. Saccharomyces boulardii alleviates ulcerative colitis carcinogenesis in mice by reducing TNF-α and IL-6 levels and functions and by rebalancing intestinal microbiota. BMC Microbiol. 2019;19:246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 144] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 20. | Gu Y, Wang C, Qin X, Zhou B, Liu X, Liu T, Xie R, Liu J, Wang B, Cao H. Saccharomyces boulardii, a yeast probiotic, inhibits gut motility through upregulating intestinal serotonin transporter and modulating gut microbiota. Pharmacol Res. 2022;181:106291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 21. | Shen ZH, Zhu CX, Quan YS, Yang ZY, Wu S, Luo WW, Tan B, Wang XY. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J Gastroenterol. 2018;24:5-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 307] [Cited by in RCA: 459] [Article Influence: 65.6] [Reference Citation Analysis (6)] |
| 22. | Seyedian SS, Nokhostin F, Malamir MD. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J Med Life. 2019;12:113-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 415] [Article Influence: 69.2] [Reference Citation Analysis (113)] |
| 23. | Li B, Zhang H, Shi L, Li R, Luo Y, Deng Y, Li S, Li R, Liu Z. Saccharomyces boulardii alleviates DSS-induced intestinal barrier dysfunction and inflammation in humanized mice. Food Funct. 2022;13:102-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 24. | Lee SD. Health Maintenance in Ulcerative Colitis. Gastroenterol Clin North Am. 2020;49:xv-xvi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Matsuoka K. Fecal microbiota transplantation for ulcerative colitis. Immunol Med. 2021;44:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Yun HF, Liu R, Han D, Zhao X, Guo JW, Yan FJ, Zhang C, Sun HW, Liang GQ, Zhang GX. Pingkui Enema Alleviates TNBS-Induced Ulcerative Colitis by Regulation of Inflammatory Factors, Gut Bifidobacterium, and Intestinal Mucosal Barrier in Rats. Evid Based Complement Alternat Med. 2020;2020:3896948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Yang L, Wu G, Wu Q, Peng L, Yuan L. METTL3 overexpression aggravates LPS-induced cellular inflammation in mouse intestinal epithelial cells and DSS-induced IBD in mice. Cell Death Discov. 2022;8:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 70] [Reference Citation Analysis (0)] |
| 28. | Dong L, Du H, Zhang M, Xu H, Pu X, Chen Q, Luo R, Hu Y, Wang Y, Tu H, Zhang J, Gao F. Anti-inflammatory effect of Rhein on ulcerative colitis via inhibiting PI3K/Akt/mTOR signaling pathway and regulating gut microbiota. Phytother Res. 2022;36:2081-2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 119] [Article Influence: 39.7] [Reference Citation Analysis (0)] |