Published online Feb 27, 2025. doi: 10.4240/wjgs.v17.i2.100728
Revised: October 8, 2024
Accepted: November 11, 2024
Published online: February 27, 2025
Processing time: 150 Days and 19.7 Hours
Gallbladder cancer (GBC) is known for its poor prognosis and challenging management. The preoperative fibrinogen to albumin ratio (FAR) has been proposed as a potential prognostic marker for predicting postoperative outcomes in GBC patients, but its efficacy and prognostic value remain underexplored.
To evaluate the prognostic value of preoperative FAR in GBC outcomes.
This retrospective cohort study included 66 patients who underwent curative surgery for GBC at our institution from January 2018 to January 2022. Preope
The cohort consisted of 36 male and 30 female patients, with a mean age of 61.81 ± 8.58 years. The optimal FAR cut-off value was determined to be 0.088, with an area under the receiver operating characteristic curve of 0.7899, sensitivity of 68.96%, and specificity of 80.01%. Patients with FAR ≤ 0.088 showed significantly better survival rates (1-year: 60.5%, 2-year: 52.6%, 3-year: 25.9%) and a median OS of 25.6 months (95% confidence interval: 18.8-30.5 months), compared to those with FAR > 0.088 who had a median OS of 10.8 months (95% confidence interval: 6.3-12.9 months).
Lower preoperative FAR is associated with longer OS in GBC patients, confirming its potential as a valuable prognostic indicator for improving outcome predictions and guiding patient management strategies in gallbladder cancer.
Core Tip: Our study explores the efficacy of the preoperative fibrinogen to albumin ratio (FAR) as a prognostic marker in gallbladder cancer (GBC), aiming to provide valuable insights into its potential utility in clinical settings. In this retro
- Citation: Wang PF, Duan YJ. Prognostic value of preoperative fibrinogen to albumin ratio in predicting postoperative outcomes in patients with gallbladder cancer. World J Gastrointest Surg 2025; 17(2): 100728
- URL: https://www.wjgnet.com/1948-9366/full/v17/i2/100728.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i2.100728
Gallbladder cancer (GBC) is acknowledged as one of the most aggressive neoplasms of the biliary tract, distinguished by its late onset and unfavorable prognosis. Notwithstanding progress in diagnostic methods and surgical procedures, the overall survival (OS) statistics for GBC remain bleak, chiefly because to late-stage detection and elevated recurrence rates post-surgery[1-3]. For patients with biliary tract cancers (BTC), including GBC, treated with gemcitabine and cisplatin (GEMCIS), the median OS and progression-free survival are reported to be 11.7 months and 8.0 months, respectively, highlighting the poor outcomes associated with standard treatment regimens[4]. Consequently, developing dependable prognostic markers that can forecast surgical outcomes is essential for enhancing treatment options and patient management.
The preoperative fibrinogen to albumin ratio (FAR) has emerged as a promising predictive biomarker in several malignancies. Fibrinogen, a soluble plasma glycoprotein, is crucial in tumor progression and metastasis by facilitating tumor cell proliferation, angiogenesis, and inflammation. Increased fibrinogen levels are frequently linked to a hypercoagulable condition and heightened cancer-related thrombosis, which can negatively impact cancer prognosis[5,6]. Conversely, albumin levels negatively correlate with systemic inflammation and nutritional status, where diminished levels signify poor systemic health and an increased risk of negative outcomes. The FAR, by integrating these two characteristics, may provide a more thorough understanding of patients’ inflammatory and nutritional status, potentially acting as a more dependable predictor of surgical outcomes than each parameter individually. In oncology, specifically regarding GBC, comprehending the ramifications of FAR could markedly improve preoperative risk assessment and inform clinical decision-making[7,8]. Patients exhibiting a high preoperative FAR may have an elevated risk of complications, recurrence, or decreased survival, hence requiring more aggressive or customized therapy strategies.
The utility of FAR beyond prediction and may impact therapeutic paradigms. A high FAR may indicate the necessity for intensified monitoring and assertive care, including the evaluation of neoadjuvant medicines that could reduce tumor stage, improve operability, and potentially boost outcomes. In contrast, a reduced FAR may pinpoint patients who could gain from conventional surgical methods with diminished risk of complications[9,10]. This paper examines the predictive significance of the preoperative FAR in forecasting postoperative outcomes in GBC patients. This work emphasizes a unique biomarker, contributing to the enhancement of predictive tools in GBC while highlighting the significance of integrative biomarkers that encompass both inflammatory and nutritional aspects of cancer patients.
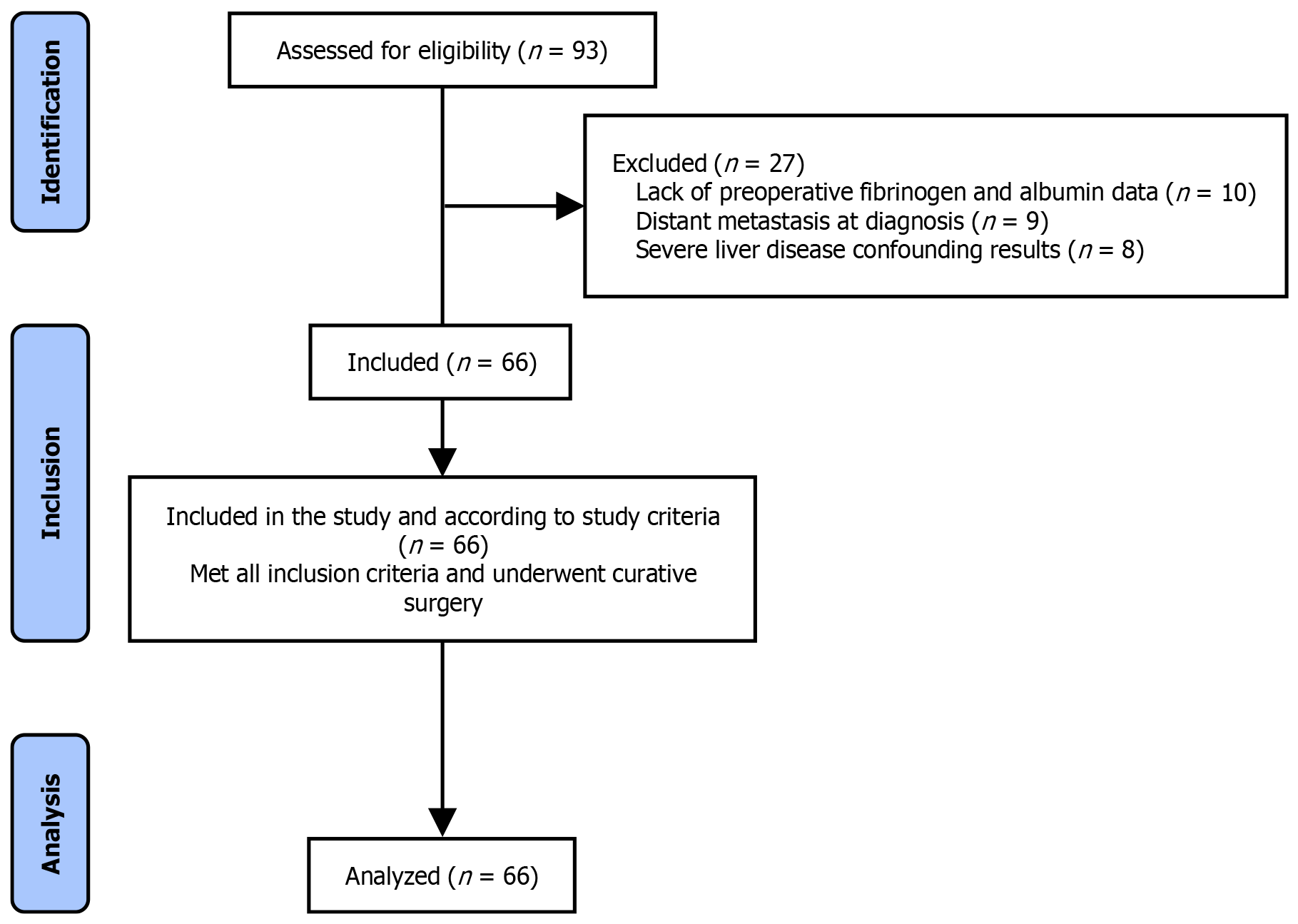
The purpose of our research was to perform a thorough retrospective analysis to evaluate the prognostic significance of the preoperative FAR in forecasting postoperative outcomes in GBC patients. This investigation was structured as a retrospective cohort analysis and conducted at our institution. The research duration extended from January 2018 to January 2022. In this period, we incorporated a cohort of 66 patients who received curative surgery for GBC at our institution (Figure 1). The patients were chosen based on their preoperative FAR values measured within one week before their surgical interventions. Before commencing the study, the research protocol underwent thorough evaluation and received approval from the hospital’s Ethics Committee to guarantee adherence to ethical norms and patient confidentiality.
Inclusion criteria: (1) Diagnosis: Patients diagnosed with GBC based on histopathological examination; (2) Surgical treatment: Patients who underwent curative-intent surgery for gallbladders cancer at our institution; (3) Preoperative laboratory tests: Availability of preoperative fibrinogen and albumin levels measured within one week prior to surgical intervention; and (4) Consent: Patients who provided written informed consent for the use of their medical records for research purposes. Exclusion criteria: (1) Metastatic disease: Patients with distant metastases at the time of diagnosis; (2) Prior malignancies: Patients with a history of other malignancies within the last 5 years, except for non-melanoma skin cancers; (3) Additional major surgery: Patients who underwent major surgical procedures within 6 months prior to the GBC surgery; and (4) Coagulopathy: Patients with known bleeding disorders or those on anticoagulant therapy which might affect fibrinogen or albumin levels. Liver disease: Patients with severe liver disease such as cirrhosis or active hepatitis, which could confound the assessment of albumin levels.
The research entailed gathering a detailed array of observational information to assess the predictive significance of the preoperative FAR in patients with GBC. The factors encompassed demographic information such as gender and age; clinical attributes such as the presence of jaundice and gallstones; and essential laboratory indicators including fibrinogen, albumin, and total bilirubin levels. Furthermore, tumor features were documented, including tumor size, pathological kind, invasion depth, differentiation grade, liver invasion, and lymph node metastasis. Preoperative blood concentrations of carbohydrate antigen 19-9 and carcinoembryonic antigen were evaluated to determine their association with clinical outcomes.
To evaluate the postoperative outcomes of patients with GBC, follow-up assessments were performed by outpatient clinic visits or telephone interviews. The principal outcome of this trial was OS, defined as the duration from the date of surgery to the date of death attributable to the tumor or to the last recorded follow-up. Survival duration was quantified in months to provide a consistent assessment of patient prognosis. This follow-up method facilitated thorough monitoring of patients’ health over time, enabling precise assessment of survival results after surgery.
Statistical analyses were performed utilizing SPSS software (version 27.0) with strict compliance to established methods. For normally distributed quantitative variables, means ± SDs were computed, and group comparisons were conducted using independent sample t-tests. Quantitative data that were not normally distributed were expressed as medians and interquartile ranges (M[P25, P75]), with the Mann-Whitney U test employed for group comparisons. Receiver operating characteristic (ROC) curve analysis was utilized to ascertain the best cutoff value for the preoperative FAR. Survival curves were generated employing the Kaplan-Meier method, and the disparities between these curves were evaluated using the Log-rank test. All statistical tests were two-tailed, with a P-value of less than 0.05 being statistically significant.
In this retrospective cohort study, a total of 66 patients diagnosed with GBC were included. The demographic breakdown revealed 36 male and 30 female patients, with ages ranging from 43 to 79 years and an average age of 61.81 ± 8.58 years. Preoperative assessment of albumin levels showed an average concentration of 39.32 ± 3.83 g/L, while the mean preoperative fibrinogen concentration was 3.51 ± 0.92 g/L. Survival analysis indicated that the postoperative 1-year, 2-year, and 3-year survival rates were 40.91%, 30.88%, and 23.16%, respectively. The median OS for the cohort was 12.63 months, with a 95% confidence interval (CI) ranging from 9.89 to 14.98 months (Table 1).
| Parameter | Details |
| Total patients | 66 |
| Gender distribution | Male: 36; Female: 30 |
| Age range, year | 43-79 |
| Average age, year | 61.81 ± 8.58 |
| Preoperative albumin (g/L) | 39.32 ± 3.83 |
| Preoperative fibrinogen (g/L) | 3.51 ± 0.92 |
| 1-year survival rate | 40.91% |
| 2-year survival rate | 30.88% |
| 3-year survival rate | 23.16% |
| Median overall survival | 12.63 months (95%CI: 9.89-14.98 months) |
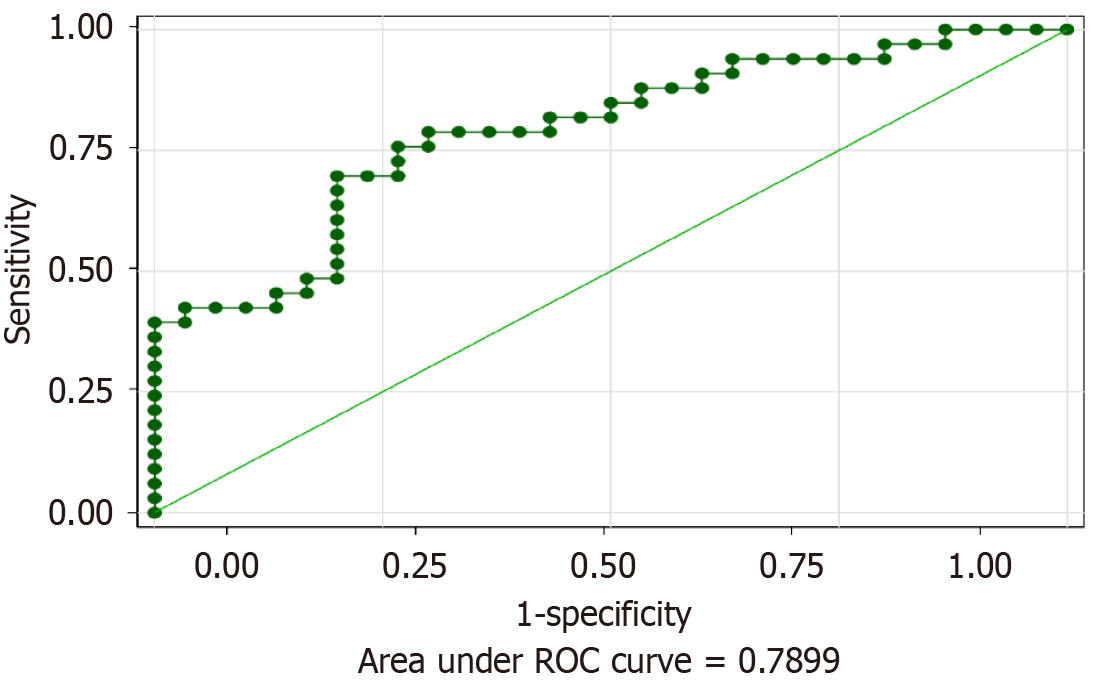
The determination of the optimal cut-off value for the preoperative FAR was a key component of our analysis aimed at predicting OS in GBC patients. ROC curve analysis was utilized to assess the predictive power of FAR with respect to postoperative outcomes. The area under the ROC curve was found to be 0.7899, indicating a good predictive ability of FAR in assessing the risk of adverse outcomes. The cut-off value for FAR was established at 0.088 based on the maximum Youden index, which balances the sensitivity and specificity of the test. At this threshold, the sensitivity was 68.96%, and the specificity was 80.01% (Figure 2). This FAR threshold of 0.088 serves as an optimal point for differentiating between higher and lower risk of poor survival outcomes in patients undergoing surgery for GBC.
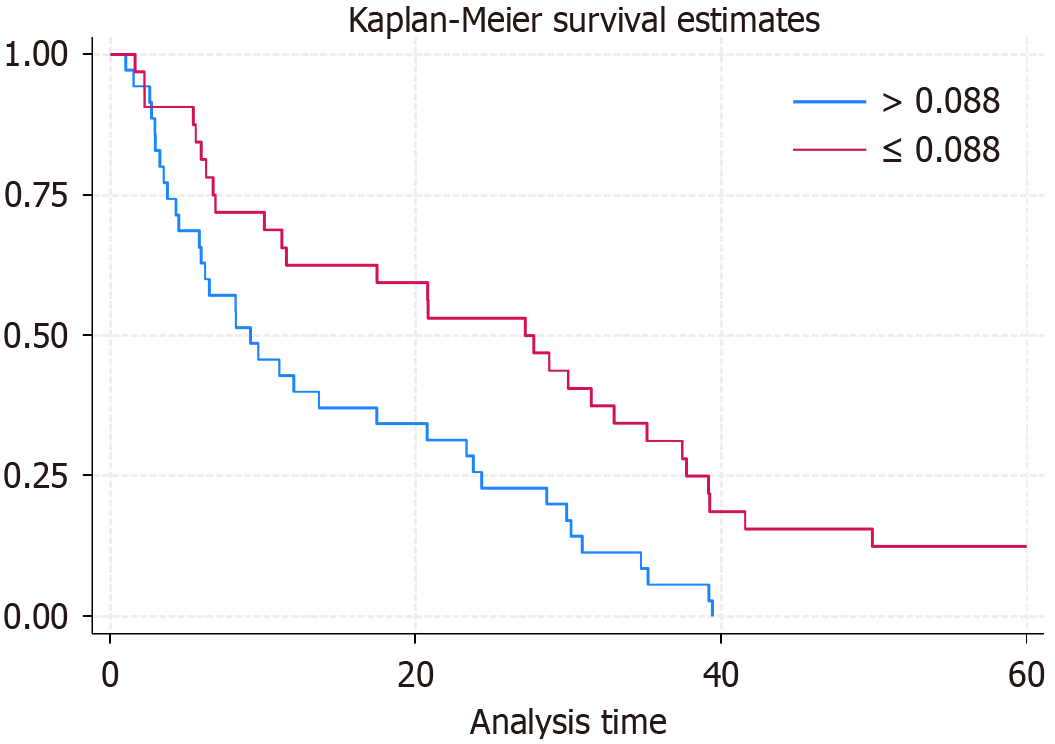
In this retrospective study, GBC patients were stratified into two groups based on the optimal cut-off value for the preoperative FAR determined by ROC curve analysis. Patients with a preoperative FAR ≤ 0.088 were categorized into the low-level group, while those with FAR > 0.088 were placed in the high-level group. The survival analysis revealed significant differences between the two groups. Patients in the low FAR group demonstrated superior OS compared to those in the high FAR group. The 1-year, 2-year, and 3-year survival rates for the low FAR group were 60.5%, 52.6%, and 25.9%, respectively, with a median survival time of 25.6 months (95%CI: 18.8-0.5 months). Conversely, the high FAR group exhibited lower survival rates of 45.7% at 1 year, 23.6% at 2 years, and 8.9% at 3 years, with a median survival time of only 10.8 months (95%CI: 6.3-12.9 months) (Figure 3). The comparison of postoperative OS between the two groups showed statistically significant differences, with a P-value of 0.001. This outcome indicates that preoperative FAR is a potent prognostic indicator for survival in patients undergoing surgery for GBC.
GBC is an extremely aggressive cancer marked by late detection and few therapeutic alternatives, resulting in low OS rates. Due to the aggressive characteristics of GBC, there is an essential requirement for dependable prognostic indicators that can forecast postoperative results and inform treatment choices[11-13]. The preoperative FAR has lately garnered interest as a potential predictive marker in oncology, illustrating the interaction among inflammation, coagulation, and nutritional status-crucial elements that affect cancer growth and patient outcomes. Fibrinogen, a coagulation factor, is increased in response to systemic inflammation frequently related to malignancies and is associated with tumor cell proliferation, migration, and metastasis. Elevated fibrinogen levels may foster a pro-thrombotic environment that facilitates tumor dissemination[14,15]. In contrast, albumin levels diminish due to systemic inflammation and malnutrition, symptoms commonly seen in cancer patients, especially those with advanced disease. Reduced albumin levels typically signify a bad prognosis in cancer, since they correlate with the dysfunction of physiological processes essential for patient recovery and resilience to intensive therapies.
This study’s findings highlight the preoperative FAR as a crucial prognostic indicator for evaluating postoperative outcomes in patients with GBC. The FAR threshold of 0.088, established using ROC curve analysis, efficiently distinguishes patients with varying risks for adverse survival outcomes, as demonstrated by significant differences in survival rates and median survival times between high and low FAR groups. Fibrinogen, a soluble plasma glycoprotein essential for clot formation, has been associated with cancer progression due to its involvement in tumor cell proliferation, migration, and metastasis[16,17]. Increased fibrinogen levels may indicate the hypercoagulable condition frequently observed in cancer patients, which can facilitate tumor development and metastasis by offering a substrate for tumor cell adherence and shielding circulating tumor cells from immune detection. Moreover, fibrinogen can stimulate angiogenesis, supplying essential nutrients and oxygen to the tumor microenvironment, hence promoting tumor growth and proliferation.
Conversely, albumin, an indicator of nutritional status and systemic inflammation, exhibits an inverse correlation with cancer severity. Reduced albumin levels may signify a systemic inflammatory response to the tumor, which is recognized to facilitate tumor growth and worse outcomes in other malignancies, including GBC. Albumin can influence the inflammatory response and maintain cellular proliferation and differentiation; hence, diminished levels may signify an aggressive tumor phenotype and an impaired host response. The predictive value of FAR in our study is substantiated by its capacity to integrate two essential dimensions-coagulation and nutrition/inflammation-into a unified composite biomarker[18,19]. This dual-function biomarker provides a more comprehensive understanding of the tumor microenvironment and the patient’s overall health than each parameter individually. The categorization of patients according to FAR values offers a clear approach for identifying those at elevated risk of unfavorable outcomes, hence facilitating more customized therapeutic interventions.
The current study’s findings regarding the prognostic significance of preoperative FAR provide important insights into survival outcomes for GBC patients[4]. When compared to previously published median OS for BTC, including GBC, treated with GEMCIS, our results demonstrated a distinct stratification based on FAR levels. For instance, the median OS of BTC patients treated with GEMCIS was 11.7 months, as reported in prior studies, which aligns closely with the median OS of 12.63 months observed in our cohort as a whole. However, the stratified analysis based on FAR levels revealed notable differences: Patients with FAR ≤ 0.088 had a markedly improved median OS of 25.6 months, compared to 10.8 months in the high FAR group[20]. These findings suggest that preoperative FAR can effectively differentiate patients with a better or worse prognosis, thus offering a potential means for refining risk stratification and personalized treatment planning in GBC[21]. Furthermore, the results from Shroff et al[22] involving the addition of nab-paclitaxel to GEMCIS showed a median OS improvement to 19.2 months. This outcome is similar to the median OS observed in our low FAR group, underscoring that preoperative FAR might serve as a useful biomarker in identifying patients likely to benefit from more intensive treatment regimens. Conversely, our high FAR group’s OS of 10.8 months indicates a worse prognosis, which is comparable to untreated BTC patients and emphasizes the need for novel treatment strategies in such cases. Thus, our study contributes to the literature by suggesting that preoperative FAR may help identify patients who could potentially achieve similar survival benefits as those reported with combination regimens such as GEMCIS with nab-paclitaxel and provides a useful tool for clinical decision-making regarding the selection of patients for aggressive treatment approaches.
The findings from this study suggest several clinical applications. Firstly, FAR can be used to guide decision-making regarding the aggressiveness of the surgical approach and the need for adjuvant therapies. For example, patients in the high FAR group might benefit from more aggressive surgical techniques or the addition of systemic therapies pre- or post-operation. Additionally, FAR could serve as a criterion for closer postoperative surveillance and follow-up in patients at higher risk of recurrence or poor survival[23,24]. For future research, it is crucial to explore the biological mechanisms that directly link FAR with survival outcomes in GBC patients. Understanding these pathways could lead to the development of targeted therapies that specifically address the processes modulated by fibrinogen and albumin. Moreover, prospective studies are needed to validate these retrospective findings and to assess the utility of FAR in a broader, more diverse patient population. One unresolved question is the underlying biological rationale for why FAR correlates with survival in GBC. Further research is needed to elucidate the mechanisms that mediate the observed relationship between FAR and survival outcomes. Additionally, it remains unclear whether this association holds true across different populations and clinical settings, which warrants investigation through multi-center studies involving diverse cohorts.
This study has several limitations that should be considered. Firstly, the retrospective design may introduce biases related to data collection and patient selection, limiting the ability to establish causation. Secondly, the sample size is relatively small, which may affect the generalizability of the findings to a broader population. Additionally, the study was conducted at a single institution, which may not reflect the variability in clinical practices and patient populations seen in different geographic regions. Furthermore, due to the retrospective nature, detailed information on tumor stage, comorbidities, systemic treatment (neoadjuvant/adjuvant), and progression-related treatments was not comprehensively documented, limiting our ability to fully assess their impact. Lastly, potential confounders such as the stage of cancer, comorbidities, and differences in treatment protocols were not fully controlled, which could influence the outcomes related to the prognostic value of FAR.
Future research should aim to address the limitations highlighted in this study. Prospective, multicenter trials involving larger and more diverse patient populations are essential to validate the prognostic value of FAR and improve the external validity of our findings. Additionally, exploring the underlying biological mechanisms that link FAR to GBC prognosis could yield insights into the processes modulated by fibrinogen and albumin, potentially leading to novel targeted therapies. Investigating the use of FAR in combination with other established biomarkers, such as carbohydrate antigen 19-9 or inflammatory markers like the neutrophil-to-lymphocyte ratio, may also enhance risk stratification and prognostic assessment in GBC. These future directions will contribute to a more comprehensive understanding of FAR and its role in guiding personalized treatment and improving patient outcomes. It should also investigate how FAR can be integrated into clinical decision-making algorithms to stratify risk and tailor therapeutic interventions for GBC patients. Defining specific FAR thresholds that may prompt different treatment strategies, such as intensified surveillance or early adjuvant therapy, could further enhance personalized care in GBC.
This study suggests that GBC patients with a lower preoperative FAR could exhibit longer postoperative OS compared to those with higher FAR values. Consequently, preoperative FAR might serve as a valuable prognostic indicator, offering potential implications for patient management and outcome prediction in GBC.
We appreciate the support provided by our laboratory’s entire clinical research personnel.
| 1. | Roa JC, García P, Kapoor VK, Maithel SK, Javle M, Koshiol J. Gallbladder cancer. Nat Rev Dis Primers. 2022;8:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 178] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 2. | Hickman L, Contreras C. Gallbladder Cancer: Diagnosis, Surgical Management, and Adjuvant Therapies. Surg Clin North Am. 2019;99:337-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 3. | Sun Y, Gong J, Li Z, Han L, Sun D. Gallbladder cancer: surgical treatment, immunotherapy, and targeted therapy. Postgrad Med. 2024;136:278-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 4. | You MS, Ryu JK, Choi YH, Choi JH, Huh G, Paik WH, Lee SH, Kim YT. Therapeutic outcomes and prognostic factors in unresectable gallbladder cancer treated with gemcitabine plus cisplatin. BMC Cancer. 2019;19:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Xu WY, Zhang HH, Yang XB, Bai Y, Lin JZ, Long JY, Xiong JP, Zhang JW, Sang XT, Zhao HT. Prognostic significance of combined preoperative fibrinogen and CA199 in gallbladder cancer patients. World J Gastroenterol. 2018;24:1451-1463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Cao P, Jiang L, Zhou LY, Chen YL. The clinical significance of preoperative serum fibrinogen levels and platelet counts in patients with gallbladder carcinoma. BMC Gastroenterol. 2021;21:366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Yang Z, Ren T, Liu S, Cai C, Gong W, Shu Y. Preoperative serum fibrinogen as a valuable predictor in the nomogram predicting overall survival of postoperative patients with gallbladder cancer. J Gastrointest Oncol. 2021;12:1661-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Shu YJ, Weng H, Bao RF, Wu XS, Ding Q, Cao Y, Wang XA, Zhang F, Xiang SS, Li HF, Li ML, Mu JS, Wu WG, Liu YB. Clinical and prognostic significance of preoperative plasma hyperfibrinogenemia in gallbladder cancer patients following surgical resection: a retrospective and in vitro study. BMC Cancer. 2014;14:566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Huang L, Deng X, Fan RZ, Hao TT, Zhang S, Sun B, Xu YH, Li SB, Feng YF. Coagulation and fibrinolytic markers offer utility when distinguishing between benign and malignant gallbladder tumors: A cross-sectional study. Clin Chim Acta. 2024;560:119751. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Liu XF, Zhou LY, Wei ZH, Liu JX, Li A, Wang XZ, Ying HQ. The diagnostic role of circulating inflammation-based biomarker in gallbladder carcinoma. Biomark Med. 2018;12:1095-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Clark KR. Gallbladder Cancer: Risk Factors, Diagnosis, and Treatment. Radiol Technol. 2021;92:305-309. [PubMed] |
| 12. | Cui X, Zhu S, Tao Z, Deng X, Wang Y, Gao Y, Liao Y, Ma W, Zhang Y, Ma X. Long-term outcomes and prognostic markers in gallbladder cancer. Medicine (Baltimore). 2018;97:e11396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Lee EC, Park SJ, Lee SD, Han SS, Kim SH. Effects of Sarcopenia on Prognosis After Resection of Gallbladder Cancer. J Gastrointest Surg. 2020;24:1082-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Sun L, Ke X, Wang D, Yin H, Jin B, Xu H, Du S, Xu Y, Zhao H, Lu X, Sang X, Zhong S, Yang H, Mao Y. Prognostic Value of the Albumin-to-γ-glutamyltransferase Ratio for Gallbladder Cancer Patients and Establishing a Nomogram for Overall Survival. J Cancer. 2021;12:4172-4182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Bao Y, Yang J, Duan Y, Chen Y, Chen W, Sun D. The C-reactive protein to albumin ratio is an excellent prognostic predictor for gallbladder cancer. Biosci Trends. 2021;14:428-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Koshiol J, Castro F, Kemp TJ, Gao YT, Roa JC, Wang B, Nogueira L, Araya JC, Shen MC, Rashid A, Hsing AW, Hildesheim A, Ferreccio C, Pfeiffer RM, Pinto LA. Association of inflammatory and other immune markers with gallbladder cancer: Results from two independent case-control studies. Cytokine. 2016;83:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Utsumi M, Aoki H, Nagahisa S, Nishimura S, Une Y, Kimura Y, Watanabe M, Taniguchi F, Arata T, Katsuda K, Tanakaya K. Preoperative C-Reactive Protein/Albumin Ratio as a Predictive Factor for Gallbladder Carcinoma. In Vivo. 2020;34:1901-1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Saqib R, Pathak S, Smart N, Nunes Q, Rees J, Finch Jones M, Poston G. Prognostic significance of pre-operative inflammatory markers in resected gallbladder cancer: a systematic review. ANZ J Surg. 2018;88:554-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Mady M, Prasai K, Tella SH, Yadav S, Hallemeier CL, Rakshit S, Roberts L, Borad M, Mahipal A. Neutrophil to lymphocyte ratio as a prognostic marker in metastatic gallbladder cancer. HPB (Oxford). 2020;22:1490-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Zhou Y, Yuan K, Yang Y, Ji Z, Zhou D, Ouyang J, Wang Z, Wang F, Liu C, Li Q, Zhang Q, Li Q, Shan X, Zhou J. Gallbladder cancer: current and future treatment options. Front Pharmacol. 2023;14:1183619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 21. | Sturm N, Schuhbaur JS, Hüttner F, Perkhofer L, Ettrich TJ. Gallbladder Cancer: Current Multimodality Treatment Concepts and Future Directions. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 22. | Shroff RT, Javle MM, Xiao L, Kaseb AO, Varadhachary GR, Wolff RA, Raghav KPS, Iwasaki M, Masci P, Ramanathan RK, Ahn DH, Bekaii-Saab TS, Borad MJ. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol. 2019;5:824-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 343] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 23. | Rai A, Tewari M, Mohapatra SC, Shukla HS. Correlation of nutritional parameters of gallbladder cancer patients. J Surg Oncol. 2006;93:705-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Medina-Franco H, Ramos-Gallardo G, Orozco-Zepeda H, Mercado-Díaz MA. [Prognostic factor in gallbladder cancer]. Rev Invest Clin. 2005;57:662-665. [PubMed] |