Published online Jan 27, 2025. doi: 10.4240/wjgs.v17.i1.99752
Revised: September 23, 2024
Accepted: October 28, 2024
Published online: January 27, 2025
Processing time: 151 Days and 0.7 Hours
Cirrhotic patients with super-giant hepatocellular carcinoma (HCC) and portal vein invasion generally have a poor prognosis. This paper presents a patient with super-giant HCC and portal vein invasion, who underwent hepatectomy followed by a combination of sorafenib and camrelizumab, resulting in complete remission (CR) for 5 years.
A 40-year-old male with compensated hepatitis B-related cirrhosis was diagnosed with HCC, Barcelona Clinic Liver Cancer stage C. Enhanced computed tomogra
The combination of hepatectomy with sorafenib and camrelizumab can achieve durable CR in patients with super-giant HCC and portal vein invasion. Further research is necessary to address these challenges and improve patient outcomes.
Core Tip: Cirrhotic patients with super-giant hepatocellular carcinoma (HCC), accompanied by portal vein invasion, generally have a poor prognosis. We report a patient with super-giant HCC and portal vein invasion, who underwent hepatectomy followed by sorafenib plus camrelizumab, resulting in complete remission for 5 years. However, two critical issues are raised: One is how to screen and manage portal hypertension in HCC, and the other is related to the optimal treatment duration of tyrosine kinase inhibitors and immune checkpoint inhibitors in HCC patients who achieve complete remission. Further research is needed to address these challenges.
- Citation: Zheng XQ, Sun LB, Jin WJ, Liu H, Song WY, Xu H, Wu JS, Wang XJ, Gou CY, Ding HG. Five-year complete remission of super-giant hepatocellular carcinoma with hepatectomy followed by sorafenib plus camrelizumab: A case report. World J Gastrointest Surg 2025; 17(1): 99752
- URL: https://www.wjgnet.com/1948-9366/full/v17/i1/99752.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i1.99752
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer, originating from hepatocytes and intrahepatic cholangiocytes. It is the fourth most common malignancy and the second leading cause of cancer-related death in China, with hepatitis B virus (HBV) infection being a major causative factor[1]. According to the guidelines, patients with super-giant HCC, portal vein invasion, and extrahepatic spread are classified as having advanced stage (Barcelona Clinic Liver Cancer stage C)[2]. Despite treatment with liver resection and transcatheter arterial chemoembolization (TACE), the long-term prognosis for these patients remains extremely poor[3,4].
The advent of multi-targeted tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs) has provided new hope for these patients. Sorafenib, a multi-targeted kinase inhibitor, has been extensively validated for the treatment of advanced HCC[5]. Camrelizumab is a humanized monoclonal antibody that targets programmed cell death receptor 1 (PD-1), blocking its interaction with ligands PD-L1 and PD-L2, and restoring T-cell anti-tumor activity. Although many studies have demonstrated the efficacy and safety of sorafenib and camrelizumab in patients with advanced HCC[6,7], long-term complete remission (CR) remains rare. Here, we present the unique case of a patient with super-giant HCC and portal vein invasion. The patient underwent hepatectomy, followed by combination therapy of sorafenib and camrelizumab, achieving a sustained CR over a 5-year follow-up period.
A 40-year-old male presented to the hospital with the complaint of abdominal pain for one day.
One day ago, the patient developed persistent pain in the right upper abdomen. A subsequent abdominal computed tomography (CT) scan revealed a hepatic space-occupying lesion. Consequently, hospital admission was deemed necessary for further assessment and treatment.
The patient had no significant medical history.
The patient had a family history of HBV infection.
Physical examination showed tenderness in the right upper abdomen.
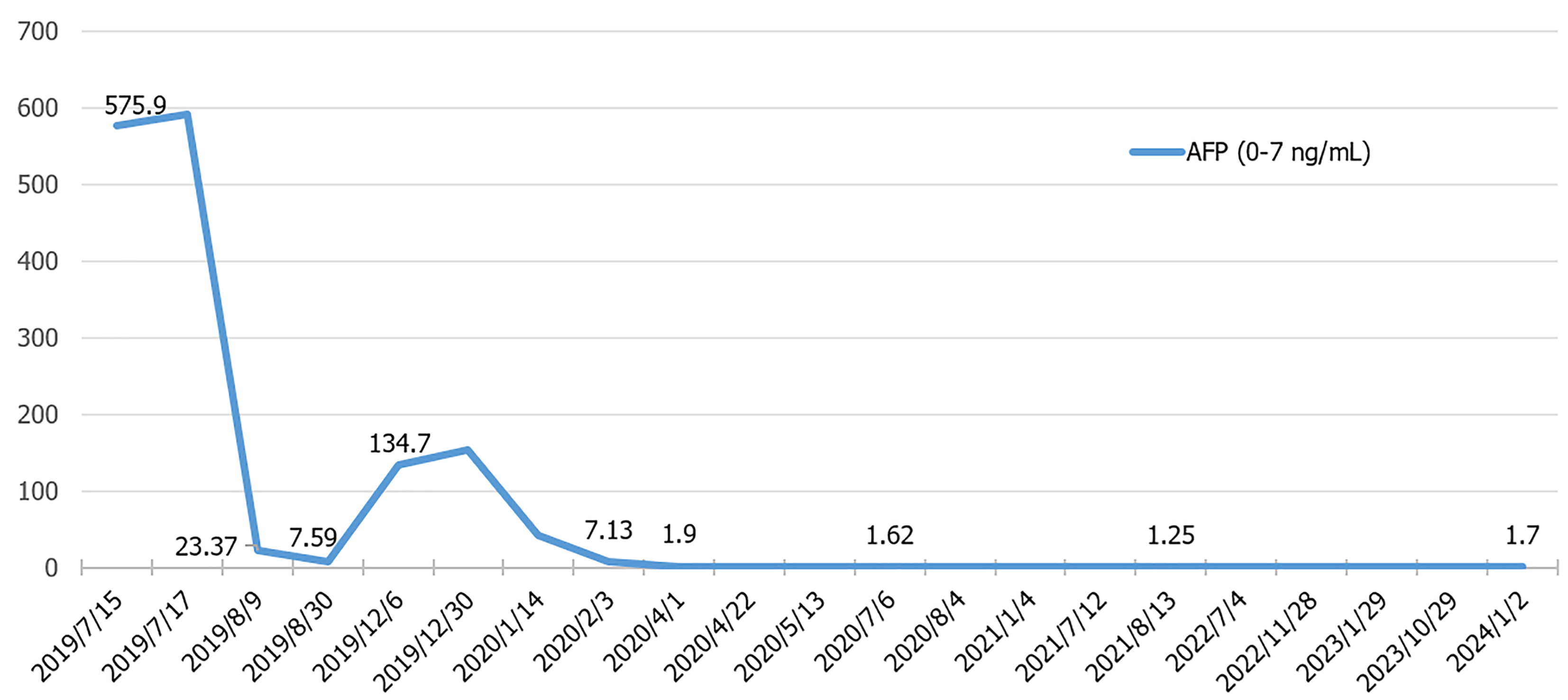
Laboratory tests indicated positive hepatitis B surface antigen, elevated HBV DNA (5.28 × 103 IU/mL), elevated α-fetoprotein (AFP) (575.9 ng/mL), and elevated AFP-L3 (206.8 ng/mL), with normal blood cell counts, liver and renal function, and coagulation tests (Table 1). The indocyanine green retention rate at 15 minutes was 7.2%.
| Variables | Baseline | Endpoint | |
| CBC test | WBC (× 109/L) | 7.51 | 3.19 |
| RBC (× 109/L) | 5.17 | 4.83 | |
| HGB (g/L) | 155 | 135 | |
| PLT (× 109/L) | 314 | 96 | |
| Coagulation test | PT (s) | 12.2 | 11.6 |
| INR | 1.09 | 1.03 | |
| Biochemistry test | ALT (U/L) | 25 | 28 |
| TBIL (μmol/L) | 23.6 | 17.4 | |
| ALP (U/L) | 454 | 58 | |
| γ-GT (U/L) | 77 | 26 | |
| ALB (g/L) | 38.2 | 40.9 | |
| CREA (μmol/L) | 65 | 57 | |
| Tumor markers | AFP (ng/mL) | 561.9 | 1.99 |
| AFP-L3 (ng/mL) | 206.8 | Less than 0.908 | |
| Serology test | HBV-DNA (IU/mL) | 5280 | Less than 10 |
| HBsAg | Positive | Negative | |
| HBsAb | Negative | Negative | |
| HBeAg | Negative | Negative | |
| HBcAb | Positive | Positive | |
| HBeAb | Positive | Positive |
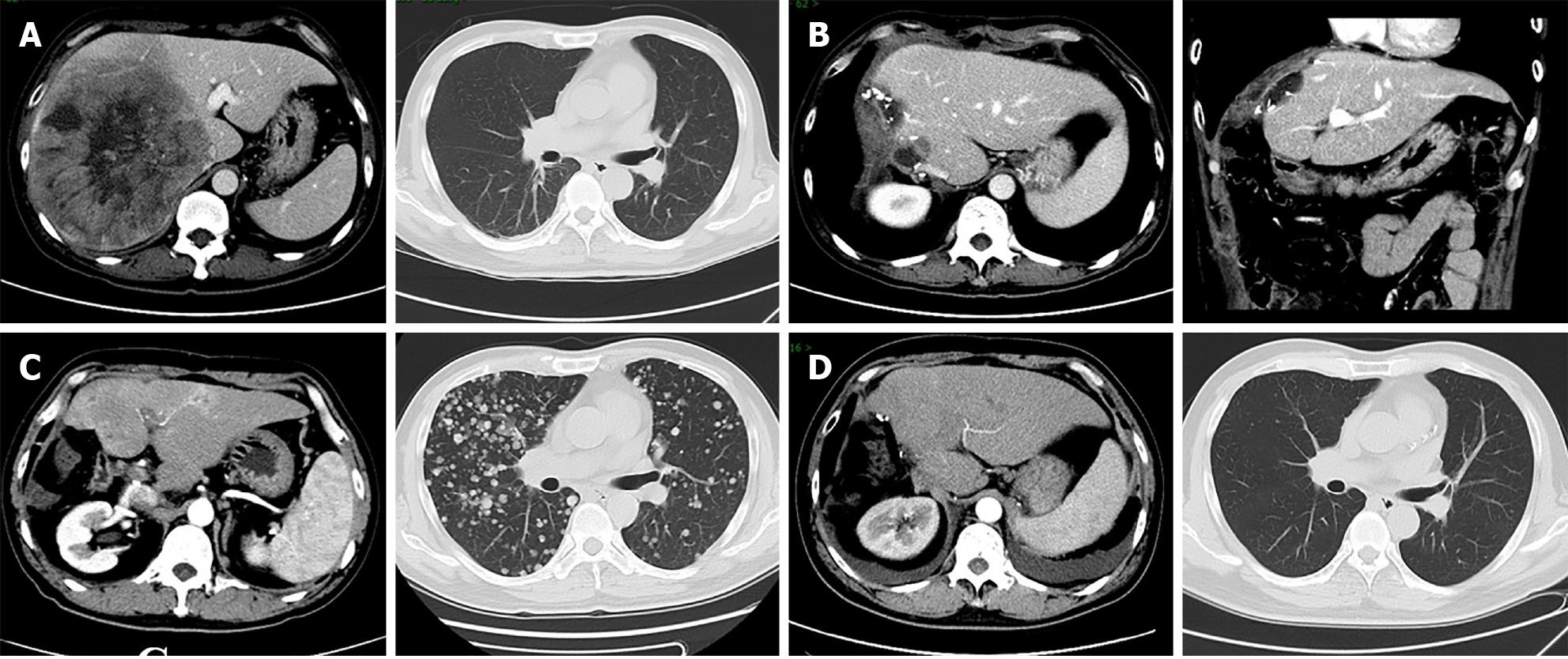
Liver stiffness measurement (LSM) was 7.9 kPa. Enhanced CT revealed a 152 mm × 171 mm liver mass in the right lobe with satellite nodules, invading the right posterior branch of the portal vein and the right hepatic vein. Thoracic CT showed no metastasis (Figure 1A).
The patient was diagnosed with HCC (Barcelona Clinic Liver Cancer stage C) and compensated hepatitis B-related cirrhosis (Child-Pugh class A, Eastern Cooperative Oncology Group Performance Status 0).
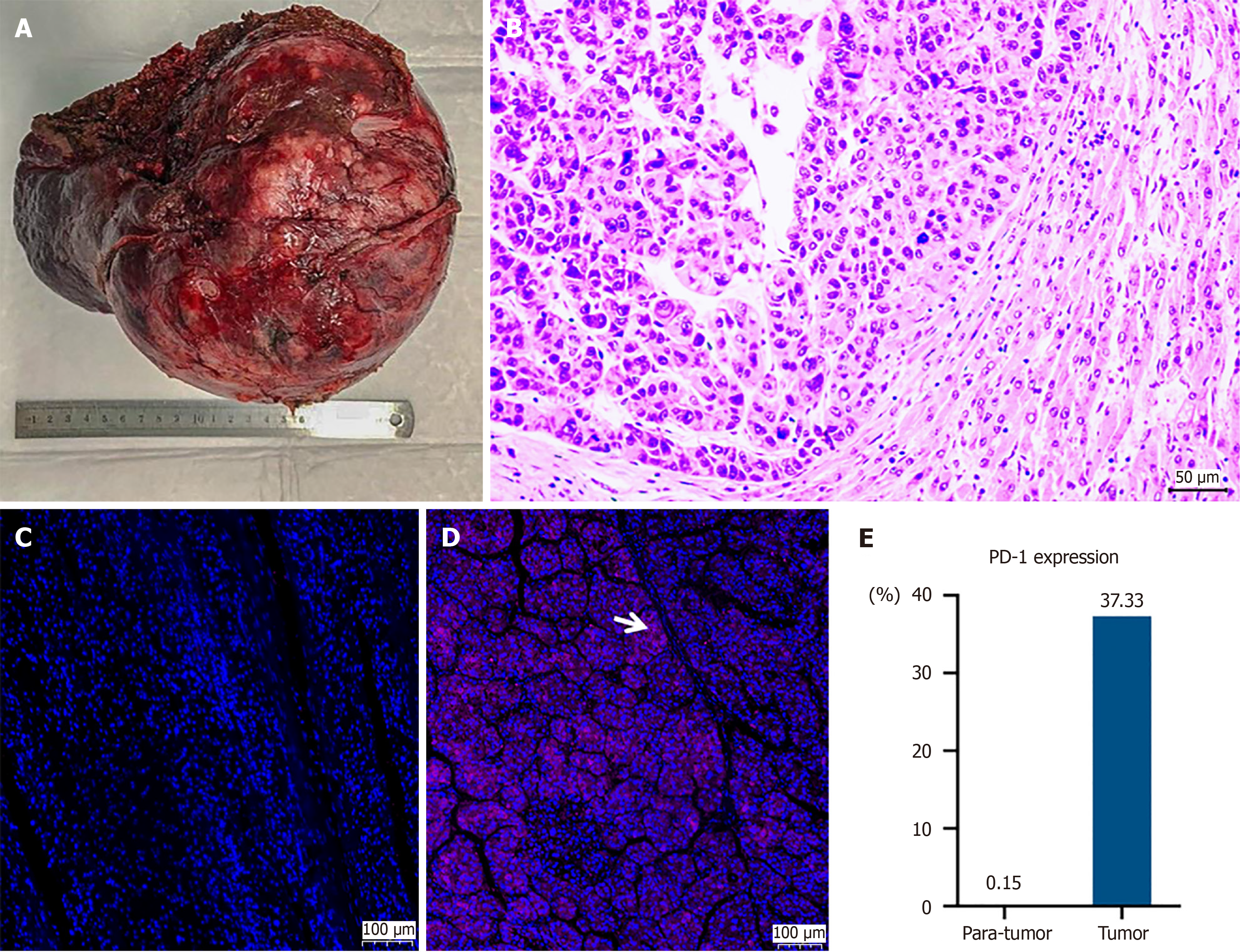
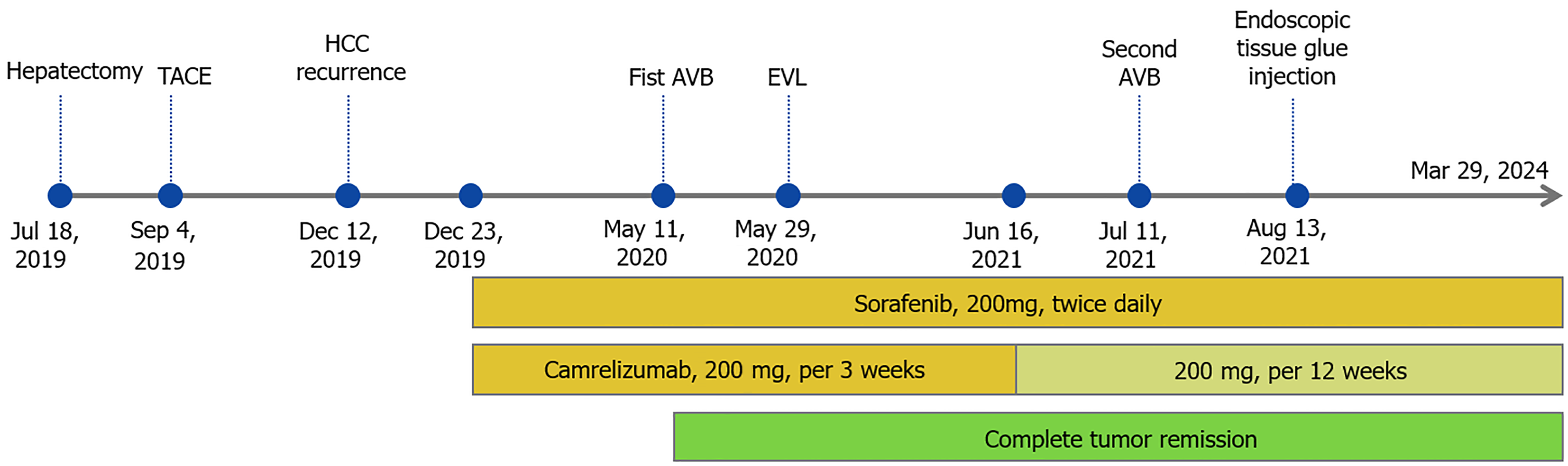
The patient was administered entecavir for HBV infection and underwent hepatectomy on July 18, 2019 (Figures 1B and 2A). Pathological examination revealed moderately to poorly differentiated HCC with tumor thrombosis in most vessels, including the portal and right hepatic veins (Figure 2B). Immunohistochemical staining showed higher PD-1 expression in the tumor compared to surrounding tissue (Figure 2C-E). On September 4, 2019, the patient underwent TACE to reduce recurrence risk, confirming no tumor remnants.
The patient recovered well after operation, and received regular follow-ups every 3 months. Serum AFP and enhanced CT scans showed no recurrence until December 2019. At that time, he reported respiratory distress and elevated serum AFP (134.7 ng/mL) and AFP-L3 (27.05 ng/mL). Enhanced CT scans identified multiple recurrent tumors in the liver and lungs (Figure 1C). The patient began treatment with sorafenib (200 mg twice daily) and camrelizumab (200 mg every 3 weeks) on December 23, 2019. Initially, he experienced mild desquamation of the feet and palms, nausea, and loss of appetite due to drug side effects but continued to improve throughout treatment. By February 12, 2020, enhanced CT scans showed significant remission with tumor markers returning to normal. CR was achieved on May 12, 2020 (Figures 1D and 3). From June 16, 2021, the dosage of camrelizumab was reduced to 200 mg every 12 weeks, with the final infusion administered on March 29, 2024.
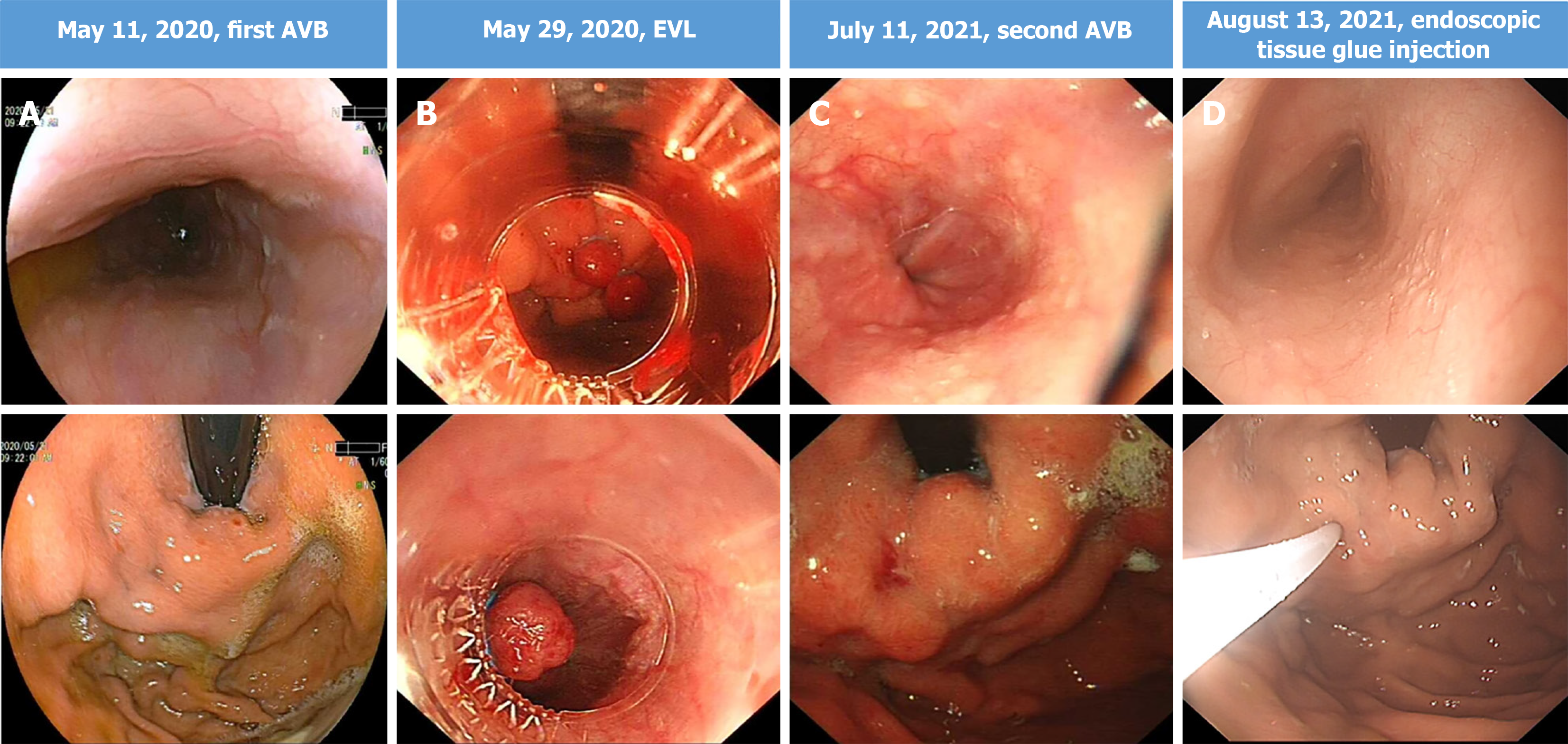
During the follow-up period, the patient experienced two episodes of gastrointestinal bleeding due to esophagogastric varices on May 11, 2020 and July 11, 2021, respectively, which was treated with endoscopic variceal ligation and endoscopic tissue glue injection on May 29, 2020 and August 13, 2021, respectively (Figure 4). During these periods, both sorafenib and camrelizumab were temporarily discontinued. Encouragingly, the patient has maintained durable CR since May 12, 2020, with regular CT scans and tumor marker tests conducted every 6 months. The complete treatment timeline is shown in Figure 5, and the dynamic change in AFP is shown in Figure 3.
The treatment of HCC has significantly advanced with the introduction of combination therapy using TKIs and ICIs[8]. Despite this progress, patients with super-giant HCC and portal vein invasion typically have a poor prognosis, and achieving CR is rare, even with combination regimens. This case report describes a patient with super-giant HCC and portal vein invasion who achieved CR and a 5-year recurrence-free survival following hepatectomy and subsequent sorafenib plus camrelizumab therapy.
Both TKIs and ICIs have dramatically improved the feasibility and effectiveness of hepatectomy for patients with super-giant HCC. This case demonstrates that in the era of TKIs plus ICIs, patients with super-giant HCC can achieve CR and long-term recurrence-free survival following hepatectomy. Generally, patients with HCC tumors larger than 10 cm have poorer prognoses compared to those with smaller tumors. Within 5 years after liver resection, the recurrence rate can be as high as 84.9%, and the overall survival (OS) rate as low as 34.0%[9]. Consequently, some patients forgo hepatectomy in favor of TACE to manage HCC progression. However, for patients with giant HCC undergoing TACE, the progression-free survival is only about 9.5 months, and OS ranges from 10.6 to 19.3 months[10]. Due to the large size of super-giant HCC, these patients are at high risk for various complications following TACE. Currently, TKIs plus ICIs is considered the first-line treatment for advanced HCC. However, as demonstrated in the COSMIC-312 study, not all combination regimens achieve OS benefits[11]. Therefore, exploring new combination treatment regimens that extend OS for super-giant HCC patients is essential. Sorafenib, a multikinase inhibitor, promotes apoptosis, reduces angiogenesis, and inhibits tumor progression. Prior to 2020, it was the only effective first-line treatment for HCC. Studies have shown that sorafenib may exert antitumor effects by normalizing tumor vasculature[12], altering macrophage polarization[13], and reducing the number of CD4+PD-1+ and CD8+PD-1+ T cells[14]. Camrelizumab, a high-affinity humanized PD-1 monoclonal antibody, can alleviate the immunosuppressive state of CD8+ T cells. These factors collectively support the idea that the combination of sorafenib and camrelizumab could benefit HCC patients by modulating antitumor immunity. Existing research has also confirmed the safety and efficacy of the combined use of sorafenib and camrelizumab in HCC patients. In a 2022 retrospective study, recurrence-free survival was significantly extended to 10.2 months in the group receiving sorafenib combined with camrelizumab, compared to 6.1 months in the sorafenib monotherapy group[7]. Despite its potential, identifying which patients will respond best to this combination remains a challenge. PD-1 inhibitors are theoretically more effective in patients with high PD-1 expression, making PD-1 levels in tumor tissues a potentially optimal biomarker for predicting treatment response[15]. In this case, we report a patient with super-giant HCC who achieved CR and long-term survival through surgical resection of the primary tumor, followed by sorafenib and camrelizumab for recurrent disease. Early in the treatment, immunohistochemistry revealed higher PD-1 expression in the tumor compared to surrounding healthy tissues, supporting the rationale for using camrelizumab. This underscores that hepatectomy can substantially reduce tumor burden, improving the sensitivity and effectiveness of subsequent sorafenib and camrelizumab combination for super-giant HCC patients. In addition, PD-1 expression levels in tumor tissues may serve as a predictive indicator for selecting patients who are likely to respond to camrelizumab therapy.
In the era of TKIs and ICIs, managing portal hypertension in HCC patients is crucial. This patient experienced bleeding twice due to mild gastroesophageal varices during systemic therapies, indicating that the Barveno VII criteria may not be appropriate for predicting esophagogastric varices or bleeding in HCC patients with compensated cirrhosis undergoing targeted therapy and immunotherapy. Based on Barveno VII criteria, patients with LSM ≤ 15 kPa and platelets (PLT) ≥ 150 × 109/L can be diagnosed without clinically significant portal hypertension and avoid endoscopy[16]. However, HCC can worsen portal hypertension and increase bleeding risk in cirrhotic patients, related to the development of arteriovenous shunts, structural liver changes, and portal vein tumor invasion[17]. There is growing concern about the potential for TKIs and ICIs to increase gastrointestinal bleeding in HCC patients. Vascular endothelial growth factor receptor inhibitors are believed to increase bleeding risk by reducing endothelial cell regenerative capacity, inhibiting angiogenesis, and decreasing collateral vessel proliferation[18-20]. This patient was not advised to undergo screening endoscopy initially because his LSM was ≤ 15 kPa and PLT were ≥ 150 × 109/L. However, he experienced esophagogastric variceal bleeding twice despite an excellent tumor response and stable liver function, with no further bleeding after endoscopic therapy. Therefore, our report suggests that the Barveno VII criteria are inadequate for screening portal hypertension in HCC patients treated with TKIs and ICIs. We recommend that HCC patients, even with LSM ≤ 15 kPa and PLT ≥ 150 × 109/L, undergo screening endoscopy before starting TKIs and ICIs. To ensure continuous and effective TKIs plus ICIs, regular gastroscopy intervals should be shortened during treatment, and necessary prophylactic treatment of esophagogastric varices should be actively pursued.
Determining the optimal duration and discontinuation strategy for TKIs plus ICIs remains challenging for clinicians. HCC patients typically discontinue TKIs and ICIs due to disease progression, drug resistance, or serious adverse events such as hand-foot skin reactions, diarrhea, liver dysfunction, and upper gastrointestinal hemorrhage[21]. With the advent of TKIs plus ICIs, CR is possible but rare in advanced HCC patients, with rates as low as 1.3% in the targeted therapies group and 2.7% in the ICIs group[22,23]. However, for patients who achieve CR, there are no guidelines on the optimal duration of TKIs plus ICIs[24]. Additionally, it remains uncertain whether HCC patients will still benefit from their original treatment regimen if the tumor recurs after discontinuation. Indefinite-duration treatment could reduce recurrence but might also lead to significant economic waste and the accumulation of drug toxicity. In this case, sorafenib plus camrelizumab has been administered continuously for over 4 years, except during periods of variceal bleeding, with favorable outcomes. The emerging issue for clinicians is determining when and how to safely discontinue the treatment of sorafenib plus camrelizumab, a topic worth discussing and investigating.
Overall, the combination of hepatectomy with sorafenib plus camrelizumab is a potentially effective treatment for super-giant HCC patients with portal vein invasion. This approach can significantly reduce tumor burden, enhance systemic therapy efficacy, and achieve CR. However, extensive use of TKIs plus ICIs combination therapy raises two critical issues: How to perform endoscopic screening and manage portal hypertension in HCC patients, and how to determine the optimal treatment duration of TKIs plus ICIs and safely discontinue the drugs in HCC patients who achieve CR. Further research is needed to address these challenges and improve patient outcomes.
| 1. | Zheng RS, Chen R, Han BF, Wang SM, Li L, Sun KX, Zeng HM, Wei WW, He J. [Cancer incidence and mortality in China, 2022]. Zhonghua Zhong Liu Za Zhi. 2024;46:221-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 145] [Reference Citation Analysis (0)] |
| 2. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2547] [Article Influence: 849.0] [Reference Citation Analysis (59)] |
| 3. | Cai X, Wu S. Transarterial chemoembolization versus surgical resection for giant hepatocellular carcinoma under the different status of capsule: a retrospective study. Transl Cancer Res. 2022;11:4359-4372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 4. | Wee JJ, Tee CL, Junnarkar SP, Low JK, Tan YP, Huey CW, Shelat VG. Outcomes of surgical resection of super-giant (≥15 cm) hepatocellular carcinoma: Volume does matter, if not the size. J Clin Transl Res. 2022;8:209-217. [PubMed] |
| 5. | Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J Hepatol. 2017;67:999-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 451] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 6. | Sun B, Chen L, Lei Y, Zhang L, Sun T, Liu Y, Zheng C. Sorafenib plus transcatheter arterial chemoembolization with or without camrelizumab for the treatment of intermediate and advanced hepatocellular carcinoma. Br J Radiol. 2024;97:1320-1327. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Liu Q, You N, Li J, Wu K, Peng X, Wang Z, Wang L, Zhu Y, Zheng L. Camrelizumab Plus Sorafenib Versus Sorafenib Monotherapy for Advanced Hepatocellular Carcinoma: A Retrospective Analysis. Front Oncol. 2021;11:694409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 8. | Solimando AG, Susca N, Argentiero A, Brunetti O, Leone P, De Re V, Fasano R, Krebs M, Petracci E, Azzali I, Nanni O, Silvestris N, Vacca A, Racanelli V. Second-line treatments for Advanced Hepatocellular Carcinoma: A Systematic Review and Bayesian Network Meta-analysis. Clin Exp Med. 2022;22:65-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 9. | Hwang S, Kim KH, Moon DB, Ahn CS, Ha TY, Song GW, Jung DH, Park GC. Prediction of Post-resection Prognosis Using the ADV Score for Huge Hepatocellular Carcinomas ≥13 cm. J Liver Cancer. 2021;21:45-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 10. | Laface C, Ranieri G, Maselli FM, Ambrogio F, Foti C, Ammendola M, Laterza M, Cazzato G, Memeo R, Mastrandrea G, Lioce M, Fedele P. Immunotherapy and the Combination with Targeted Therapies for Advanced Hepatocellular Carcinoma. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Reference Citation Analysis (0)] |
| 11. | Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin S, Zhu AX, Chan SL, Melkadze T, Sukeepaisarnjaroen W, Breder V, Verset G, Gane E, Borbath I, Rangel JDG, Ryoo BY, Makharadze T, Merle P, Benzaghou F, Banerjee K, Hazra S, Fawcett J, Yau T. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23:995-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 347] [Article Influence: 115.7] [Reference Citation Analysis (1)] |
| 12. | He Y, Zhan L, Shi J, Xiao M, Zuo R, Wang C, Liu Z, Gong W, Chen L, Luo Y, Zhang S, Wang Y, Chen L, Guo H. The Combination of R848 with Sorafenib Enhances Antitumor Effects by Reprogramming the Tumor Immune Microenvironment and Facilitating Vascular Normalization in Hepatocellular Carcinoma. Adv Sci (Weinh). 2023;10:e2207650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Sprinzl MF, Puschnik A, Schlitter AM, Schad A, Ackermann K, Esposito I, Lang H, Galle PR, Weinmann A, Heikenwälder M, Protzer U. Sorafenib inhibits macrophage-induced growth of hepatoma cells by interference with insulin-like growth factor-1 secretion. J Hepatol. 2015;62:863-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Kalathil SG, Lugade AA, Miller A, Iyer R, Thanavala Y. PD-1(+) and Foxp3(+) T cell reduction correlates with survival of HCC patients after sorafenib therapy. JCI Insight. 2016;1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 15. | Macek Jilkova Z, Aspord C, Decaens T. Predictive Factors for Response to PD-1/PD-L1 Checkpoint Inhibition in the Field of Hepatocellular Carcinoma: Current Status and Challenges. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 16. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1454] [Article Influence: 484.7] [Reference Citation Analysis (2)] |
| 17. | Thabut D, Kudo M. Treatment of portal hypertension in patients with HCC in the era of Baveno VII. J Hepatol. 2023;78:658-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 18. | Thabut D, Shah V. Intrahepatic angiogenesis and sinusoidal remodeling in chronic liver disease: new targets for the treatment of portal hypertension? J Hepatol. 2010;53:976-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 218] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 19. | Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R, Chinot OL. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat Rev. 2020;86:102017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 714] [Article Influence: 142.8] [Reference Citation Analysis (0)] |
| 20. | Ellis LM, Curley SA, Grothey A. Surgical resection after downsizing of colorectal liver metastasis in the era of bevacizumab. J Clin Oncol. 2005;23:4853-4855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 132] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Fulgenzi CAM, Scheiner B, Korolewicz J, Stikas CV, Gennari A, Vincenzi B, Openshaw MR, Silletta M, Pinter M, Cortellini A, Scotti L, D'Alessio A, Pinato DJ. Efficacy and safety of frontline systemic therapy for advanced HCC: A network meta-analysis of landmark phase III trials. JHEP Rep. 2023;5:100702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 22. | Park JG. Long-term outcomes of patients with advanced hepatocellular carcinoma who achieved complete remission after sorafenib therapy. Clin Mol Hepatol. 2015;21:287-294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, Kudo M, Harding JJ, Merle P, Rosmorduc O, Wyrwicz L, Schott E, Choo SP, Kelley RK, Sieghart W, Assenat E, Zaucha R, Furuse J, Abou-Alfa GK, El-Khoueiry AB, Melero I, Begic D, Chen G, Neely J, Wisniewski T, Tschaika M, Sangro B. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 729] [Article Influence: 182.3] [Reference Citation Analysis (0)] |
| 24. | Rose MG, Kennedy EB, Abou-Alfa GK, Finn RS, Gade T, Kelley RK, Taddei T, Gordan JD. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline Update Clinical Insights. JCO Oncol Pract. 2024;20:1035-1039. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |