Published online Jan 27, 2025. doi: 10.4240/wjgs.v17.i1.99495
Revised: September 22, 2024
Accepted: October 30, 2024
Published online: January 27, 2025
Processing time: 156 Days and 20.6 Hours
Intraoperative and postoperative biliary injuries remain significant complications of laparoscopic common bile duct exploration (LCBDE). Indocyanine green (ICG) has been shown to significantly reduce injuries caused by intraoperative opera
To evaluate the efficacy of ICG fluorescence imaging In LCBDE and J-tube drai
We retrospectively collected the clinical case data of patients who were treated at the Hepatobiliary Surgery Department of the Third People’s Hospital of Nantong, affiliated with Nantong University, from January 2016 to January 2021 due to gallbladder stones with choledocholithiasis and who underwent LCBDE com
A total of 198 patients (112 males and 86 females) were included in the study, with 74 patients in the WL + T-tube, 47 in the WL + J-tube, 42 in the ICG + T-tube, and 35 in the ICG + J-tube. Compared with the other groups, the ICG + J had significantly shorter operation time (114 minutes, P = 0.001), less blood loss (42 mL, P = 0.02), shorter postoperative hospital stays (7 days, P = 0.038), and lower surgical costs (China yuan 30178, P = 0.001). Fur
ICG fluorescence imaging in laparoscopic cholecystectomy with common bile duct exploration and J-tube drainage facilitates rapid identification of biliary anatomy and variations, reducing intraoperative bile duct injury, blood loss, surgery duration, and postoperative bile duct stenosis rates, supporting its clinical adoption.
Core Tip: In this work, we innovatively analyzed the short-term complication indicators of patients by assessing the diameter of the common bile duct preoperatively and postoperatively, as well as the short-term recurrence rate of common bile duct stones after surgery, reflecting the mechanical damage to the inner wall of the common bile duct caused by T-tubes and J-tubes, thereby demonstrating the advantages of J-tubes. At the same time, we demonstrated the superiority of indocyanine green guidance through a series of intraoperative indicator comparisons.
- Citation: Wang ZH, Yan S, Wang R, Chen L, Wu JZ, Cai WH. Clinical application of indocyanine green fluorescence imaging in laparoscopic cholecystectomy with common bile duct exploration and J-Tube drainage. World J Gastrointest Surg 2025; 17(1): 99495
- URL: https://www.wjgnet.com/1948-9366/full/v17/i1/99495.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i1.99495
In recent years, the incidence of choledocholithiasis (CBDS) has been rising globally, accounting for approximately 10%-18% of biliary tract diseases[1]. Timely diagnosis is crucial for management, as disease progression can lead to complications such as pancreatitis and suppurative cholangitis, significantly increasing mortality. Conservative treatments for CBDS are of limited benefit, making surgery and the increasingly popular endoscopic approaches the core diagnostic and treatment strategies. With the continuous advancement of medical technologies like laparoscopy and endoscopy, surgical methods have diversified. Developing minimally invasive surgical plans without compromising treatment efficacy and safety has become a clinical focus[2]. Therefore, a clear understanding of the intricate biliary anatomy remains a primary research interest for hepatobiliary surgeons seeking to reduce intraoperative injuries[3].
Traditional open common bile duct (CBD) exploration (OCBDE) remains the preferred treatment method in some hospitals[4]. Despite the disadvantages of OCBDE, such as greater surgical trauma, longer recovery time, and higher complication risks compared to laparoscopic CBD exploration (LCBDE), OCBDE provides a broader operational space through manual palpation and assistant exposure, particularly for complex CBDS or abnormal biliary anatomy[5]. Fluorescence laparoscopy (FLC) has partially replaced open surgery in recent years. Endoscopic retrograde cholangiopancreatography (ERCP) combined with endoscopic sphincterotomy and/or endoscopic nasobiliary drainage, as a first-line treatment for CBDS due to its effectiveness, currently leads in managing CBDS. However, studies have shown post-ERCP recurrence and residual stone rates of 4%-25%[6,7], with residual fragments and biliary sludge being significant risk factors for recurrence[8]. Some patients, as a result, suffer multiple surgical interventions, causing significant psychological and physiological distress. To address this, many hospitals combine endoscopic treatment with surgery, per
Compared to traditional open surgery, laparoscopic techniques offer benefits such as less trauma and faster recovery[9,10]. These advantages are particularly crucial for elderly patients, significantly improving stone clearance rates and reducing intraoperative and postoperative complications[11,12]. During bile duct exploration, T-tubes are often placed for biliary support and decompression, facilitating postoperative cholangiography[13]. However, prolonged T-tube usage can lead to complications such as retrograde infections, electrolyte and nutritional disturbances, early tube removal causing bile leaks, and thicker drainage tubes causing biliary sludge and even strictures[14-16]. No widely accepted solutions to significantly reduce T-tube-related injuries have been documented, but studies suggest that ureteral stents can significantly decrease postoperative urinary fistula and strictures[17,18]. Therefore, we explored the use of a modified J-tube suitable for the CBD to investigate whether it could serve as an alternative drainage option to the T-tube, thereby reducing the rates of postoperative bile leaks and biliary strictures. Indocyanine green (ICG) fluorescence imaging technology has been widely adopted in recent years for various surgical procedures. ICG is primarily used to diagnose various liver diseases and for vascular imaging[19]. Upon intravenous injection, ICG rapidly binds to plasma proteins and is swiftly distributed throughout the body’s vascular system. It is efficiently and selectively taken up by hepatocytes and excreted in free form into bile, passing through the bile ducts into the intestines and eventually excreted in feces. Due to its rapid excretion, approximately 97% of ICG is cleared from the blood within 20 minutes post-injection in normal individuals. It does not participate in chemical reactions within the body, undergo enterohepatic circulation (ICG entering the intestines is not reabsorbed into the bloodstream), or experience lymphatic backflow, nor is it excreted by non-hepatic organs like the kidneys. Within 2 to 3 minutes of injection, ICG achieves uniform distribution and dynamic equilibrium, and its blood concentration decreases exponentially, disappearing at a first-order rate within approximately 20 minutes[20-22]. Compared to traditional iodine-based contrast agents, ICG offers significantly improved clinical safety with a reduced likelihood of allergic reactions. Clinically validated studies have demonstrated that ICG is particularly valuable for the clear identification of anatomical structures and precise operations in cases involving anatomical variations or severe adhesions during bile duct surgeries[23,24]. This significantly reduces the incidence of intraoperative bile duct injuries and the need for reoperations. By preoperatively injecting ICG intravenously and leveraging its excretion through the biliary system, surgeons can achieve real-time intraoperative visualization of the biliary tract, allowing for clearer identification of bile duct anatomy[25]. In recent years, ICG has gradually been applied in clinical practice. Accordingly, our team at Affiliated Nantong Hospital Third of Nantong University retrospectively analyzed 198 patients diagnosed with gallbladder stones coexisting with CBDS. We applied ICG fluorescence imaging technology during laparoscopic cholecystectomy (LC) combined with choledochotomy and J-tube drainage, addressing the challen
This study included patients who underwent LCBDE at the Third People’s Hospital of Nantong City for gallbladder stones with CBDS between January 2017 and December 2022. Preoperative diagnosis and confirmation of CBDS were achieved by B-mode ultrasonography (US) and either plain or enhanced magnetic resonance imaging. A total of 198 patients were enrolled and divided into four groups according to the surgical method: White-light laparoscopy (WL) + T-tube, WL + J-tube, ICG + T-tube, and ICG + J-tube, meeting the following inclusion criteria: (1) No history of upper abdominal surgery; (2) Satisfactory condition of the Oddi sphincter; (3) Presence of CBD dilation: Diameter > 0.7 cm; (4) No prior ERCP treatment; and (5) No allergy to iodine agents or ICG. The exclusion criteria were as follows: (1) Acute suppurative obstructive cholangitis and unstable circulation upon admission; (2) Multiple hepatic stones; and (3) Distal CBD stricture with a diameter < 0.7 mm. Among the enrolled patients, 133 patients presented with preoperative abdominal pain, 47 had elevated preoperative body temperature (> 37.3 °C), 33 displayed preoperative jaundice (scleral icterus was observable upon examination), and 24 showed preoperative vomiting (Table 1). All symptoms improved after receiving symptomatic treatment before surgery.
| ICG + T-tube | ICG + J-tube | WL + J-tube | WL + T-tube | |
| Age (year) | 65 (56.5, 73.25) | 65 (55, 76) | 68 (55, 75) | 65 (58, 75) |
| Gender | ||||
| Male | 24 (57.1) | 23 (63.9) | 29 (61.7) | 37 (50) |
| Female | 18 (42.9) | 13 (36.1) | 18 (38.3) | 37 (50) |
| CBD diameter (mm) | 1.15 (0.9, 1.325) | 1.2 (1, 1.3) | 1.1 (0.9, 1.2) | 1.1 (0.9, 1.2) |
| Number of stones | ||||
| 1-2 | 22 (52.3) | 30 (83.3) | 27 (56.2) | 46 (61.3) |
| 3-5 | 9 (21.4) | 4 (11.1) | 4 (8.3) | 15 (20) |
| > 5 | 11 (26.2) | 2 (5.5) | 16 (33.3) | 13 (17.3) |
| fever | 8 (19) | 5 (13.9) | 6 (12.8) | 15 (20.3) |
| Jaundice | 7 (16.7) | 5 (13.9) | 9 (19.1) | 12 (16.2) |
| Fatigue | 8 (19) | 5 (13.9) | 8 (17) | 15 (20.3) |
| Nausea and vomiting | 6 (14.3) | 1 (2.8) | 5 (10.6) | 8 (10.8) |
| Abdominal pain | 35 (83.3) | 28 (77.8) | 44 (93.6) | 60 (81.1) |
| Postoperative complications | 3 (7.1) | 1 (2.8) | 2 (4.2) | 7 (9.4) |
Ethical approval for the study was obtained from the Ethics Committee of the Affiliated Nantong Hospital Third of Nantong University, and we confirm that informed consent was obtained from all participants and/or their legal guardians. All procedures complied with ethical standards and the Helsinki Declaration.
Routine laparoscopic procedure and T-tube placement: A 1 cm incision was made below the umbilicus for trocar insertion into the abdomen to establish pneumoperitoneum with a pressure of 12 cm Hg. A laparoscope was then inserted. Under laparoscopic guidance, puncture holes were made 1 cm below the xiphoid process, 0.5 cm along the anterolateral line below the right costal margin, and 0.5 cm below the costal margin along the right midclavicular line to insert the laparoscopic instruments. The gallbladder and CBD were explored, and Calot’s triangle was dissected to expose the cystic artery and duct. The cystic duct was clamped proximally with two hemlock clips and distally with one, without cutting. The cystic artery was sealed proximally with a clip and coagulated distally for haemostasis. The CBD was longitudinally opened 1 cm below the confluence with the cystic duct, creating a 1.0 cm opening. A choledochoscope (OLYMPUS CHF-V, model: OEV262H) was used to inspect the left and right hepatic ducts upwards and the distal CBD downwards, removing stones with a retrieval basket. The choledochoscope revealed no abnormalities at the lower end of the CBD or the ampulla. The area was flushed with saline to ensure that no residual stones remained. A 22-24 French unit T-tube was routinely placed, and 4-0 absorbable sutures were used to intermittently secure the T-tube without any leakage at the suture or puncture site. The cystic duct was severed, and the gallbladder was completely dissected from the fossa and removed. Haemostasis was achieved at the operative site, with no active bleeding or bile leakage noted. A negative pressure drainage tube was placed under the right liver and fixed through the right puncture hole, while the T-tube was exteriorized through the right midclavicular puncture hole. After confirming the correct number of sponges and instruments, we released the pneumoperitoneum, withdrawn the instruments, and the incisions were sutured. Depending on the specific situation of the patient, we will remove the T-tube approximately 2-3 weeks after surgery. By this time, the bile duct scar has already contracted, and the possibility of bile duct stricture and obstruction is relatively low.
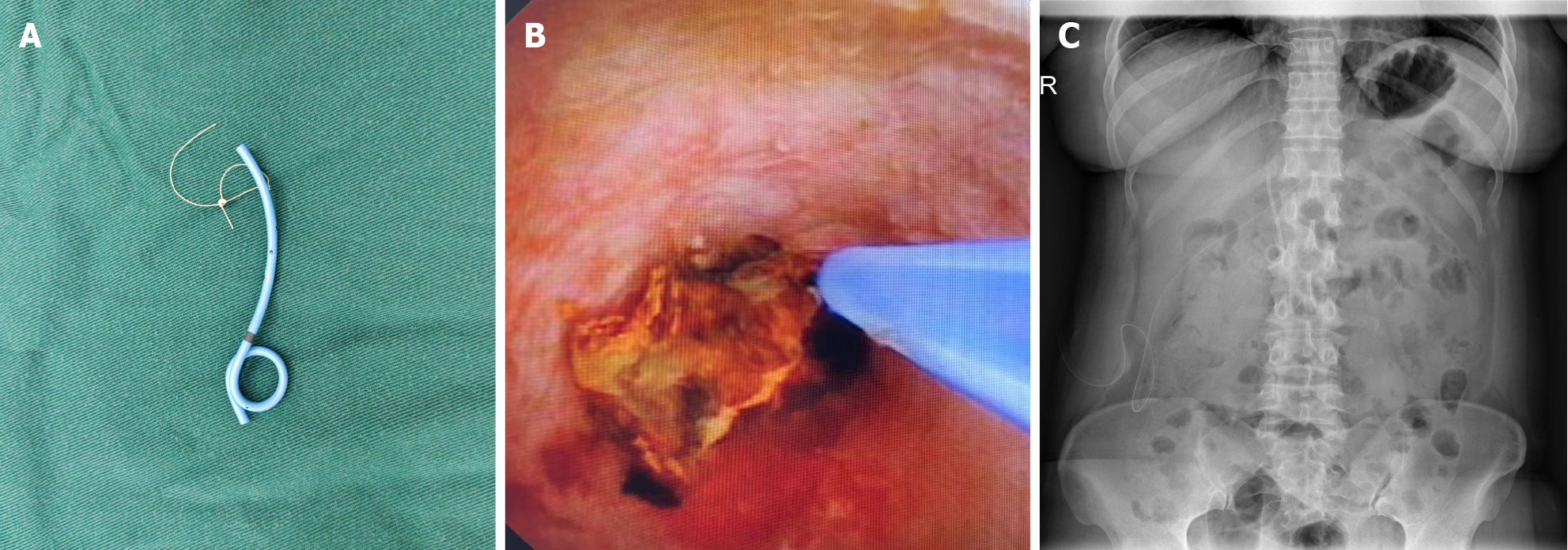
ICG imaging technique: Patients selected for intraoperative fluorescence imaging were administered ICG via intravenous injection. Thirty minutes before surgery, depending on the patient’s weight, 0.3‰ ICG solution was prepared and administered through peripheral venous injection[23,26]. Inject an ICG solution equivalent to 0.5 mg per kilogram of body weight via the elbow vein, while observing the patient’s reactions, and administer slowly, generally completing the injection within 10 seconds. Fluorescence imaging scope was used as the observation lens during the procedure. Lifting the gallbladder, Calot’s triangle and the course of the CBD were visualized (Figure 1A). Upon completion of the surgery, a J-tube or T-tube was placed as dictated by the actual conditions (Figure 1B). The fluorescence was then reactivated to inspect the integrity of the CBD incision and to check for any potential bile leakage around the area, as indicated by the escape of green fluorescent fluid (Figure 1C).
J-tube placement technique: Under direct vision, a guidewire was inserted into the CBD and through the duodenal papilla into the duodenum. After removing the choledochoscope, a homemade biliary J-shaped stent-approximately 12 cm in length (Figure 2A), with an absorbable suture loop at the end (model: W9918, 4-0; Johnson and Johnson)-was threaded over the zebra guidewire and advanced into the distal CBD until the stent passed through the duodenal papilla. The guidewire was then removed, and stent placement was reconfirmed with a choledochoscope (Figure 2B). The sidewall incision of the CBD was continuously sutured with an absorbable suture passed through the loop of the same material using a 4-0 V-Lok suture with both margin and stitch spacing of 2.5 mm. A standing abdominal X-ray was performed one week postoperatively to determine the position of the internal drainage stent (Figure 2C). Postoperative follow-up one month later by phone to observe whether the stent has been excreted with feces.
The following parameters were analyzed across the different surgical groups: Intraoperative blood loss, surgery duration (from skin incision to closure), hospitalization costs, intraoperative bile duct injuries, average postoperative hospital stay, postoperative complications, preoperative and postoperative Day 3 inflammatory markers (C-reactive protein, white blood cell count, percentage of neutrophils, etc.), liver function tests (gamma-glutamyl transferase, alanine aminotransferase, aspartate aminotransferase, total bilirubin, direct bilirubin, etc.), and postoperative pain scores. Ultrasound was performed one and three months post-surgery to assess the diameter of the CBD and to monitor for any recurrence of CBDS.
The data were processed using statistical product and service solutions 26.0 software. Nonnormally distributed con
This study included 198 patients, with 112 males and 86 females. The median age was 66 years (range: 22 to 89 years). The decision to use fluorescence imaging-guided technology was considered crucial for patients with complicating factors, such as Mirizzi syndrome, acute cholangitis or cholecystitis, or anatomical variations affecting the exposure of Calot’s triangle. All patients underwent LCBDE, and the postoperative drainage type was selected based on the severity of inflammation in the CBD wall and the characteristics of the stones. T-tube drainage was preferred for sludge-like stones, and patients were expected to have incomplete stone removal; a clearer biliary tract environment led to the choice of J-tube drainage. There were no significant differences in the baseline characteristics, such as sex, age, CBD diameter, or incidence of clinical symptoms, between the groups before surgery (Table 1).
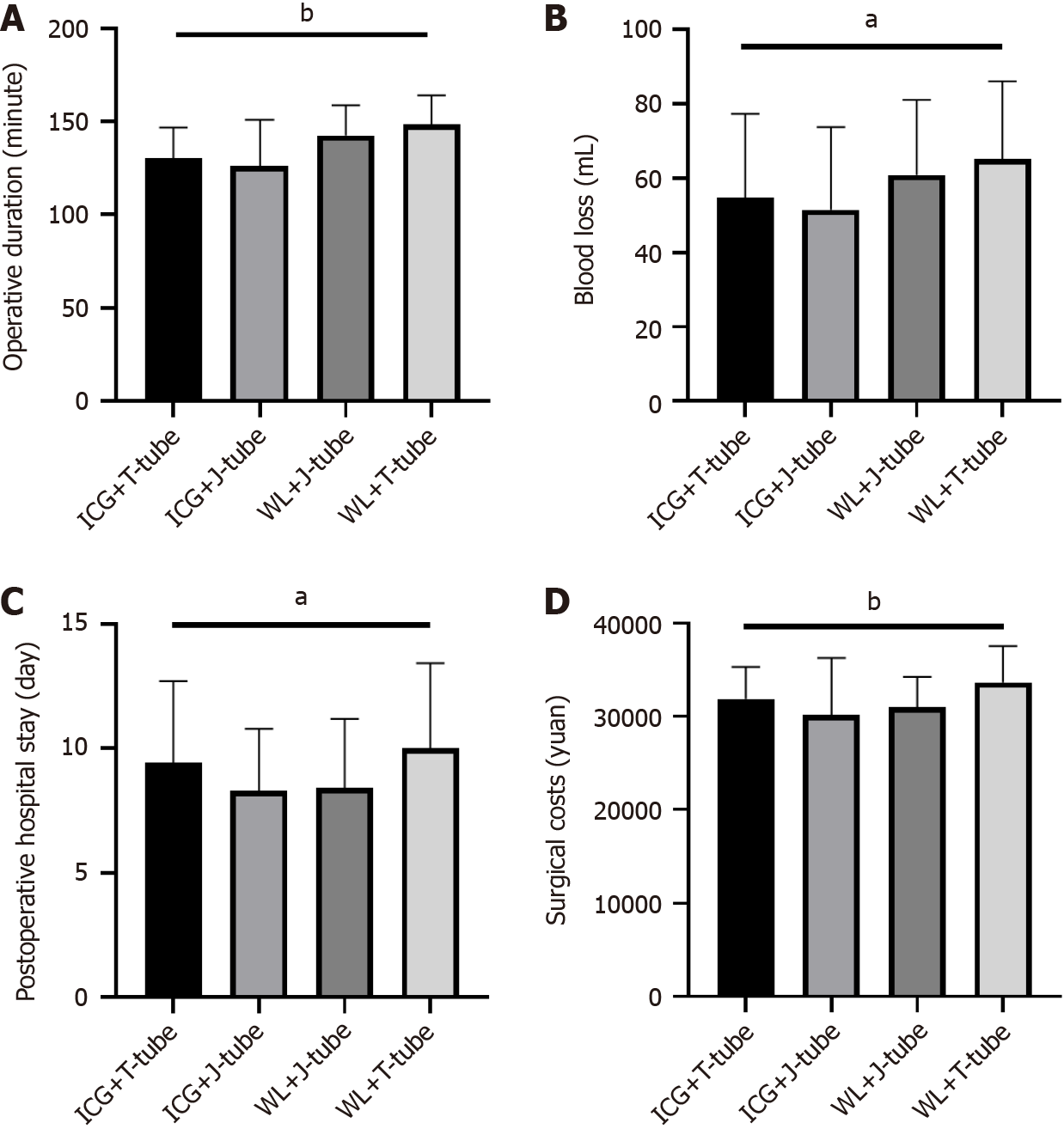
In this study, a total of 198 enrolled patients were divided into four groups: WL + T-tube (74 patients), WL + J-tube (47 patients), ICG + T-tube (42 patients), and ICG + J-tube (36 patients). Compared to the WL + T-tube and WL + J-tube groups, the ICG + T-tube and ICG + J-tube groups demonstrated a significant reduction in blood loss (P = 0.02) (Figure 3A) and a more pronounced decrease in operative time (P = 0.001) (Figure 3B). No significant differences were observed between the groups in terms of postoperative hospital stay or hematological parameters (Figure 3C). No significant differences were observed between the groups in terms of postoperative hospital stay (Figure 3D).
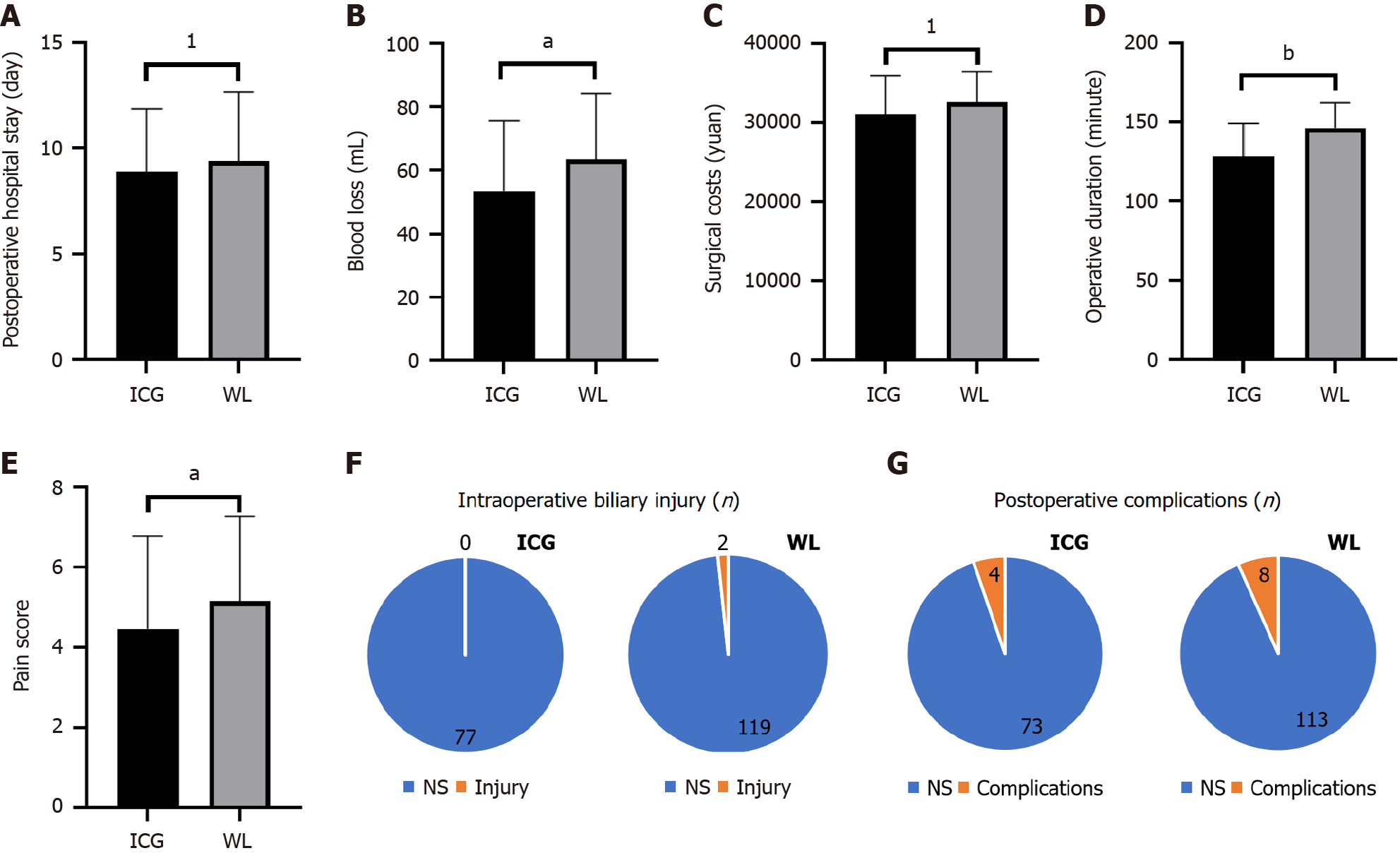
Further analysis comparing all patients who underwent ICG imaging (regardless of whether they received a T-tube or J-tube) with those who did not receive ICG imaging (white light group) revealed that the ICG group had significantly lower intraoperative blood loss (P = 0.030) (Figure 4A) and overall operative time (P = 0.001) (Figure 4B) compared to the white light group. Additionally, the ICG group reported lower numerical rating scale pain scores on the first postope
Compared to traditional T-tube drainage, J-tube drainage reduced the incidence of postoperative complications, although the difference was not statistically significant. However, the use of J-tubes not only significantly reduced surgical costs (P = 0.001) (Table 2) and shortened hospital stays (P = 0.001) (Table 2), but also facilitated faster recovery. Moreover, laboratory results on the third postoperative day indicated that patients who underwent J-tube drainage had better liver function recovery, as indicated by significant improvements in total bilirubin, direct bilirubin, alanine aminotransferase, and aspartate aminotransferase levels (P < 0.001) (Table 2).
| T-tube | J-tube | P value | |
| Preoperative indicators, CRP (mg/L) | 25.45 (9.05, 57.33) | 19.40 (6.96, 44.41) | 0.246 |
| GGT (U/L) | 563.00 (184.00, 1062.00) | 342.00 (123.00, 658.00) | 0.004 |
| TBIL (μmol/L) | 30.2500 (19.50, 42.85) | 29.75 (19.00, 45.20) | 0.958 |
| DBIL (μmol/L) | 14.2000 (9.85, 21.15) | 13.00 (7.60, 22.60) | 0.509 |
| AST (U/L) | 126.00 (75.50, 218.50) | 98.00 (65.00, 155.00) | 0.074 |
| ALT (U/L) | 135.50 (83.50, 196.00) | 97.50 (51.00, 199.00) | 0.082 |
| WBC (109/L) | 10.99 (9.27, 12.39) | 10.76 (8.15, 12.62) | 0.463 |
| NE (%) | 79.75 (71.85, 87.45) | 79.05 (71.60, 87.00) | 0.776 |
| Postoperative indicators | |||
| TBIL (μmol/L) | 22.55 (16.55, 34.75) | 14.30 (11.20, 20.00) | < 0.001 |
| DBIL (μmol/L) | 14.00 (10.05, 22.15) | 7.34 (4.90, 14.20) | < 0.001 |
| AST (U/L) | 68.50 (41.00, 81.00) | 40.50 (29.00, 68.000) | < 0.001 |
| ALT (U/L) | 68.50 (38.50, 81.50) | 34.50 (23.00, 51.00) | < 0.001 |
| WBC (109/L) | 8.99 (7.39, 10.63) | 8.86 (7.06, 10.31) | 0.329 |
| NE (%) | 76.15 (62.30, 90.45) | 74.05 (64.82, 83.00) | 0.308 |
| Biliary diameter discrepancy one month, (mm) | 0.30 (0.20, 0.30) | 0.30 (0.20, 0.40) | 0.295 |
| Three months | 0.50 (0.40, 0.70) | 0.40 (0.30, 0.60) | 0.0002 |
| Recurrence one month, n (%) | 2 (1.6) | 1 (1.3) | 0.874 |
| Three months, n (%) | 6 (4.7) | 3 (3.9) | 0.068 |
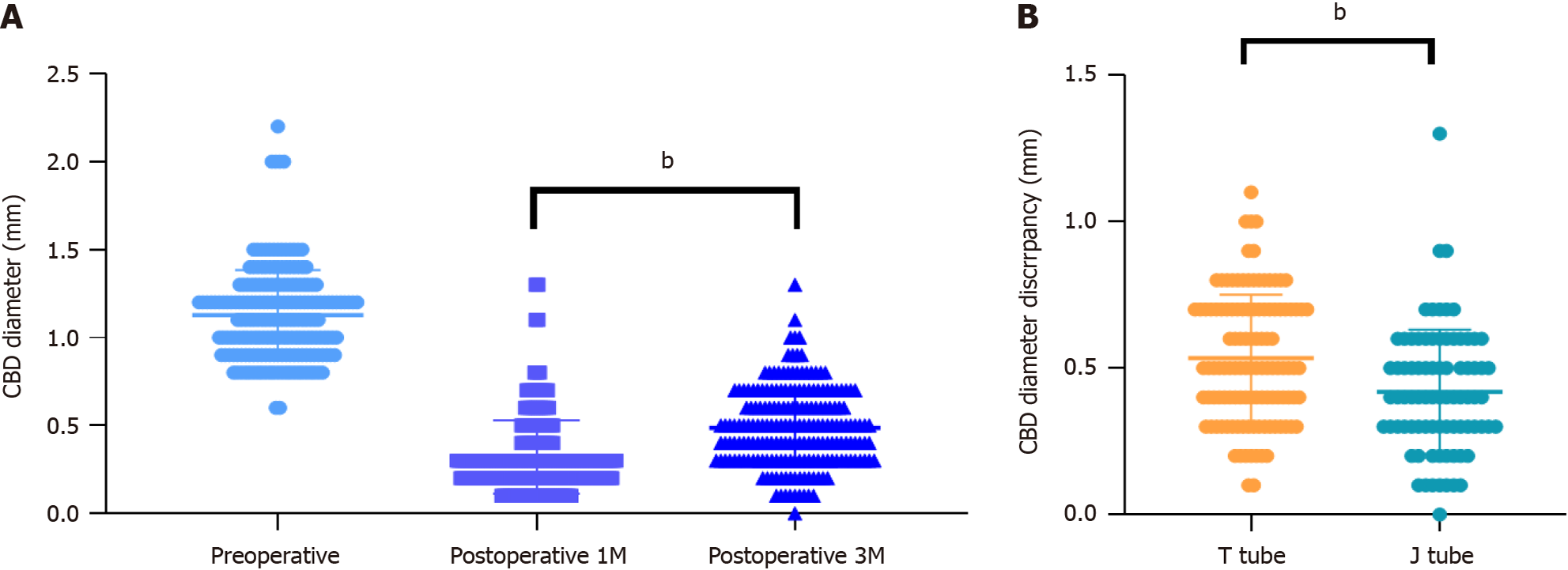
Additionally, we conducted a follow-up of patients one and three months postoperatively using imaging studies to compare instances of biliary stricture and the recurrence of gallstones. US was utilized to measure the diameter of the CBD 1 cm below the junction of the cystic and hepatic ducts-corresponding to the site of the intraoperative choledochotomy, and compare it with the preoperative diameter to assess biliary stricture. We also checked for the recurrence of gallstones within the CBD. The results indicated that patients who underwent J-tube drainage experienced significantly less biliary stricture three months postoperatively than did those who underwent T-tube drainage (P = 0.002) (Figure 5).
Gallstone disease is a prevalent digestive system disorder. Globally, approximately 10%-18% of patients with gallbladder stones also suffer from CBD stones[27]. In China, the co-occurrence rate of gallbladder and CBD stones is notably high, ranging from 8% to 25%, which is potentially related to dietary patterns or drinking culture. Symptoms include abdo
Our study thoroughly compared ICG fluorescence-guided FLC with traditional white light laparoscopy (WLC) for LCBDE in terms of operative time, blood loss, and intraoperative biliary injury. The results clearly demonstrated that FLC-LCBDE offers significant advantages over WLC-LCBDE, particularly in reducing operative time, minimizing blood loss. These benefits are primarily due to FLC’s high-definition visualization and enhanced contrast of biliary structures[31], yielding the following advantages: (1) Unlike traditional minimally invasive surgery, FLC allows clearer visualization of minute biliary structures, enabling surgeons to more quickly and accurately identify relevant anatomy, even in complex cases, thereby shortening operative time and reducing unnecessary damage; and (2) Previous studies suggest that shorter operative times and precise surgical techniques can alleviate postoperative discomfort and reduce the need for postoperative analgesia and associated costs. In practice, some patients did not use the fluorescence imaging mode continuously during FLC, only intermittently for cystic duct localization and to ensure its complete closure, which extended the operative time and led to intraoperative biliary injuries in two cases, which were promptly repaired during the procedure with no bile leakage upon closure. In this study, bile leaks occurred during surgery in two patients using WLC due to the significant thickening of the cystic duct caused by severe inflammation, leading the surgeon to mistakenly identify it as the CBD and cut it, resulting in bile leakage during the operation. The literature reports an intraoperative bile duct injury incidence of about 0.4%-0.6%, but this study, constrained by a smaller case number, observes a rate slightly higher than the global average. Additionally, for patients with thickened CBD walls, surgeons, despite identifying the bile duct, continued to search for it due to the absence of bile flow after multiple electrocauteries, increasing the likelihood of prolonged surgery and biliary injury. We observed no statistically significant difference in postoperative hospital stay between the ICG and WL groups, likely because our department is in the early stages of implementing FLC surgery and took a conservative approach in assessing postoperative recovery, opting for longer hospital stays. However, surgeries in the past two years have shown that shorter operative times and reduced blood loss typically result in faster recovery and less pain from surgical trauma. These findings not only enrich the technical options in hepatobiliary surgery but also provide valuable guidance for clinical practice.
Although ICG navigation demonstrates significant advantages in LCBDE, its use is limited. However, further research may expand its applications. On one hand, it requires advanced medical facilities, such as a sophisticated fluorescence imaging system and a cholangioscope less than 8 mm in diameter. It also demands a higher skill level from surgeons, who must be proficient in both traditional LC and CBDS removal, as well as detailed endoscopic manoeuvres[23]. On the other hand, the patient’s biliary condition is crucial for surgical success. Acute purulent obstructive cholangitis, for instance, can cause severe inflammation and oedema, narrowing the bile ducts and complicating surgery due to large or numerous stones. Anatomical variations between the cystic and CBD significantly increase surgical difficulty and risk[24]. In our hospital practice, we also found that stone impaction in the upper CBD could prevent ICG excretion, rendering fluorescence imaging impossible. Choosing the appropriate timing and dosage for administering fluorescence agents can be particularly challenging in cases with CBDS or compromised liver function due to cirrhosis, affecting imaging outcomes. Therefore, a comprehensive assessment of the patient’s overall condition is necessary to devise an appropriate treatment plan for optimal results.
Our study’s findings echo recent meta-analyses and clinical consensus, which also emphasize the importance of gentler internal drainage strategies post-LCBDE. For example, improper T-tube management can increase the risk of nosocomial infections, and patients with significant preoperative CBD oedema are more susceptible to postoperative complications from T-tube mechanical damage[4,32].
When comparing T-tubes with J-tubes, T-tubes have conventionally been favoured due to their ability to decompress the biliary tract postoperatively and facilitate cholangiography. However, with advancements in technology and a better understanding of postoperative recovery, J-tubes are considered to potentially cause less mechanical damage to the biliary tract, thus reducing the risks of postoperative liver dysfunction and complications. Analysis of postoperative hospital stay, total hospital costs, and complication rates indicates that patients with J-tubes recover faster than those with T-tubes. This is attributed to the J-tube’s role as an internal stent, encouraging early ambulation and reducing bed rest, which not only avoids complications associated with prolonged immobilization but also shortens hospital stays and costs. Although there was no difference in the rate of postoperative complications observed between the two groups in this study, in practice, fewer complications were observed in the J-tube group. Yu et al[32] also confirmed significantly lower total incidence of postoperative bile leaks, cholangitis, pancreatitis, and bile duct stricture in the J-tube group, credited to the effective internal drainage by the J-tube within the CBD and duodenum. This drainage approach supports electrolyte balance and internal environment stability, ensuring normal absorption of intestinal nutrients and promoting rapid gastrointestinal function recovery.
Previous research and meta-analyses have typically focused on perioperative metrics and patient outcomes, such as surgical success rates, postoperative morbidity, stone clearance, mortality, conversions to open surgery, total operative time, and economic benefits[10,33,34]. Innovatively, this study analyzed short-term complication indicators by measuring CBD diameters preoperatively and postoperatively, reflecting the mechanical damage to the CBD wall by T-tubes vs J-tubes. The suture spacing during placement of different drainage tubes also varied, with J-tubes allowing for wider and more even spacing compared to T-tubes. We observed that three months postoperatively, the reduction in CBD diameter (preoperative diameter minus diameter at three months postop) was significantly less in the J-tube group. This suggests that long-term biliary strictures and scar formation may be less likely in patients with J-tubes than in those with T-tubes, which also impacts the recurrence rate of gallstones.
It is important to note that while our study tends to support the use of J-tubes, T-tubes may still be necessary in certain circumstances. For example, in patients with complex biliary anatomy or those anticipated to require postoperative biliary interventions, T-tubes can provide access for subsequent endoscopic or radiologic procedures. Additionally, in developing countries with limited medical facilities, the use of T-tubes cannot be entirely dismissed. Therefore, the choice of drainage method should be tailored based on the specific conditions of the patient and surgical details.
ICG-guided FLC and J-tube drainage in biliary surgery offers multiple advantages. Firstly, this technology allows for more rapid identification of the biliary anatomy, helping surgeons to clearly understand anatomical variations in the bile ducts, thereby enhancing the precision and safety of the surgery. This accurate localization not only significantly reduces the incidence of intraoperative bile duct injuries but also effectively decreases intraoperative blood loss, ensuring a smoother surgical process. Moreover, by minimizing unnecessary exploration and maneuvers, ICG fluorescence imaging technology can significantly shorten the duration of the surgery, reducing the patient’s surgical burden and anesthesia risks.
Importantly, J-tube also demonstrates clear advantages in postoperative management. The application of this technology can lower the incidence of postoperative bile duct strictures, thereby improving the long-term outcomes for patients. These clinical benefits not only result in a smoother postoperative recovery for patients but also reduce the utilization of medical resources and associated healthcare costs.
In summary, the application of ICG-guided FLC and J-tube drainage in biliary surgery holds significant clinical promotional value. As the technology continues to mature and clinical experience accumulates, it has the potential to become the standard operating procedure in bile duct surgery, providing better treatment outcomes and quality of life for a wide range of patients.
Although the results of this study confirm the potential advantages of ICG-guided FLC and J-tube drainage in biliary surgery, they represent only preliminary explorations. Future research is needed on multiple levels to optimize drainage strategies and improve clinical outcomes in biliary surgeries.
First, this study is primarily based on retrospective data from a single centre, constrained by sample size and regional characteristics, which may affect the generalizability and applicability of the results. Future studies should employ prospective, multicentre, randomized controlled trial designs to validate the robustness and transferability of these findings. These studies should include a broader demographic, patients from different geographical backgrounds, and long-term follow-up results to comprehensively assess the impacts of drainage method choice on postoperative recovery and long-term quality of life.
Second, while J-tubes have shown advantages in some areas, detailed exploration is required regarding the specific applications of T-tube vs J-tube drainage across various clinical scenarios. Future research should focus on the effectiveness and safety of different drainage methods within special patient groups such as patients with high-risk factors like liver cirrhosis and coagulopathy.
Technical innovation is also an important aspect of future prospects. With advancements in medical imaging technologies and fluorescent dyes, we anticipate the development of a wider variety of dyes and advanced imaging systems to further improve the visualization quality of biliary anatomy during surgery, thereby reducing surgical risks. Addi
Last, the refinement and optimization of J-tube drainage are also focal points for future research. Exploring the impacts of different types of J-tube materials and designs on surgical outcomes, as well as developing new types of drainage tubes that can be more easily degraded within or removed from the body, could further reduce patient discomfort and the risk of complications.
In summary, future research will be multifaceted and interdisciplinary, aiming to advance the quality and safety of biliary surgery treatments through precise technologies and detailed patient analyses.
| 1. | Cianci P, Restini E. Management of cholelithiasis with choledocholithiasis: Endoscopic and surgical approaches. World J Gastroenterol. 2021;27:4536-4554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 99] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (15)] |
| 2. | Jorba R, Pavel MC, Llàcer-Millán E, Estalella L, Achalandabaso M, Julià-Verdaguer E, Nve E, Padilla-Zegarra ED, Badia JM, O'Connor DB, Memba R. Contemporary management of concomitant gallstones and common bile duct stones: a survey of Spanish surgeons. Surg Endosc. 2021;35:5024-5033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Geng Y, Chen S, Yang Y, Miao H, Li X, Li G, Ma J, Zhang T, Ren T, Li Y, Li L, Liu L, Yang J, Wang Z, Zou L, Liu K, Li Y, Yan S, Cui X, Sun X, Yang B, Zhang L, Han X, Wang C, Chen B, Yue X, Liang W, Ren J, Jia J, Gu J, Li Z, Zhao T, Wang P, Wei D, Qiu S, Xiang D, Xu X, Chen W, He M, Yang L, Wang H, Chen T, Hua R, Wang X, Wu X, Gong W, Wang G, Li M, Zhang W, Shao R, Wu W, Liu Y. Long-term exposure to genistein inhibits the proliferation of gallbladder cancer by downregulating the MCM complex. Sci Bull (Beijing). 2022;67:813-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Rudiman R, Hanafi RV, Halim F. Complications of biliary stenting versus T-tube insertion after common bile duct exploration: A systematic review and meta-analysis. PLoS One. 2023;18:e0280755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 5. | Wills VL, Gibson K, Karihaloot C, Jorgensen JO. Complications of biliary T-tubes after choledochotomy. ANZ J Surg. 2002;72:177-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Wu Y, Xu CJ, Xu SF. Advances in Risk Factors for Recurrence of Common Bile Duct Stones. Int J Med Sci. 2021;18:1067-1074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 7. | Zhang Z, Li X, Guo J, He B, Wu L, Yang R, Li X, Fang D, Yang X, Yang D, Wang F, Tang M, Han Y, Jose PA, Wang H, Zeng C. β-aminoisobutyrics acid, a metabolite of BCAA, activates the AMPK/Nrf-2 pathway to prevent ferroptosis and ameliorates lung ischemia-reperfusion injury. Mol Med. 2023;29:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 8. | Lin YY, Wang YD, Yue P, Zhang XZ, Leung JW, Jiao PP, Yang M, Wang HP, Bai B, Liu Y, Zhang JD, Chen HB, Meng WB, Li X. Could saline irrigation clear all residual common bile duct stones after lithotripsy? A self-controlled prospective cohort study. World J Gastroenterol. 2021;27:358-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Zhang Z, Ji H, Chen G, Hou Y. A comparative study of laparoscopic choledocholithotomy with primary suture and T-tube drainage. Medicine (Baltimore). 2024;103:e36757. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Zhao C, Xu Z, Hu W, Ge C, Zhang Z, Dai Z, Zhang S, Tang N, Wang W, Gu J, Chen C, He S. A Retrospective Study on the Three-Port Technique of Laparoscopic Common Bile Duct Exploration for the Management of Cholelithiasis and Choledocholithiasis. Int J Gen Med. 2023;16:3435-3445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 11. | Luo T, Huang Y, Wang S, Yang T, Gong J, Zhou B, Song Z, Meng H, Xu B. Laparoscopic common bile duct exploration with primary closure is preferred for selected elderly individuals with choledocholithiasis. Ann Gastroenterol Surg. 2023;7:772-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Zhuang L, Li Y, Zhang L, Xu X, Sun D, Xi D, Lu Y. A comparison of the therapeutic outcomes between primary duct closure and T-tube drainage after laparoscopic common bile duct exploration: a single-centre retrospective study. Wideochir Inne Tech Maloinwazyjne. 2023;18:108-116. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Padmore G, Sutherland FR, Ball CG. The art and craft of biliary T-tube Use. J Trauma Acute Care Surg. 2021;91:e46-e49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Xie W, Yu W, Zhang Z, Ma Z, Song Z, Yang T. Is T-tube drainage no longer needed for laparoscopic common bile duct exploration? A retrospective analysis and literature review. Wideochir Inne Tech Maloinwazyjne. 2023;18:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Gurusamy KS, Koti R, Davidson BR. T-tube drainage versus primary closure after laparoscopic common bile duct exploration. Cochrane Database Syst Rev. 2013;CD005641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Lu J, Zhang X, Zeng C, Gu JT, Cai H. Clinical Analysis of Laparoscopic Common Bile Duct Primary Suture and T-Tube Drainage in the Treatment of Common Bile Duct Stones. J Laparoendosc Adv Surg Tech A. 2023;33:622-625. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Kim HS, Lee BK, Jung JW, Lee JK, Byun SS, Lee SE, Jeong CW. J-tube technique for double-j stent insertion during laparoscopic upper urinary tract surgical procedures. J Endourol. 2014;28:1278-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Xie W, Zhang S, Li X, Liu Y, Yang J, Liu P, Zeng K. Effect of indwelling time of double J tube on infected ureteral calculi and the distribution of pathogenic characteristics in diabetics. Am J Transl Res. 2021;13:5685-5690. [PubMed] |
| 19. | de'Angelis N, Catena F, Memeo R, Coccolini F, Martínez-Pérez A, Romeo OM, De Simone B, Di Saverio S, Brustia R, Rhaiem R, Piardi T, Conticchio M, Marchegiani F, Beghdadi N, Abu-Zidan FM, Alikhanov R, Allard MA, Allievi N, Amaddeo G, Ansaloni L, Andersson R, Andolfi E, Azfar M, Bala M, Benkabbou A, Ben-Ishay O, Bianchi G, Biffl WL, Brunetti F, Carra MC, Casanova D, Celentano V, Ceresoli M, Chiara O, Cimbanassi S, Bini R, Coimbra R, Luigi de'Angelis G, Decembrino F, De Palma A, de Reuver PR, Domingo C, Cotsoglou C, Ferrero A, Fraga GP, Gaiani F, Gheza F, Gurrado A, Harrison E, Henriquez A, Hofmeyr S, Iadarola R, Kashuk JL, Kianmanesh R, Kirkpatrick AW, Kluger Y, Landi F, Langella S, Lapointe R, Le Roy B, Luciani A, Machado F, Maggi U, Maier RV, Mefire AC, Hiramatsu K, Ordoñez C, Patrizi F, Planells M, Peitzman AB, Pekolj J, Perdigao F, Pereira BM, Pessaux P, Pisano M, Puyana JC, Rizoli S, Portigliotti L, Romito R, Sakakushev B, Sanei B, Scatton O, Serradilla-Martin M, Schneck AS, Sissoko ML, Sobhani I, Ten Broek RP, Testini M, Valinas R, Veloudis G, Vitali GC, Weber D, Zorcolo L, Giuliante F, Gavriilidis P, Fuks D, Sommacale D. 2020 WSES guidelines for the detection and management of bile duct injury during cholecystectomy. World J Emerg Surg. 2021;16:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 20. | Pal R, Lwin TM, Krishnamoorthy M, Collins HR, Chan CD, Prilutskiy A, Nasrallah MP, Dijkhuis TH, Shukla S, Kendall AL, Marshall MS, Carp SA, Hung YP, Shih AR, Martinez-Lage M, Zukerberg L, Sadow PM, Faquin WC, Nahed BV, Feng AL, Emerick KS, Mieog JSD, Vahrmeijer AL, Rajasekaran K, Lee JYK, Rankin KS, Lozano-Calderon S, Varvares MA, Tanabe KK, Kumar ATN. Fluorescence lifetime of injected indocyanine green as a universal marker of solid tumours in patients. Nat Biomed Eng. 2023;7:1649-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 39] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 21. | Ma C, Zhang L, Wen J, Zhang W, Chen H. Indocyanine Green Imaging in Laparoscopic Cholecystectomy Plus Laparoscopic Common Bile Duct Exploration: A Suitable Option for Patients With Difficult Exploration (With Videos). Surg Laparosc Endosc Percutan Tech. 2023;33:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Morales-Conde S, Licardie E, Alarcón I, Balla A. Indocyanine green (ICG) fluorescence guide for the use and indications in general surgery: recommendations based on the descriptive review of the literature and the analysis of experience. Cir Esp (Engl Ed). 2022;100:534-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Dip F, Aleman R, Frieder JS, Gomez CO, Menzo EL, Szomstein S, Rosenthal RJ. Understanding intraoperative fluorescent cholangiography: ten steps for an effective and successful procedure. Surg Endosc. 2021;35:7042-7048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Huang H, Du D, Wang Z, Xie Y, Ni Z, Li X, Jin H. Application of Intraoperative Fluorescence Imaging with Indocyanine Green in the Difficult Gallbladder: A Comparative Study between Indocyanine Green-Guided Fluorescence Cholangiography and Conventional Surgery. J Laparoendosc Adv Surg Tech A. 2023;33:404-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 25. | Matsumura M, Kawaguchi Y, Kobayashi Y, Kobayashi K, Ishizawa T, Akamatsu N, Kaneko J, Arita J, Kokudo N, Hasegawa K. Indocyanine green administration a day before surgery may increase bile duct detectability on fluorescence cholangiography during laparoscopic cholecystectomy. J Hepatobiliary Pancreat Sci. 2021;28:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Huang Y, Chen Q, Kuang J, Zhang S, Weng J, Lai Y, Liu H, Wu Z, Huang D, Lin F, Zhu G, Cao T, Gu W. Real-time fluorescent cholangiography with indocyanine green in laparoscopic cholecystectomy: a randomized controlled trial to establish the optimal indocyanine green dose within 30 min preoperatively. Surg Today. 2023;53:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Dasari BV, Tan CJ, Gurusamy KS, Martin DJ, Kirk G, McKie L, Diamond T, Taylor MA. Surgical versus endoscopic treatment of bile duct stones. Cochrane Database Syst Rev. 2013;CD003327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Choe JW, Kim SY, Lee DW, Hyun JJ, Ahn KR, Yoon I, Jung SW, Jung YK, Koo JS, Yim HJ, Lee SW. Incidence and risk factors for postoperative common bile duct stones in patients undergoing endoscopic extraction and subsequent cholecystectomy. Gastrointest Endosc. 2021;93:608-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | De Silva HM, Howard T, Yong T, Hodgson R. Comparing Stone Recurrence Following Surgical Common Bile Duct Exploration or Endoscopic Stone Extraction for Patients with Common Bile Duct Stones. J Laparoendosc Adv Surg Tech A. 2023;33:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 30. | Fujita N, Yasuda I, Endo I, Isayama H, Iwashita T, Ueki T, Uemura K, Umezawa A, Katanuma A, Katayose Y, Suzuki Y, Shoda J, Tsuyuguchi T, Wakai T, Inui K, Unno M, Takeyama Y, Itoi T, Koike K, Mochida S. Evidence-based clinical practice guidelines for cholelithiasis 2021. J Gastroenterol. 2023;58:801-833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 51] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 31. | Cassinotti E, Al-Taher M, Antoniou SA, Arezzo A, Baldari L, Boni L, Bonino MA, Bouvy ND, Brodie R, Carus T, Chand M, Diana M, Eussen MMM, Francis N, Guida A, Gontero P, Haney CM, Jansen M, Mintz Y, Morales-Conde S, Muller-Stich BP, Nakajima K, Nickel F, Oderda M, Parise P, Rosati R, Schijven MP, Silecchia G, Soares AS, Urakawa S, Vettoretto N. European Association for Endoscopic Surgery (EAES) consensus on Indocyanine Green (ICG) fluorescence-guided surgery. Surg Endosc. 2023;37:1629-1648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 53] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 32. | Yu M, Xue H, Shen Q, Zhang X, Li K, Jia M, Jia J, Xu J. Primary Closure Following Laparoscopic Common Bile Duct Exploration Combined with Intraoperative Choledochoscopy and D-J Tube Drainage for Treating Choledocholithiasis. Med Sci Monit. 2017;23:4500-4505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Zou Q, Ding Y, Li CS, Yang XP. A randomized controlled trial of emergency LCBDE + LC and ERCP + LC in the treatment of choledocholithiasis with acute cholangitis. Wideochir Inne Tech Maloinwazyjne. 2022;17:156-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 34. | Zhu JG, Wu S, Feng Q, Li F, Han W, Xiu D, Tan H, Fu J, Li X, Shang D, Liu H, Li B, Yang L, Kong Y, Zhan S, Guo W, Zhang ZT. Protocol for the CREST Choles (Chinese REgistry Study on Treatment of Cholecysto-Choledocholithiasis) study: an ambispective, multicenter, observational, open-cohort study. BMJ Open. 2019;9:e030293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |