Published online Feb 27, 2025. doi: 10.4240/wjgs.v17.i2.101239
Revised: November 8, 2024
Accepted: December 17, 2024
Published online: February 27, 2025
Processing time: 135 Days and 18.3 Hours
Progressive familial intrahepatic cholestasis type 1 (PFIC-1) is a genetic cholestatic disease causing end-stage liver disease, which needs liver transplantation (LT). Simultaneous biliary diversion (BD) was recommended to prevent allograft steatosis after transplantation, while increasing the risk of infection. Here, an attempt was made to perform BD using appendix to prevent bacterial translocation after LT.
An 11-month-old boy diagnosed with PFIC-1 received ABO compatible living donor LT due to refractory jaundice and pruritus. His mother donated her left lateral segment with a graft-to-recipient weight ratio of 2.9%. Internal BD was constructed during LT using the appendix by connecting its proximal end with the intrahepatic biliary duct and the distal end with colon. Biliary leakage was suspected on the 5th day after transplantation and exploratory laparotomy indicated biliary leakage at the cutting surface of liver. The liver function returned to normal on the 9th day post-operation and maintained normal during the 15-month follow-up. Cholangiography at 10 months after transplantation confirmed the direct secretion of bile into colon. Computerized tomography scan (4 months and 10 months) and liver biopsy (10 months) indicated no steatosis in the allograft. No complaint of recurrent diarrhea, infection or growth retardation was reported during follow-up.
Internal BD using appendix during LT is effective in preventing allograft steatosis and post-transplant infection in PFIC-1 recipients.
Core Tip: Liver transplantation indicated in end-stage liver disease caused by progressive familial intrahepatic cholestasis type 1 is often performed with biliary diversion (BD). Although effectively preventing allograft steatosis, traditional BD increases post-operative infection risk. Here, we present a case of an 11-month-old progressive familial intrahepatic cholestasis type 1 patient receiving liver transplantation and BD using appendix to prevent post-operative infection. The proximal end and distal end of appendix were connected with intrahepatic biliary duct and colon respectively. Post-operative exams confirmed successful BD and absence of allograft steatosis. Thus, using appendix for BD might be a new surgical approach preventing allograft steatosis and post-transplant infection.
- Citation: Song JQ, Zhou T, Luo Y, Liu Y. Internal biliary diversion using appendix during liver transplantation for progressive familial intrahepatic cholestasis type 1: A case report. World J Gastrointest Surg 2025; 17(2): 101239
- URL: https://www.wjgnet.com/1948-9366/full/v17/i2/101239.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i2.101239
Progressive familial intrahepatic cholestasis (PFIC) is a group of rare genetic liver disorders characterized by progressive cholestasis in childhood, eventually leading to liver fibrosis and end-stage liver disease[1]. Among the 6 subtypes of PFIC, PFIC type 1 (PFIC-1) is caused by mutation in ATP8B1, a gene encoding a P-type aminophospholipid flippase (ATPase) called FIC1[2,3]. Mutation of ATP8B1 impairs the transportation of phospholipid and results in cholestasis[4]. Liver transplantation (LT) is indicated in patients with end-stage liver disease. However, PFIC-1 patients are susceptible to persistent chronic diarrhea and allograft steatosis after transplantation, which may lead to graft dysfunction[5-7]. Therefore, internal biliary diversion (BD) is recommended to skip the absorption of biliary acid in distal ileum and alleviate chronic diarrhea and hepatic steatosis[8-11]. However, direct connection between biliary duct and the colon may expose the hepatobiliary system to gut microbiome which may lead to bacterial translocation and infection[11]. Here, we report a novel surgical method using the appendix for biliary reconstruction. The appendix possesses Gerlach’s valve, which can act as a check valve to prevent intestinal contents regurgitation and gut microbiome translocation, which might reduce the infection risk of BD after LT.
An 11-month-old boy developed progressively worsening jaundice and sclera for over 11 months.
The patient was male, 11 months old, full term with normal delivery. After birth, he developed jaundice and the whole-exon test revealed a mutation in the ATP8B1 gene (c.1798C>T), supporting the diagnosis of PFIC-1.
The baby was born by uneventful spontaneous delivery. The results of the prenatal examination showed no abnormalities.
The patient had no family genetic history of related liver diseases.
The vital signs of the patients were within normal range, including respiratory rate, heart rate, blood pressure, body temperature and oxygen saturation. The patient had jaundice and sclera.
Laboratory tests showed severe cholestasis with total bilirubin: 313.3 μmol/L and direct bilirubin: 224.4 μmol/L.
Preoperative ultrasonography indicated elevated liver elasticity (mean value: 10.0 kPa, median value: 10.1 kPa).
According to the provided medical history, the final diagnosis was PFIC-1.
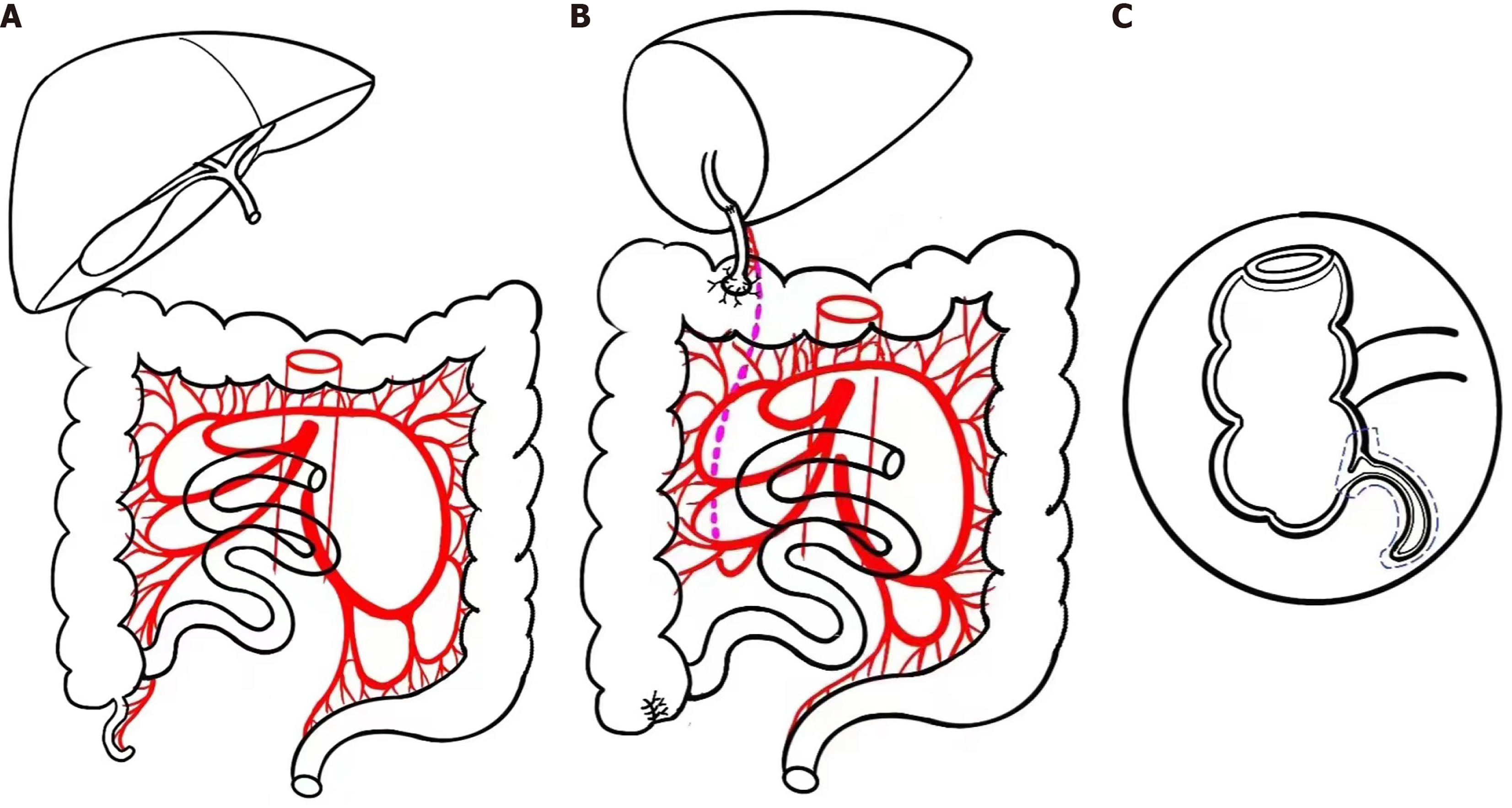
This patient then received ABO compatible living donor LT, in which his mother donated the left lateral segment, weighted 251 g with graft-to-recipient weight ratio as 2.9%. The operation was uneventful with bleeding of 50 mL. To conduct the internal BD, the free appendix was cut off from the ileocecal junction area with the blood supply vessels connected. Then the appendix was reconstructed at the junction of transverse and ascending colon, with the distal end was anastomosed with the bile duct (Figure 1).
The recovery of this patient after transplantation was smooth. His tracheal intubation was removed 3 days after operation with a length of stay in computerized tomography of 8 days. Biliary leakage was suspected on the 5th day after transplantation and exploratory laparotomy indicated a small amount of bile-like effusion at the cutting surface of the allograft. No abnormality was found at the appendiceal-colonic and biliary-appendiceal anastomotic stomas. The liver function returned to normal on the 9th day post-operation and this patient was discharged on the 18th day after transplantation. The maintenance immunosuppressive strategy was tacrolimus and prednisone.
During the 15-month follow up, the patient had no complaint of recurrent diarrhea or growth retardation. Biochemical parameters for liver function were routinely tested during follow-up visits and no obvious abnormality was reported. Upper abdomen computerized tomography was carried out in the 4th and 10th months after LT operation and no graft steatosis was found, as presented in Figure 2A and B. Fine needle liver biopsy, performed 10 months after LT, showed no damage of hepatic lobular structure and no steatosis. A radionuclide cholangiography, performed 10 months after LT, showed that bile was excreted into the transverse colon directly with no expansion of intrahepatic biliary tract (Figure 2C). The postoperative blood vitamin E and vitamin K1 levels were below normal. Thus, regular intramuscular vitamin E and vitamin K1 injections were prescribed and the subsequent lipid soluble vitamin concentrations were closely monitored.
PFIC-1 patients who receive LT treatment encounter high risk of allograft steatosis and refractory diarrhea, which makes BD a common and necessary surgical procedure during LT[5,6]. However, traditional internal BD may expose the liver directly to the excessive gut microbiome in the colon, leading to microbiome translocation and post-operation infection such as cholangitis[11]. In this study, we used appendix to construct a novel “appendiceal-colonic anastomosis” in an 11-month-old boy with PFIC-1 during LT to prevent the translocation of colon microbiome. The recipient experienced normal recovery after transplantation and no steatosis or diarrhea was found during the follow-up. Cholangiography also indicated the drainage of bile flow directly into colon. Our report has provided a novel approach for BD in PFIC-1 LT recipients with less post-transplant infection risk.
To reduce the risk of infection while preventing allograft steatosis, using the appendix to replace the bile duct for BD has become a new exploration, which is expected to prevent colonic microbiota translocation and reduce the risk of infection through the Gerlach valve. In this study, replacing the bile duct with the appendix for biliary enteric anastomosis can achieve normal BD function. This successful case may provide a new treatment strategy for PFIC-1 Liver transplant recipients.
Here, we would like to express our sincere gratitude to the patients’ families and patients for their support of our work.
| 1. | van Wessel DBE, Thompson RJ, Gonzales E, Jankowska I, Shneider BL, Sokal E, Grammatikopoulos T, Kadaristiana A, Jacquemin E, Spraul A, Lipiński P, Czubkowski P, Rock N, Shagrani M, Broering D, Algoufi T, Mazhar N, Nicastro E, Kelly D, Nebbia G, Arnell H, Fischler B, Hulscher JBF, Serranti D, Arikan C, Debray D, Lacaille F, Goncalves C, Hierro L, Muñoz Bartolo G, Mozer-Glassberg Y, Azaz A, Brecelj J, Dezsőfi A, Luigi Calvo P, Krebs-Schmitt D, Hartleif S, van der Woerd WL, Wang JS, Li LT, Durmaz Ö, Kerkar N, Hørby Jørgensen M, Fischer R, Jimenez-Rivera C, Alam S, Cananzi M, Laverdure N, Targa Ferreira C, Ordonez F, Wang H, Sency V, Mo Kim K, Chen HL, Carvalho E, Fabre A, Quintero Bernabeu J, Alonso EM, Sokol RJ, Suchy FJ, Loomes KM, McKiernan PJ, Rosenthal P, Turmelle Y, Rao GS, Horslen S, Kamath BM, Rogalidou M, Karnsakul WW, Hansen B, Verkade HJ; Natural Course and Prognosis of PFIC and Effect of Biliary Diversion Consortium. Impact of Genotype, Serum Bile Acids, and Surgical Biliary Diversion on Native Liver Survival in FIC1 Deficiency. Hepatology. 2021;74:892-906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 2. | Bull LN, van Eijk MJ, Pawlikowska L, DeYoung JA, Juijn JA, Liao M, Klomp LW, Lomri N, Berger R, Scharschmidt BF, Knisely AS, Houwen RH, Freimer NB. A gene encoding a P-type ATPase mutated in two forms of hereditary cholestasis. Nat Genet. 1998;18:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 505] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 3. | Elferink RO, Groen AK. Genetic defects in hepatobiliary transport. Biochim Biophys Acta. 2002;1586:129-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Andersen JP, Vestergaard AL, Mikkelsen SA, Mogensen LS, Chalat M, Molday RS. P4-ATPases as Phospholipid Flippases-Structure, Function, and Enigmas. Front Physiol. 2016;7:275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 237] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 5. | Nicastro E, Stephenne X, Smets F, Fusaro F, de Magnée C, Reding R, Sokal EM. Recovery of graft steatosis and protein-losing enteropathy after biliary diversion in a PFIC 1 liver transplanted child. Pediatr Transplant. 2012;16:E177-E182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Miyagawa-Hayashino A, Egawa H, Yorifuji T, Hasegawa M, Haga H, Tsuruyama T, Wen MC, Sumazaki R, Manabe T, Uemoto S. Allograft steatohepatitis in progressive familial intrahepatic cholestasis type 1 after living donor liver transplantation. Liver Transpl. 2009;15:610-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Egawa H, Yorifuji T, Sumazaki R, Kimura A, Hasegawa M, Tanaka K. Intractable diarrhea after liver transplantation for Byler's disease: successful treatment with bile adsorptive resin. Liver Transpl. 2002;8:714-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Alrabadi LS, Morotti RA, Valentino PL, Rodriguez-Davalos MI, Ekong UD, Emre SH. Biliary drainage as treatment for allograft steatosis following liver transplantation for PFIC-1 disease: A single-center experience. Pediatr Transplant. 2018;22:e13184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Mali VP, Fukuda A, Shigeta T, Uchida H, Hirata Y, Rahayatri TH, Kanazawa H, Sasaki K, de Ville de Goyet J, Kasahara M. Total internal biliary diversion during liver transplantation for type 1 progressive familial intrahepatic cholestasis: a novel approach. Pediatr Transplant. 2016;20:981-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Shanmugam N, Menon J, Vij M, Rammohan A, Rajalingam R, Rela M. Total Internal Biliary Diversion for Post-Liver Transplant PFIC-1-Related Allograft Injury. J Clin Exp Hepatol. 2022;12:212-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Kavallar AM, Messner F, Scheidl S, Oberhuber R, Schneeberger S, Aldrian D, Berchtold V, Sanal M, Entenmann A, Straub S, Gasser A, Janecke AR, Müller T, Vogel GF. Internal Ileal Diversion as Treatment for Progressive Familial Intrahepatic Cholestasis Type 1-Associated Graft Inflammation and Steatosis after Liver Transplantation. Children (Basel). 2022;9:1964. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |