Published online Feb 27, 2025. doi: 10.4240/wjgs.v17.i2.101897
Revised: December 12, 2024
Accepted: December 25, 2024
Published online: February 27, 2025
Processing time: 76 Days and 2.1 Hours
An increasing number of studies to date have found preoperative magnetic resonance imaging (MRI) features valuable in predicting the prognosis of rectal cancer (RC). However, research is still lacking on the correlation between pre
To investigate the correlation between preoperative MRI parameters and the risk of recurrence after radical resection of RC to provide an effective tool for predicting postoperative recurrence.
The data of 90 patients who were diagnosed with RC by surgical pathology and underwent radical surgical resection at the Second Affiliated Hospital of Bengbu Medical University between May 2020 and December 2023 were collected through retrospective analysis. General demographic data, MRI data, and tumor markers levels were collected. According to the reviewed data of patients six months after surgery, the clinicians comprehensively assessed the recurrence risk and divided the patients into high recurrence risk (37 cases) and low recurrence risk (53 cases) groups. Independent sample t-test and χ2 test were used to analyze differences between the two groups. A logistic regression model was used to explore the risk factors of the high recurrence risk group, and a clinical prediction model was constructed. The clinical prediction model is presented in the form of a nomogram. The receiver operating characteristic curve, Hosmer-Lemeshow goodness of fit test, calibration curve, and decision curve analysis were used to evaluate the efficacy of the clinical prediction model.
The detection of positive extramural vascular invasion through preoperative MRI [odds ratio (OR) = 4.29, P = 0.045], along with elevated carcinoembryonic antigen (OR = 1.08, P = 0.041), carbohydrate antigen 125 (OR = 1.19, P = 0.034), and carbohydrate antigen 199 (OR = 1.27, P < 0.001) levels, are independent risk factors for increased postoperative recurrence risk in patients with RC. Furthermore, there was a correlation between magnetic resonance based T staging, magnetic resonance based N staging, and circumferential resection margin results determined by MRI and the postoperative recurrence risk. Additionally, when extramural vascular invasion was integrated with tumor markers, the resulting clinical prediction model more effectively identified patients at high risk for postoperative recurrence, thereby providing robust support for clinical decision-making.
The results of this study indicate that preoperative MRI detection is of great importance for predicting the risk of postoperative recurrence in patients with RC. Monitoring these markers helps clinicians identify patients at high risk, allowing for more aggressive treatment and monitoring strategies to improve patient outcomes.
Core Tip: Currently, a growing focus exists on the application of preoperative magnetic resonance imaging (MRI) in patients with rectal cancer (RC). However, there remains a lack of sufficient research on the correlation between preoperative MRI features and the risk of recurrence after radical resection of RC, necessitating further in-depth exploration. In this study, a retrospective analysis was conducted on preoperative MRI data from 90 patients with RC, combined with their basic characteristics and tumor markers. The findings revealed that preoperative MRI is of great significance in predicting the risk of postoperative recurrence in patients with RC.
- Citation: Wu L, Zhu JJ, Liang XH, Tong H, Song Y. Predictive value of magnetic resonance imaging parameters combined with tumor markers for rectal cancer recurrence risk after surgery. World J Gastrointest Surg 2025; 17(2): 101897
- URL: https://www.wjgnet.com/1948-9366/full/v17/i2/101897.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i2.101897
Rectal cancer (RC) is a malignant tumor that emerges between the sigmoido-rectal junction and the dentate line, mainly arising from the rectal mucosal epithelium[1]. As one of the most common malignant tumors in the world, RC has high morbidity and mortality, which seriously threaten human health and quality of life[2]. The main treatment methods for RC usually include surgery, radiotherapy, chemotherapy, and targeted drug therapy[3]. In the absence of surgical contraindications, surgical excision remains the preferred treatment option[4]. Despite significant improvements in the survival rate of patients because of advancements in surgical techniques and the optimization of comprehensive treatment strategies, postoperative recurrence remains a crucial factor influencing the long-term quality of life and prognosis of patients[5]. Previous studies have indicated that the recurrence rate of RC following radical surgery can be higher than 30%, particularly local recurrence[6,7]. Local recurrence not only significantly undermines the quality of life of patients but is also correlated with higher morbidity and mortality[8]. Therefore, early and accurate recurrence risk prediction after radical resection of RC is of paramount importance for formulating personalized treatment, optimizing postoperative monitoring strategies, and enhancing patient prognosis.
Magnetic resonance imaging (MRI) is a medical imaging test that utilizes the phenomenon of nuclear magnetic resonance[9]. Among many imaging methods, MRI, with its advantages of high soft tissue resolution, multi-parameter imaging, and non-invasiveness, has shown unique advantages in the preoperative diagnosis, staging, and postoperative follow-up of RC[10]. In addition to clearly displaying the anatomical relationship between tumors and surrounding tissues, MRI also reflects the internal biological characteristics of tumors through functional imaging technologies, providing more comprehensive and accurate preoperative information for clinical use[11]. Especially in the preoperative evaluation of RC, MRI clearly shows the location, size, depth of invasion, and the relationship between the tumor and surrounding tissues, providing an important basis for the formulation of surgical protocols[12]. To date, an increasing number of studies have focused on the application value of preoperative MRI features in patients with RC, such as forecasting postoperative tumor deposits and lymph node metastasis[13,14]. However, research is still lacking on the correlation between preoperative MRI features and the risk of recurrence after radical resection of RC. Therefore, further in-depth exploration is urgently needed.
The purpose of this study was to retrospectively analyze preoperative MRI data of patients with RC, combined with their basic characteristics and tumor markers data, and comprehensively explore their role in assessing the postoperative recurrence risk of RC. In this study, we first described in detail the inclusion and exclusion criteria of the study, ensuring homogeneity of the study subjects. Second, we adopted a standardized MRI scanning protocol and image evaluation process to reduce operator variance. In addition, we rigorously and clearly defined the risk of RC recurrence, ensuring the validity of the study grouping. By analyzing the correlation between preoperative MRI features and the risk of recurrence following radical resection of RC, the results of this study will provide a scientific basis for clinical screening of patients with a high risk of recurrence and formulating individualized treatment strategies. Furthermore, this study will provide new insights and approaches for using MRI in the preoperative evaluation of RC, promoting the improvement of the diagnostic and treatment strategies of RC.
Ninety patients diagnosed with RC by surgical pathology, and who underwent radical surgical resection at the Second Affiliated Hospital of Bengbu Medical University between May 2020 and December 2023 were retrospectively enrolled in this study. The inclusion criteria for this study were: (1) Patients diagnosed with RC by pathology who underwent surgery; (2) Patients who had undergone preoperative abdominal MRI examination with complete image data; (3) Patients with complete clinical data; and (4) Patients with complete reexamination results within 6 months. The exclusion criteria were: (1) Patients with other tumors or serious diseases; (2) Patients with uneven lesion location or unclear preoperative MRI scans; (3) Patients with liver and kidney dysfunction; and (4) Patients with mental health problems. This study was conducted in strict accordance with the ethical guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Second Affiliated Hospital of Bengbu Medical University, approval No. [2024]KY047. All patients voluntarily signed informed consent forms.
We collected general demographic data, preoperative MRI data, and preoperative tumor markers. General demographic data included age, sex, body mass index, smoking, alcohol consumption, family history of tumors, and complications. MRI data included maximum tumor length (MTL), tumor location, magnetic resonance based T (mrT) stage, magnetic resonance based N (mrN) stage, circumferential resection margin (CRM), and extramural vascular invasion (EMVI). The tumor marker data were obtained from the preoperative examination results of the patients, including carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 125, and CA 199.
All patients were examined using a GE3.0T MRI scanner and 16-channel body surface coil before surgery. The inspection sequence included readout segmentation of long variable echo-train diffusion-weighted imaging (DWI), T2-weighted fast spin-echo sequences of DWI, and slant-axis adipose-free inhibitors. After the examination, images were transmitted to the GE Advantage Workstation 4.7, and the data were measured and analyzed by two abdominal radiologists with more than 8 years of work experience who were blinded to the diagnosis based on MRI and pathological findings. The region of interest was delineated, and the MTL and distance to the anal verge were measured using sagittal and DWI sequences. Distance to the anal verges of < 5 cm, 5-10 cm, and > 10 cm were considered low, middle, and high RCs, respectively. In the mrT1 stage, the tumor was confined to the inner wall of the rectum. The mrT2 stage comprised an enhanced tumor signal that broke through the submucosa into the muscle layer. In the mrT3 stage, the tumor signal extended beyond the muscle layer but did not break through to the serosal layer. The mrT4 stage showed a significantly enhanced tumor signal that broke through the serous membrane layer, with an unclear boundary with the surrounding structure[15]. The mrN0 stage showed no apparant lymph node enlargement or abnormal signal. In the mrN1 stage, enlarged lymph nodes associated with the tumor were observed. The mrN2 stage showed multiple enlarged lymph nodes with enhanced signal and wide distribution. When the distance between the tumor and the CRM was ≤ 1 mm, the CRM was positive, whereas a CRM > 1 mm was negative[15]. A MRI-defined EMVI (mrEMVI) score based on high-resolution MRI was used to assess the extent of EMVI. A MRI-defined EMVI score of 3-4 was considered positive, and a score of 0-2 was considered negative[16].
This study assessed the risk of recurrence based on the clinical symptoms of the patient combined with reexamination results within 6 months. Patients were assigned to the high recurrence risk (HRR) group if reexamination showed tumor recurrence at the primary site or metastasis to distant organs such as the liver, lung, and bone. In addition, evidence of poor prognosis included the following: (1) Postoperative pathological examination showing local invasion or distant metastasis of the tumor; (2) Levels of tumor markers that did not decrease significantly or even increased; (3) Patients that did not respond significantly to postoperative chemotherapy or radiotherapy or had severe adverse reactions; (4) Patients with elevated inflammatory markers and abnormal liver and kidney function; and (5) Patients with significant weight loss, decreased hemoglobin concentration, and decreased albumin levels. After a comprehensive assessment by two clinicians, patients with four or more of these five factors were classified as HRR. The remaining patients were classified as the low recurrence risk (LRR) group. The postoperative rehabilitation treatment of patients was comprehensively assessed by two clinical doctors based on the condition of the patient, with no subjective bias in the treatment between patients.
Univariate logistic regression analysis was used to screen risk factors for recurrence. Variables with P < 0.05 in the univariate logistic regression analysis were included in the multivariate logistic regression model to construct the recurrence risk prediction model. The variance inflation factor (VIF) was used to evaluate multicollinearity between variables. The prediction model was visualized in the form of a nomogram. The area under the curve of receiver operating characteristic was used to evaluate the differentiation of prediction models. The calibration curve and Hosmer-Lemeshow goodness of fit tests were used to evaluate the calibration degree of the prediction model. Decision curve analysis was used to describe the change in net return value under intervention conditions according to the predicted value of the model as the risk probability threshold changed. In addition, the best truncation value of the prediction model was determined according to the Jorden index, accuracy, sensitivity, specificity, positive predictive value, and negative predictive value.
Continuous variables are presented as mean ± SD, and the independent sample t-test was used for difference analysis between the two groups. Categorical variables are presented as frequency and percentage, and the Pearson χ2 test was used for difference analysis. The Pearson correlation test was used for continuity variables, while the Spearman correlation test was used for categorical variables. Univariate logistic regression analysis was used for preliminary screening of risk factors. Variables with P < 0.05 in the univariate logistic regression analysis were included in the multivariate logistic regression model for correction, and VIF values were calculated to determine collinearity. All statistical tests were performed using SPSS version 27.0. P < 0.05 was considered statistically significant.
Ninety postoperative patients with RC were included in this study. Of these, 53 (58.89%) were classified as the LRR group and 37 (41.11%) as the HRR group. The mean age of the LRR group was 64.66 ± 10.98 years, and that of the HRR group was 65.30 ± 9.75 years, with no significant difference between the two groups (P = 0.778) (Table 1). The mean body mass index of the LRR group was 25.08 ± 2.90, and that of the HRR group was 25.63 ± 2.92, with no significant difference (P = 0.380) (Table 1). In addition, no significant differences were observed between the two groups in terms of sex composition or the rates of smoking, alcohol consumption, family history of cancer, and comorbidities (P > 0.05) (Table 1).
| Variables | Total (n = 90) | LRR group (n = 53) | HRR group (n = 37) | Statistic | P value |
| Age (year), mean ± SD | 64.92 ± 10.44 | 64.66 ± 10.98 | 65.30 ± 9.75 | t = -0.28 | 0.778 |
| Sex, n (%) | χ² = 1.79 | 0.180 | |||
| Female | 29 (32.22) | 20 (37.74) | 9 (24.32) | - | - |
| Male | 61 (67.78) | 33 (62.26) | 28 (75.68) | - | - |
| BMI, mean ± SD | 25.31 ± 2.90 | 25.08 ± 2.90 | 25.63 ± 2.92 | t = -0.88 | 0.380 |
| Smoking, n (%) | χ² = 0.37 | 0.541 | |||
| No | 65 (72.22) | 37 (69.81) | 28 (75.68) | - | - |
| Yes | 25 (27.78) | 16 (30.19) | 9 (24.32) | - | - |
| Drinking, n (%) | χ² = 0.11 | 0.737 | |||
| No | 59 (65.56) | 34 (64.15) | 25 (67.57) | - | - |
| Yes | 31 (34.44) | 19 (35.85) | 12 (32.43) | - | - |
| Family history, n (%) | χ² = 0.00 | 1.000 | |||
| No | 80 (88.89) | 47 (88.68) | 33 (89.19) | - | - |
| Yes | 10 (11.11) | 6 (11.32) | 4 (10.81) | - | - |
| Complication, n (%) | χ² = 0.32 | 0.571 | |||
| No | 47 (52.22) | 29 (54.72) | 18 (48.65) | - | - |
| Yes | 43 (47.78) | 24 (45.28) | 19 (51.35) | - | - |
| MTL, mean ± SD | 3.98 ± 1.29 | 3.82 ± 1.30 | 4.22 ± 1.25 | t = -1.48 | 0.141 |
| Tumor location, n (%) | χ² = 0.48 | 0.787 | |||
| Low | 32 (35.56) | 20 (37.74) | 12 (32.43) | - | - |
| Median | 39 (43.33) | 23 (43.40) | 16 (43.24) | - | - |
| High | 19 (21.11) | 10 (18.87) | 9 (24.32) | - | - |
| MrT staging, n (%) | χ² = 2.81 | 0.094 | |||
| I or II | 46 (51.11) | 31 (58.49) | 15 (40.54) | - | - |
| III or IV | 44 (48.89) | 22 (41.51) | 22 (59.46) | - | - |
| MrN staging, n (%) | χ² = 4.43 | 0.035 | |||
| 0 or I | 46 (51.11) | 32 (60.38) | 14 (37.84) | - | - |
| II or III | 44 (48.89) | 21 (39.62) | 23 (62.16) | - | - |
| CRM, n (%) | χ² = 8.25 | 0.004 | |||
| Negative | 38 (42.22) | 29 (54.72) | 9 (24.32) | - | - |
| Positive | 52 (57.78) | 24 (45.28) | 28 (75.68) | - | - |
| EMVI, n (%) | - | - | - | χ² = 4.50 | 0.034 |
| Negative | 60 (66.67) | 40 (75.47) | 20 (54.05) | - | - |
| Positive | 30 (33.33) | 13 (24.53) | 17 (45.95) | - | - |
| CEA, mean ± SD | 40.08 ± 11.25 | 35.57 ± 9.31 | 46.55 ± 10.71 | t = -5.17 | < 0.001 |
| CA125, mean ± SD | 11.72 ± 5.25 | 11.07 ± 5.28 | 12.66 ± 5.13 | t = -1.42 | 0.160 |
| CA199, mean ± SD | 56.58 ± 11.18 | 50.44 ± 7.85 | 65.39 ± 9.19 | t = -8.29 | < 0.001 |
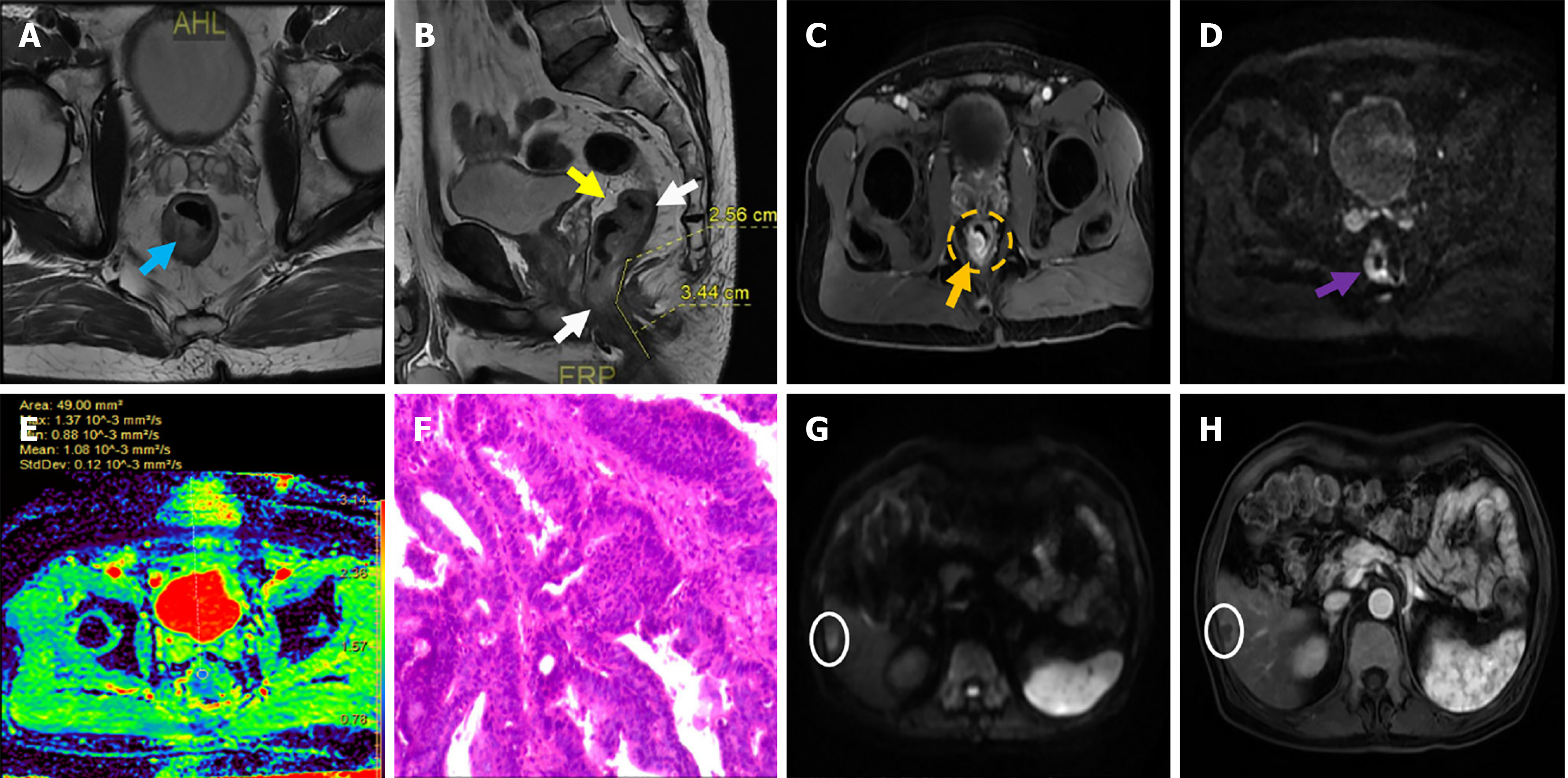
The mean value of MTL in the LRR group was 3.82 ± 1.30, while that in the HRR group was 4.22 ± 1.25, with no significant difference (P = 0.141) (Table 1). After preoperative MRI evaluation, 22 patients (41.51%) in the LRR group and 22 patients (59.46%) in the HRR group were diagnosed with mrT stage III and IV, respectively, but the difference was not statistically significant (P = 0.094) (Table 1). The proportion of mrN2 or mrN3 stage (39.62% vs 62.16%, P = 0.035), CRM-positive (45.28% vs 75.68%, P = 0.004), and EMVI-positive (24.53% vs 45.95%, P = 0.019) patients in the LRR and HRR groups differed significantly (Table 1). In addition, significant differences were observed in CEA levels (35.57 vs 46.55, P < 0.001) and CA199 levels (50.44 vs 65.39, P < 0.001) between the LRR and HRR groups (Table 1). Regarding other characteristics, no significant differences were found between the two groups. Figure 1 shows the MRI findings of the patient who was pathologically diagnosed with moderately differentiated adenocarcinoma of the rectum.
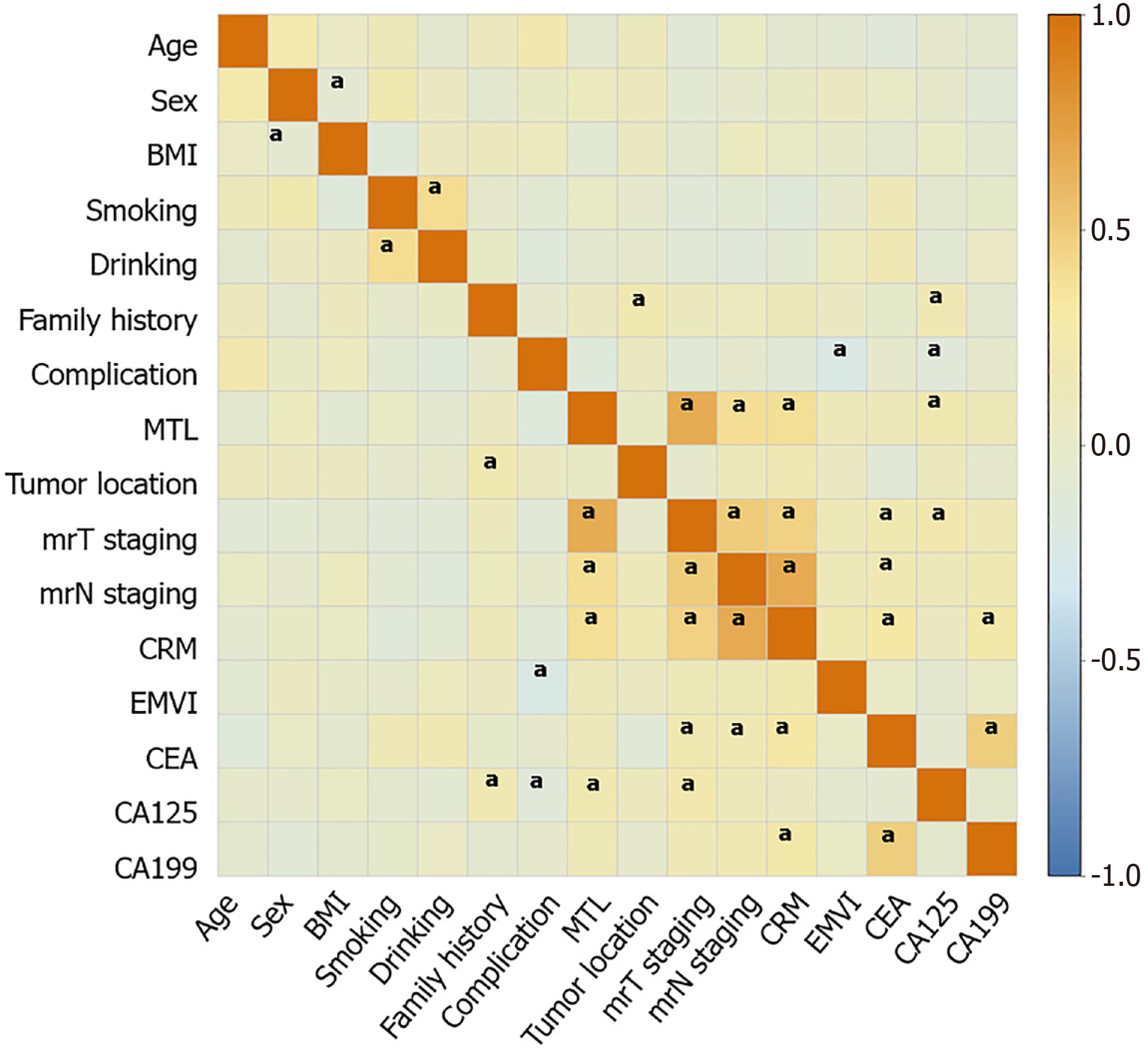
Correlation analysis showed significant associations between tumor location, elevated CA125 levels, and family history (P < 0.05) (Figure 2). A correlation was observed between greater MTL and elevated CA125 levels, positive CRM, and worse mrT and mrN staging (P < 0.05) (Figure 2). Moreover, poor mrT staging was associated with elevated CA125 levels, elevated CEA levels, positive CRM, and poor mrN staging (P < 0.05) (Figure 2). The mrN stage was correlated with an increase in CA199 and CEA levels (P < 0.05) (Figure 2). In addition, a significant positive correlation was observed between CEA and CA199 levels (P < 0.05) (Figure 2).
Results showed a statistical trend in the association between poor mrT stage and a high risk of recurrence in patients [odds ratio (OR) = 2.07, 95% confidence interval (CI): 0.88-4.85, P = 0.096] (Table 2). A significant association was observed between poor mrN staging and a high risk of recurrence (OR = 2.50, 95%CI: 1.06-5.93, P = 0.037) (Table 2). Positive CRM (OR = 3.76, 95%CI: 1.49-9.49, P = 0.005) and EMVI (OR = 2.62, 95%CI: 1.06-6.43, P = 0.036) were also significantly associated with a higher risk of recurrence. In addition, increased CEA levels (OR = 1.12, 95%CI: 1.06-1.18, P < 0.001) and CA199 levels (OR = 1.24, 95%CI: 1.14-1.35, P < 0.001) were all significantly associated with a higher risk of postoperative recurrence in patients with RC (Table 2).
| Variables | β | SE | Z | P value | OR (95%CI) |
| Age | 0.01 | 0.02 | 0.29 | 0.775 | 1.01 (0.97-1.05) |
| Sex | |||||
| Female | - | - | - | - | 1.00 (Reference) |
| Male | 0.63 | 0.48 | 1.33 | 0.183 | 1.89 (0.74-4.80) |
| BMI | 0.07 | 0.08 | 0.88 | 0.377 | 1.07 (0.92-1.24) |
| Smoking | |||||
| No | - | - | - | - | 1.00 (Reference) |
| Yes | -0.30 | 0.49 | -0.61 | 0.542 | 0.74 (0.29-1.93) |
| Drinking | |||||
| No | - | - | - | - | 1.00 (Reference) |
| Yes | -0.15 | 0.45 | -0.34 | 0.737 | 0.86 (0.35-2.09) |
| Family history | |||||
| No | - | - | - | - | 1.00 (Reference) |
| Yes | -0.05 | 0.68 | -0.08 | 0.940 | 0.95 (0.25-3.63) |
| Complication | |||||
| No | - | - | - | - | 1.00 (Reference) |
| Yes | 0.24 | 0.43 | 0.57 | 0.571 | 1.28 (0.55-2.96) |
| MTL | 0.26 | 0.17 | 1.47 | 0.143 | 1.29 (0.92- 1.82) |
| Tumor location | |||||
| Low | - | - | - | - | 1.00 (Reference) |
| Median | 0.15 | 0.49 | 0.30 | 0.762 | 1.16 (0.44-3.02) |
| High | 0.41 | 0.59 | 0.69 | 0.490 | 1.50 (0.47-4.74) |
| MrT staging | |||||
| I or II | - | - | - | - | 1.00 (Reference) |
| III or IV | 0.73 | 0.44 | 1.67 | 0.096 | 2.07 (0.88-4.85) |
| MrN staging | |||||
| 0 or I | - | - | - | - | 1.00 (Reference) |
| II or III | 0.92 | 0.44 | 2.08 | 0.037 | 2.50 (1.06-5.93) |
| CRM | |||||
| Negative | - | - | - | - | 1.00 (Reference) |
| Positive | 1.32 | 0.47 | 2.80 | 0.005 | 3.76 (1.49-9.49) |
| EMVI | |||||
| Negative | - | - | - | - | 1.00 (Reference) |
| Positive | 0.96 | 0.46 | 2.09 | 0.036 | 2.62 (1.06-6.43) |
| CEA | 0.11 | 0.03 | 4.06 | < 0.001 | 1.12 (1.06-1.18) |
| CA125 | 0.06 | 0.04 | 1.40 | 0.161 | 1.06 (0.98-1.15) |
| CA199 | 0.22 | 0.04 | 4.95 | < 0.001 | 1.24 (1.14-1.35) |
Multivariate logistic regression analysis showed that positive EMVI was an independent risk factor for the increased risk of postoperative recurrence in patients with RC (OR = 4.29, 95%CI: 1.04-17.76, P = 0.045) (Table 3). In addition, increased CEA (OR = 1.08, 95%CI: 1.01-1.15, P = 0.041), CA125 (OR = 1.19, 95%CI: 1.01-1.40, P = 0.034), and CA199 (OR = 1.27, 95%CI: 1.13-1.44 P < 0.001) levels were also independent risk factors for increased risk of recurrence (Table 3). All variables in the multivariate logistic regression model passed the collinearity diagnosis (VIF < 5), and interference of multicollinearity on the stability of the model was eliminated.
| Variables | β | SE | Z | P value | OR (95%CI) | VIF |
| EMVI | - | - | - | - | - | - |
| Negative | - | - | - | - | 1.00 (Reference) | - |
| Positive | 1.46 | 0.72 | 2.01 | 0.045 | 4.29 (1.04-17.76) | 2.31 |
| CEA | 0.07 | 0.04 | 2.04 | 0.041 | 1.08 (1.01-1.15) | 1.60 |
| CA125 | 0.18 | 0.08 | 2.13 | 0.034 | 1.19 (1.01-1.40) | 1.32 |
| CA199 | 0.24 | 0.06 | 3.95 | < 0.001 | 1.27 (1.13-1.44) | 1.49 |
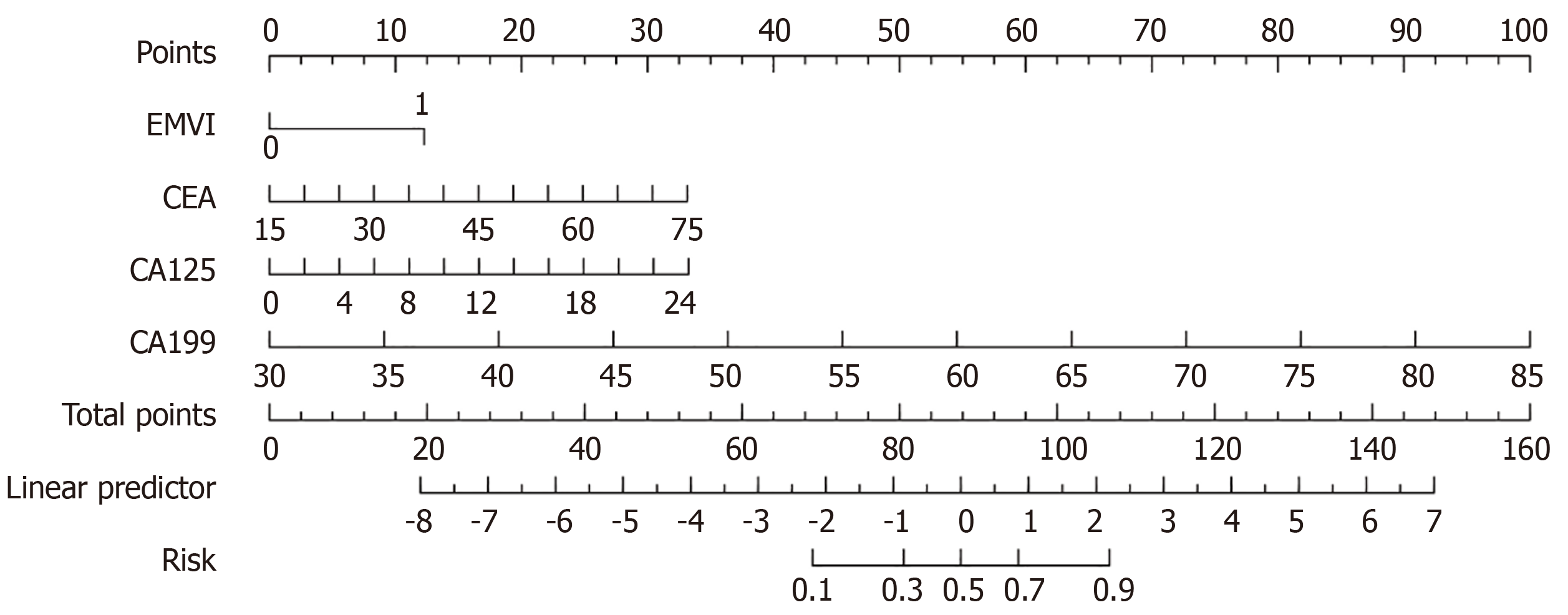
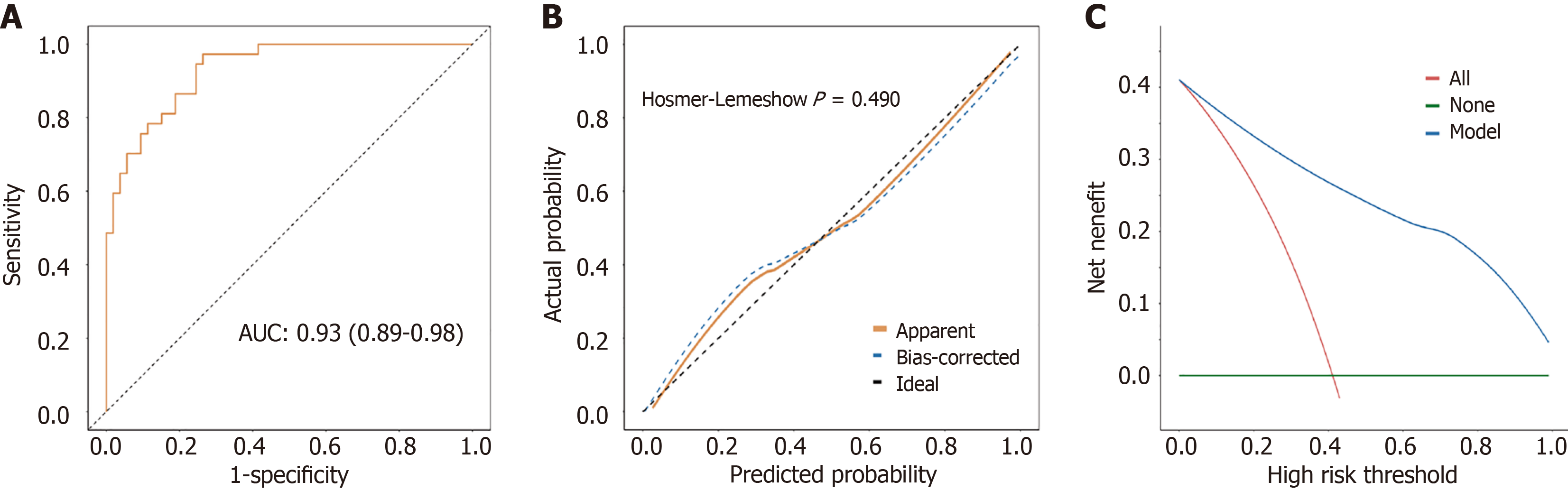
Based on multivariate logistic regression analysis, a clinical prediction model was constructed to predict the increased risk of recurrence in patients after RC surgery. The prediction model comprised four variables, namely EMVI, CEA levels, CA125 levels, and CA199 levels (Figure 3). Figure 4A shows the receiver operating characteristic curve plotted by combining the four variables in the prediction model. The prediction model had an area under the curve of 0.93 (95%CI: 0.89-0.98), indicating a degree of differentiation for patients at high risk of recurrence (Figure 4A). Hosmer-Lemeshow goodness of fit test and calibration curve results showed no significant difference between the predicted and observed values, and the model had a good fit (P = 0.490) (Figure 4B). The decision curve analysis curve was drawn with the risk probability threshold as the horizontal coordinate and the net rate of return as the vertical coordinate. The results showed that under the risk probability threshold of 0.0-1.0, the intervention measures for positive patients could obtain a better net benefit (Figure 4C). Under the optimal cut-off value, the accuracy of the model was 0.83 (95%CI: 0.74-0.90), sensitivity was 0.74 (95%CI: 0.62-0.85), specificity was 0.97 (95%CI: 0.92-1.00), positive predictive value was 0.98 (95%CI: 0.93-1.00), and negative predictive value was 0.72 (95%CI: 0.60-0.84) (Table 4).
| AUC (95%CI) | Accuracy (95%CI) | Sensitivity (95%CI) | Specificity (95%CI) | PPV (95%CI) | NPV (95%CI) | Cut off |
| 0.93 (0.89-0.98) | 0.83 (0.74-0.90) | 0.74 (0.62-0.85) | 0.97 (0.92-1.00) | 0.98 (0.93-1.00) | 0.72 (0.60-0.84) | 0.145 |
RC is a common gastrointestinal malignancy, and its postoperative recurrence is an important factor affecting the prognosis of patients[17]. Therefore, accurate assessment of the postoperative recurrence risk of patients with RC is of great value for formulating individualized treatment plans and optimizing patient management strategies. This study aimed to explore the association between MRI test indicators and the risk of recurrence in patients with RC after surgery, and to analyze the risk factors of HRR in patients with RC, combined with the demographic characteristics of patients and the content of tumor markers. In addition, the risk factors were included in the nomogram analysis, and a predictive model was constructed to identify people with a high risk of recurrence after RC surgery. The results of this study indicate that preoperative MRI detection of positive EMVI and elevated CEA, CA125, and CA199 levels may be independent risk factors for increased recurrence risk. In addition, a certain correlation may exist between the mrT stage, mrN stage, and CRM results detected by MRI and the risk of postoperative recurrence. Moreover, when EMVI is combined with tumor markers, the constructed clinical prediction model can more effectively identify postoperative patients with RC with a high risk of recurrence, providing strong support for clinical decision-making.
As an important manifestation of RC invasiveness, an EMVI-positive status often indicates that the tumor has broken through the rectal wall and invaded the peripheral vascular system[18]. This study found that positive EMVI diagnosed by MRI before surgery was significantly associated with the risk of postoperative recurrence in patients with RC. This finding is consistent with previous studies suggesting that vascular invasion is an important pathway for RC progression and recurrence[19,20]. The presence of EMVI may reflect the aggressiveness and biological behavior of the tumor, demonstrating the likelihood that the tumor had already developed micrometastases before surgery[21]. This increases the difficulty of surgery and may promote the spread of tumor cells through the blood system to distant organs, resulting in postoperative recurrence and metastasis[22]. From a biological perspective, the occurrence of EMVI may be closely related to the aggressiveness of tumor cells, the expression of angiogenic factors, and the tumor microenvironment[23]. Tumor cells secrete matrix metalloproteinases and other hydrolases to degrade the extracellular matrix and induce neovascularization simultaneously, providing conditions for tumor cell migration and diffusion[24]. Therefore, the EMVI status detected by preoperative MRI is of great value for evaluating patient prognosis and developing treatment strategies. However, the specific mechanisms must be explored further.
Tumor markers, a type of bioactive substances that reflect the presence and growth of tumors, play an important role in the diagnosis, prognosis evaluation, and efficacy monitoring of RC[25]. Tumor markers, such as CEA, CA125, and CA199 levels, used in this study have been confirmed to be closely related to the occurrence and development of RC. Tumor markers reflect the biological behavior and metabolic activity of the tumor, and their elevated levels may predict the aggressiveness and poor prognosis of the tumor[25]. For instance, increased CEA levels after surgery may indicate a higher number of residual tumor cells or a more aggressive biological behavior of tumor cells, which may lead to a higher risk of recurrence[26]. In addition, elevated CEA may indicate that tumor cells escaped the immune surveillance of the body to an extent that allows tumor cells to survive and proliferate in the body, increasing the risk of recurrence[27]. Elevated CA125 and CA199 levels may indicate that preoperative or postoperative adjuvant therapy (chemotherapy or radiotherapy) has not effectively eliminated all tumor cells. It is important to note that elevated CA125 and CA199 levels early after surgery may better predict recurrence risk than late elevations, as early elevations may reflect tumor cells that were not completely eradicated by surgery.
In this study, a clinical prediction model was constructed by combining the EMVI status assessed by preoperative MRI with the level of tumor markers. This model can evaluate the recurrence risk of patients with RC more comprehensively and accurately by considering the local invasion and systemic biological characteristics of the tumor. Our study results show that the model has high sensitivity and specificity in identifying postoperative patients with RC with a high risk of recurrence. This finding provides valuable reference information for clinicians and a scientific basis for formulating individualized treatment plans and optimizing patient management strategies. In clinical practice, this model can be used to evaluate the risk of recurrence of patients before surgery to guide the choice of surgical protocol and the decision of postoperative adjuvant therapy. More active surgical strategies and enhanced postoperative adjuvant therapy can be adopted to reduce the risk of recurrence and improve the therapeutic effect for patients with a high risk of recurrence.
Although this study has some strengths, it has some limitations. First, this was a single-center study with a relatively limited sample size, which may affect the generalizability of the results. Future studies should expand the sample size and include patient data from multiple centers and regions to enhance the reliability and generalization of conclusions. Second, because this study was conducted retrospectively, it may have problems inherent to that design, such as selection bias and information bias. Prospective randomized controlled trials can be conducted to validate the findings of this study further. In addition, the prognosis of patients after RC surgery is affected by many factors, and the results of this study need to be analyzed according to the specific clinical manifestations of patients. Finally, with continual advancements in imaging technology and molecular biology research, more new imaging techniques and biomarkers can be explored in the future to evaluate the prognosis of RC. At the same time, in-depth research on the mechanism of EMVI and the relationship between tumor markers and recurrence risk should be strengthened to provide theoretical support for the development of new treatment strategies and prediction models.
In this study, a retrospective analysis was performed that included 90 patients with RC to investigate the high-risk factors for recurrence after radical RC surgery. A multifactorial logistic regression analysis revealed that EMVI positivity was an independent risk factor for the increased risk of postoperative recurrence in patients, while elevated levels of CEA, CA125, and CA199 were also significantly associated with an increased risk of recurrence. Based on these findings, we constructed a joint prediction model for predicting the risk of postoperative recurrence in patients with RC. The model demonstrated high sensitivity and specificity and effectively identified patients with a high risk of recurrence after RC surgery, providing strong support for clinicians to develop personalized treatment plans and optimize patients’ postoperative management strategies. Future studies should expand the sample size and include data from multiple centers to validate further, optimizing the clinical prediction model, and providing a more accurate and reliable tool for prognostic assessment and recurrence risk management in patients with RC.
| 1. | Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Jeck W, Johung KL, Kirilcuk N, Krishnamurthi S, Maratt JK, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stotsky-Himelfarb E, Tavakkoli A, Willett CG, Gregory K, Gurski L. Rectal Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:1139-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 405] [Article Influence: 135.0] [Reference Citation Analysis (0)] |
| 2. | Lu L, Mullins CS, Schafmayer C, Zeißig S, Linnebacher M. A global assessment of recent trends in gastrointestinal cancer and lifestyle-associated risk factors. Cancer Commun (Lond). 2021;41:1137-1151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 183] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 3. | Koukourakis IM, Kouloulias V, Tiniakos D, Georgakopoulos I, Zygogianni A. Current status of locally advanced rectal cancer therapy and future prospects. Crit Rev Oncol Hematol. 2023;186:103992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 4. | Ryan OK, Ryan ÉJ, Creavin B, Rausa E, Kelly ME, Petrelli F, Bonitta G, Kennelly R, Hanly A, Martin ST, Winter DC. Surgical approach for rectal cancer: A network meta-analysis comparing open, laparoscopic, robotic and transanal TME approaches. Eur J Surg Oncol. 2021;47:285-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Agger EA, Jörgren FH, Lydrup MA, Buchwald PL. Risk of local recurrence of rectal cancer and circumferential resection margin: population-based cohort study. Br J Surg. 2020;107:580-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Ahiko Y, Shida D, Kudose Y, Nakamura Y, Moritani K, Yamauchi S, Sugihara K, Kanemitsu Y; Japanese Study Group for Postoperative Follow-up of Colorectal Cancer. Recurrence hazard of rectal cancer compared with colon cancer by adjuvant chemotherapy status: a nationwide study in Japan. J Gastroenterol. 2021;56:371-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Okamura R, Itatani Y, Fujita Y, Hoshino N, Okumura S, Nishiyama K, Hida K, Obama K. Postoperative recurrence in locally advanced rectal cancer: how does neoadjuvant treatment affect recurrence pattern? World J Surg Oncol. 2023;21:247. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Renehan AG. Techniques and Outcome of Surgery for Locally Advanced and Local Recurrent Rectal Cancer. Clin Oncol (R Coll Radiol). 2016;28:103-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Bruno F, Arrigoni F, Mariani S, Splendiani A, Di Cesare E, Masciocchi C, Barile A. Advanced magnetic resonance imaging (MRI) of soft tissue tumors: techniques and applications. Radiol Med. 2019;124:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 10. | Bates DDB, Homsi ME, Chang KJ, Lalwani N, Horvat N, Sheedy SP. MRI for Rectal Cancer: Staging, mrCRM, EMVI, Lymph Node Staging and Post-Treatment Response. Clin Colorectal Cancer. 2022;21:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 11. | Jo N. DWI and Dynamic Contrast-enhanced Perfusion MRI for Differentiation of Common Skull Base Tumors. Radiol Imaging Cancer. 2023;5:e239009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Hou M, Sun JH. Emerging applications of radiomics in rectal cancer: State of the art and future perspectives. World J Gastroenterol. 2021;27:3802-3814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Li H, Chen XL, Liu H, Liu YS, Li ZL, Pang MH, Pu H. MRI-based multiregional radiomics for preoperative prediction of tumor deposit and prognosis in resectable rectal cancer: a bicenter study. Eur Radiol. 2023;33:7561-7572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 14. | Niu Y, Yu X, Wen L, Bi F, Jian L, Liu S, Yang Y, Zhang Y, Lu Q. Comparison of preoperative CT- and MRI-based multiparametric radiomics in the prediction of lymph node metastasis in rectal cancer. Front Oncol. 2023;13:1230698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Horvat N, Carlos Tavares Rocha C, Clemente Oliveira B, Petkovska I, Gollub MJ. MRI of Rectal Cancer: Tumor Staging, Imaging Techniques, and Management. Radiographics. 2019;39:367-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 312] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 16. | Lee HG, Kim CW, Jang JK, Park SH, Kim YI, Lee JL, Yoon YS, Park IJ, Lim SB, Yu CS, Kim JC. Pathologic Implications of Magnetic Resonance Imaging-detected Extramural Venous Invasion of Rectal Cancer. Clin Colorectal Cancer. 2023;22:129-135. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Zhao M, Feng L, Zhao K, Cui Y, Li Z, Ke C, Yang X, Qiu Q, Lu W, Liang Y, Xie C, Wan X, Liu Z. An MRI-based scoring system for pretreatment risk stratification in locally advanced rectal cancer. Br J Cancer. 2023;129:1095-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 18. | Kim TH, Firat C, Thompson HM, Gangai N, Zheng J, Capanu M, Bates DDB, Paroder V, García-Aguilar J, Shia J, Gollub MJ, Horvat N. Extramural Venous Invasion and Tumor Deposit at Diffusion-weighted MRI in Patients after Neoadjuvant Treatment for Rectal Cancer. Radiology. 2023;308:e230079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Chand M, Bhangu A, Wotherspoon A, Stamp GWH, Swift RI, Chau I, Tekkis PP, Brown G. EMVI-positive stage II rectal cancer has similar clinical outcomes as stage III disease following pre-operative chemoradiotherapy. Ann Oncol. 2014;25:858-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 20. | Abe T, Yasui M, Imamura H, Matsuda C, Nishimura J, Haraguchi N, Nakai N, Wada H, Takahashi H, Omori T, Miyata H, Ohue M. Combination of extramural venous invasion and lateral lymph node size detected with magnetic resonance imaging is a reliable biomarker for lateral lymph node metastasis in patients with rectal cancer. World J Surg Oncol. 2022;20:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 21. | Tripathi P, Rao SX, Zeng MS. Clinical value of MRI-detected extramural venous invasion in rectal cancer. J Dig Dis. 2017;18:2-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Tang C, Lu G, Xu J, Kuang J, Xu J, Wang P. Diffusion kurtosis imaging and MRI-detected extramural venous invasion in rectal cancer: correlation with clinicopathological prognostic factors. Abdom Radiol (NY). 2023;48:844-854. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Tan JJ, Carten RV, Babiker A, Abulafi M, Lord AC, Brown G. Prognostic Importance of MRI-Detected Extramural Venous Invasion in Rectal Cancer: A Literature Review and Systematic Meta-Analysis. Int J Radiat Oncol Biol Phys. 2021;111:385-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 24. | Wang Q, Wang K, Tan X, Li Z, Wang H. Immunomodulatory role of metalloproteases in cancers: Current progress and future trends. Front Immunol. 2022;13:1064033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 25. | Sun R, Zeng Y, Fan Y, Wang X. A new post-operative prognostic System Combining CEA and CA199 for locally advanced rectal cancer patients undergoing neoadjuvant chemoradiotherapy followed by total mesorectal excision. Asian J Surg. 2023;46:2819. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Duffy MJ. Carcinoembryonic antigen as a marker for colorectal cancer: is it clinically useful? Clin Chem. 2001;47:624-630. [PubMed] |
| 27. | Candeias SM, Gaipl US. The Immune System in Cancer Prevention, Development and Therapy. Anticancer Agents Med Chem. 2016;16:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |