Published online Feb 27, 2025. doi: 10.4240/wjgs.v17.i2.101775
Revised: November 13, 2024
Accepted: December 18, 2024
Published online: February 27, 2025
Processing time: 118 Days and 11.4 Hours
Inferior vena cava (IVC) leiomyosarcomas are rare and aggressive tumors. Complete cure depends on achieving R0 resection, which often requires circumferential resection and reconstruction. Synthetic grafts have traditionally been used when venous continuity must be restored. However, the use of cadaveric IVC grafts for reconstruction has not been widely reported.
Herein, we present the case of a 64-year-old woman diagnosed with an intrahepatic IVC leiomyosarcoma with local invasion. The patient responded favorably to chemotherapy and subsequently underwent an en bloc right hepa
Cadaveric IVC grafts are an alternative to synthetic grafts for reconstruction, with acceptable outcomes. Larger, long-term studies are necessary to validate these findings.
Core Tip: We share our experience with an unprecedented approach using a cadaveric venous graft to reconstruct the inferior vena cava (IVC) after IVC leiomyosarcoma resection. The unavailability of a synthetic graft of an appropriate size led us to use the cadaveric graft, which showed durability and patency over a 27-month period, encouraging us to share our results.
- Citation: AlOmran HA, AlMatar B, AlMonsained M, Bojal S, Momani H, AlQahtani MS. Novel surgical approach - cadaveric inferior vena cava graft reconstruction following leiomyosarcoma resection: A case report. World J Gastrointest Surg 2025; 17(2): 101775
- URL: https://www.wjgnet.com/1948-9366/full/v17/i2/101775.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i2.101775
Inferior vena cava (IVC) leiomyosarcomas are rare yet aggressive tumors originating from the endothelium of a vein[1]. Although systemic treatment plays a crucial role in managing these patients, complete cure depends on achieving R0 resection, which often requires circumferential resection and reconstruction[1,2]. Synthetic grafts have traditionally been used when venous continuity must be restored. However, the use of cadaveric IVC grafts for reconstruction has not been widely reported[3].
Here, we present the case of a 64-year-old woman diagnosed with an intrahepatic IVC leiomyosarcoma with local invasion. The patient responded favorably to chemotherapy and subsequently underwent an en bloc right hepatectomy, retrohepatic IVC resection, and reconstruction with an interpositional cadaveric IVC graft.
The patient had pain in the right hypochondriac.
The patient was a 64-year-old woman who was referred to our hepatobiliary surgery service in 2019. She had a 5-month history of worsening right hypochondriac pain, with no accompanying nausea, vomiting, altered bowel habits, jaundice, fever, or weight loss.
The patient had type 2 diabetes mellitus.
The personal and family history was unremarkable.
Physical examination showed no remarkable findings.
All initial laboratory results were within normal ranges.
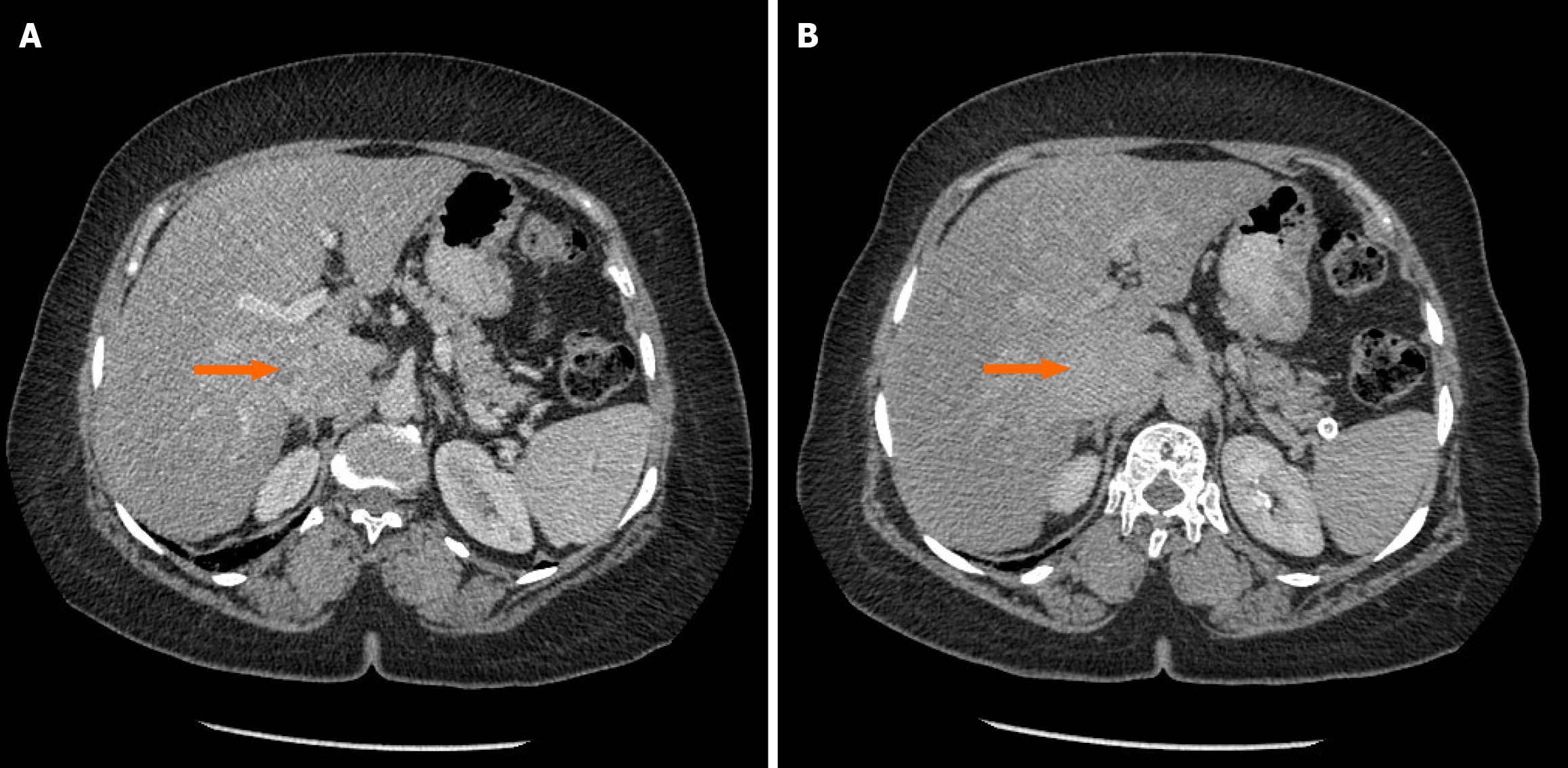
Computed tomography (CT) of the chest, abdomen, and pelvis revealed a heterogeneously enhancing, lobulated soft tissue mass measuring 4.6 cm × 3 cm × 6 cm. The mass involved the intrahepatic IVC, surrounding liver tissue, and part of the right adrenal gland. The lungs were free of metastatic deposits (Figure 1).
Magnetic resonance imaging confirmed that the lesion originated from the intrahepatic segment of the IVC and invaded segments 6 and 7 of the liver, as well as the right crus of the diaphragm.
The case was reviewed by a multidisciplinary tumor board comprising hepatobiliary surgeons, radiologists, pathologists, and medical oncologists. Owing to the tumor’s abutment to the IVC and the involvement of the left, middle, and right hepatic veins, the tumor was deemed unresectable. Systemic chemotherapy with doxorubicin, ifosfamide, and Mesna was initiated.
Following four cycles of chemotherapy, CT revealed mild progression of the tumor and necrosis in the liver lesion. The patient was switched to a second-line chemotherapy regimen of gemcitabine and docetaxel for six additional cycles. Subsequent imaging revealed tumor stabilization. One year after the initial diagnosis, follow-up imaging demonstrated a significant reduction in the tumor size (from 6 cm × 4.6 cm to 2.8 cm × 1.8 cm), with no evidence of systemic metastatic spread. Given the significant response to systemic therapy, the tumor board reconsidered the patient and recommended curative surgical resection.
An intrahepatic IVC leiomyosarcoma with invasion of the diaphragm, liver, and right adrenal gland, along with hepatic metastatic deposits.
The patient underwent an L-shaped laparotomy, en bloc right hepatectomy, caudate lobectomy, right adrenalectomy, and retrohepatic IVC resection. Reconstruction was performed using a cadaveric IVC graft obtained from a deceased donor liver. The decision to use a cadaveric graft with an acceptable diameter was made because a synthetic conduit of the proper size was unavailable.
Our transplant anesthesia team was skilled in managing intraoperative IVC clamping and performed the procedure without the need for cardiopulmonary bypass. The patient was stabilized using intravenous fluids and inotropes before clamping, and invasive monitoring was performed throughout the operation. The anastomosis extended from the infrahepatic IVC, including the right renal vein, to the suprahepatic segment.
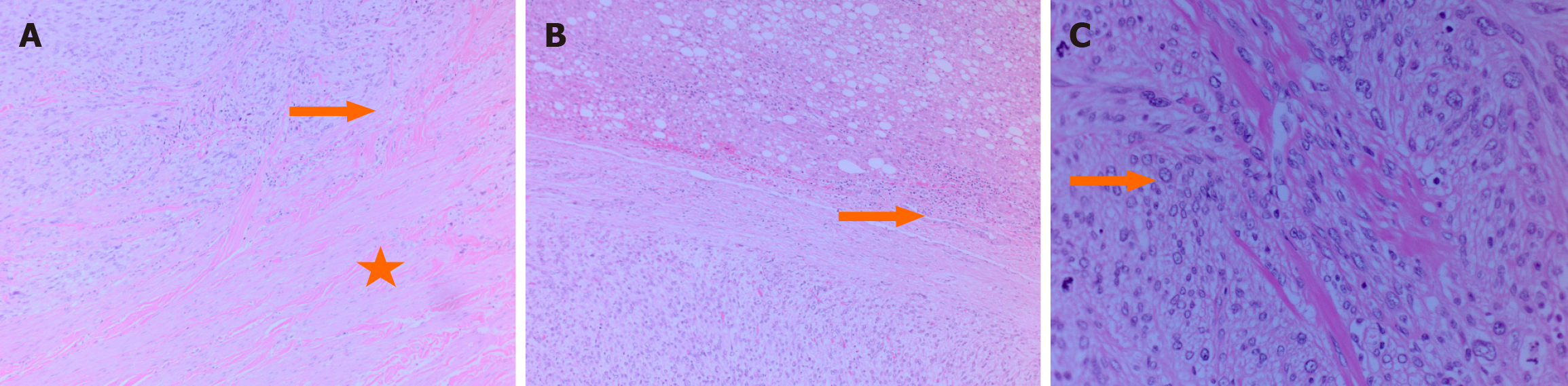
Postoperative histopathological examination confirmed leiomyosarcoma of the IVC with negative surgical margins. Severe hepatic steatosis was observed in the excised liver tissue (Figure 2).
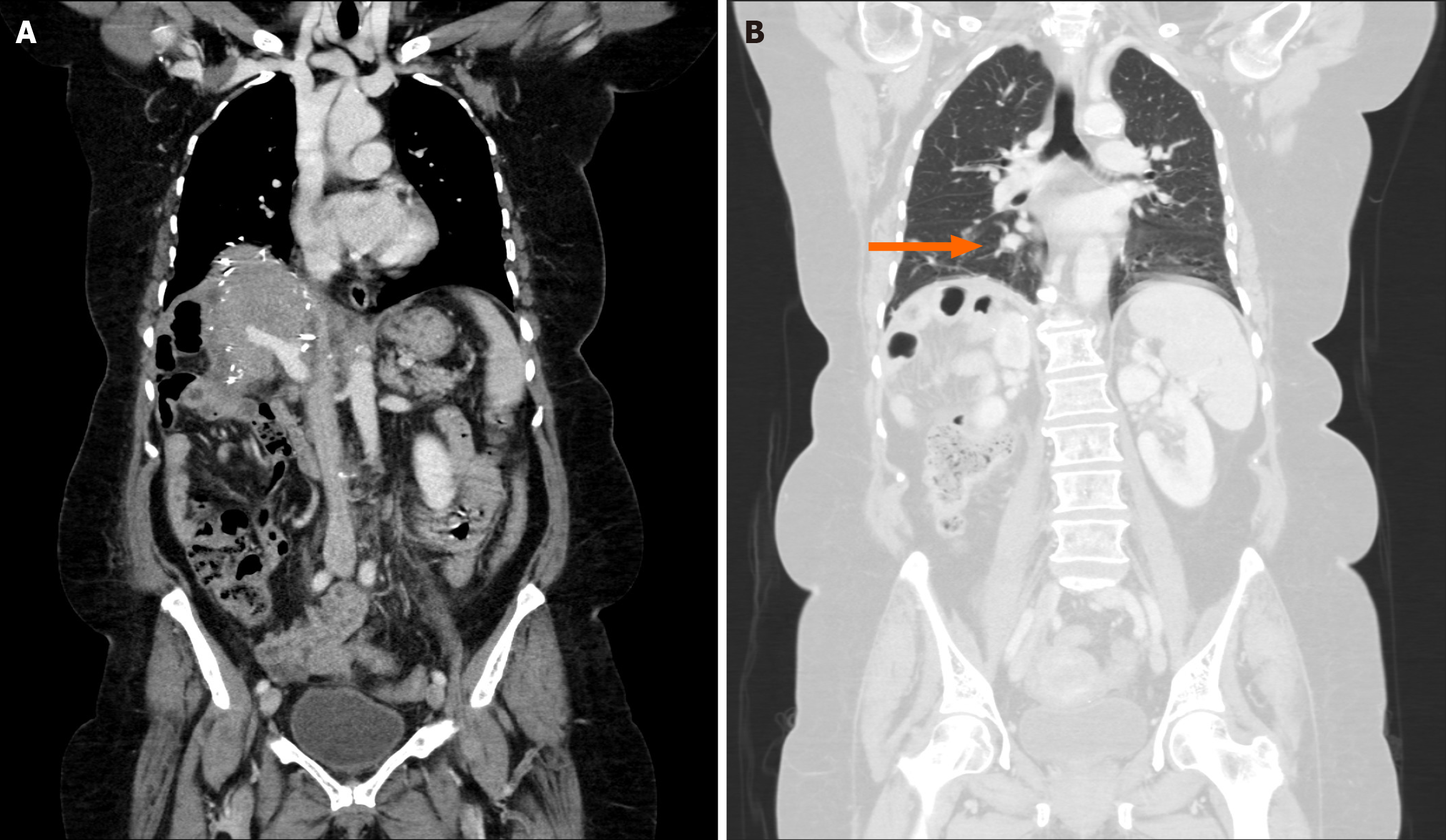
The patient was monitored regularly during clinical and radiological follow-up to assess the graft patency and detect recurrence. One month post-surgery, follow-up CT revealed suspicious pulmonary nodules in the right lower lobe but no evidence of recurrence at the surgical site. The IVC and hepatic veins remained patent (Figure 3). The patient was administered six additional cycles of gemcitabine and dacarbazine to reduce the risk of recurrence.
Unfortunately, the pulmonary nodules progressed despite chemotherapy. The tumor board recommended a right thoracotomy and resection of the metastatic lesion, followed by stereotactic body radiation therapy for a left-sided lung lesion.
Despite all locoregional and systemic treatments, the patient later developed bilateral pulmonary and duodenal metastases, and after receiving three cycles of eribulin, her condition worsened due to pneumonia, ascites, and anemia. She died 27 months after the abdominal surgery, following a family meeting that resulted in a do-not-resuscitate order.
Vascular leiomyosarcomas are slow-growing rare tumors that account for 0.5%-1% of all soft tissue sarcomas in adults. IVC leiomyosarcomas are the most common primary tumors of the IVC. Despite their rarity, the prognosis is generally poor, with a 5-year survival rate of only 31% after complete resection. Negative surgical margins are the most important prognostic factors for survival[4,5].
Leiomyosarcomas typically affect women, with a 3:1 female-to-male ratio. The median age at diagnosis is in the fifth decade of life[1,2]. Symptoms are usually nonspecific and depend on the location of IVC involvement. Patients with hepatic vein involvement or extension into the right atrium often have a worse prognosis[4,6].
Complete surgical resection with free margins remains the only curative option, while chemotherapy and radiation play uncertain roles. Studies have shown that incomplete resection or nonsurgical treatment alone results in 0% survival at 5 years.
The segment-based classification of IVC involvement helps guide surgical approaches[2-5]. Our case involved a segment III tumor necessitating right hepatectomy and adrenalectomy, along with IVC resection and reconstruction[6,7].
Various synthetic and autologous grafts have been used for IVC reconstruction, including Dacron, polytetrafluoroethylene (PTFE), reversed superficial femoral vein grafts, and common autologous venous tube grafts[6-10]. However, PTFE has been the graft of choice, owing to its superior patency rate due to its compression-resistant rings[3,5,9]. Several studies have reported complications related to the use of synthetic grafts, including leakage, graft-related infections, pseudointima formation, thrombosis, stenosis, venous hypertension, pulmonary embolism, and anticoagulation-related complications[1,8,9,10]. Moreover, graft size selection plays an important role in the development of thrombotic complications[11]. While there is no universal formula dictating an ideal graft size, a large graft diameter has been shown to result in thrombotic events due to low blood velocity via the conduit; in contrast, although a small graft diameter may enhance the blood flow and mitigate blood stagnation, it may eventually develop a thick pseudointima and subsequent thrombosis or occlusion[11,12]. The synthetic graft available at the time of the operation was too small for the already widened IVC and precluded safe anastomosis formation, which caused us to deviate from the usual practice of using a synthetic graft in light of the availability of a potential alternative with an acceptable diameter.
Cadaveric venous grafts, which are not commonly reported, have been effective in selected patients with higher infection resistance and patency rates[13-18]. In our patient, the cadaveric IVC graft demonstrated long-term patency with no complications related to graft thrombosis or infection during the 27 months of follow-up.
Cadaveric IVC grafts, although not readily available, are viable alternatives for reconstruction following IVC resection. Our patient demonstrated successful graft patency for two years. However, larger controlled studies are necessary to validate these findings and assess long-term outcomes.
| 1. | Teixeira FJR Jr, do Couto Netto SD, Perina ALF, Torricelli FCM, Ragazzo Teixeira L, Zerati AE, Ferreira FO, Akaishi EH, Nahas WC, Utiyama EM. Leiomyosarcoma of the inferior vena cava: Survival rate following radical resection. Oncol Lett. 2017;14:3909-3916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Sephien A, Mousa MS, Bui MM, Kedar R, Thomas K. Leiomyosarcoma of the Inferior Vena Cava with Hepatic and Pulmonary Metastases: Case Report. J Radiol Case Rep. 2019;13:30-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Kieffer E, Alaoui M, Piette JC, Cacoub P, Chiche L. Leiomyosarcoma of the inferior vena cava: experience in 22 cases. Ann Surg. 2006;244:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 198] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 4. | Keller K, Jacobi B, Jabal M, Stavrou GA. Leiomyosarcoma of the inferior vena cava: A case report of a rare tumor entity. Int J Surg Case Rep. 2020;71:50-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Graves A, Longoria J, Graves G, Ianiro C. Leiomyosarcoma of the inferior vena cava: a case report. J Surg Case Rep. 2020;2020:rjaa479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Rusu CB, Gorbatâi L, Szatmari L, Koren R, Bungărdean CI, Feciche BO, Bumbuluţ C, Andraş IM, Rahotă R, Telecan T, Coman I, Rath-Wolfson L, Crişan N. Leiomyosarcoma of the inferior vena cava. Our experience and a review of the literature. Rom J Morphol Embryol. 2020;61:227-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Alexander A, Rehders A, Raffel A, Poremba C, Knoefel WT, Eisenberger CF. Leiomyosarcoma of the inferior vena cava: radical surgery and vascular reconstruction. World J Surg Oncol. 2009;7:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Quinones-Baldrich W, Alktaifi A, Eilber F, Eilber F. Inferior vena cava resection and reconstruction for retroperitoneal tumor excision. J Vasc Surg. 2012;55:1386-93; discussion 1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 9. | Papamichail M, Marmagkiolis K, Pizanias M, Koutserimpas C, Heaton N. Safety and Efficacy of Inferior Vena Cava Reconstruction During Hepatic Resection. Scand J Surg. 2019;108:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Goto H, Hashimoto M, Akamatsu D, Shimizu T, Miyama N, Tsuchida K, Tajima Y, Ohuchi N. Surgical resection and inferior vena cava reconstruction for treatment of the malignant tumor: technical success and outcomes. Ann Vasc Dis. 2014;7:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Ruiz CS, Kalbaugh CA, Browder SE, McGinigle KL, Kibbe MR, Farber MA, Crowner JR, Marston WA, Pascarella L. Operative strategies for inferior vena cava repair in oncologic surgery. J Vasc Surg Venous Lymphat Disord. 2020;8:396-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Pantoja JL, Patel RP, Baril DT, Quinones-Baldrich W, Lawrence PF, Woo K. Caval Reconstruction with Undersized Ringed Graft after Resection of Inferior Vena Cava Leiomyosarcoma. Ann Vasc Surg. 2020;65:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Kwon H, Kwon H, Hong JP, Han Y, Park H, Song GW, Kwon TW, Cho YP. Use of cryopreserved cadaveric arterial allograft as a vascular conduit for peripheral arterial graft infection. Ann Surg Treat Res. 2015;89:51-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Furlough CL, Jain AK, Ho KJ, Rodriguez HE, Tomita TM, Eskandari MK. Peripheral artery reconstructions using cryopreserved arterial allografts in infected fields. J Vasc Surg. 2019;70:562-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Castier Y, Francis F, Cerceau P, Besnard M, Albertin J, Fouilhe L, Cerceau O, Albaladejo P, Lesèche G. Cryopreserved arterial allograft reconstruction for peripheral graft infection. J Vasc Surg. 2005;41:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Patel R, Marks NA, Hingorani AP, Ascher E. Outcomes of Cadaveric Veins as Conduits for Lower Extremity Arterial Bypass. J Vasc Surg. 2022;75:12S. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Randon C, Jacobs B, De Ryck F, Beele H, Vermassen F. Fifteen years of infrapopliteal arterial reconstructions with cryopreserved venous allografts for limb salvage. J Vasc Surg. 2010;51:869-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Hartranft CA, Noland S, Kulwicki A, Holden CR, Hartranft T. Cryopreserved saphenous vein graft in infrainguinal bypass. J Vasc Surg. 2014;60:1291-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |