Published online Jan 27, 2025. doi: 10.4240/wjgs.v17.i1.98891
Revised: September 26, 2024
Accepted: November 18, 2024
Published online: January 27, 2025
Processing time: 171 Days and 23.6 Hours
Cystic lymphangioma is a rare hamartoma that is especially found in the adult gastrointestinal tract. In the early stage, most patients are asymptomatic; after the onset of symptoms, there is often no specificity regarding symptoms.
Here we report the endoscopic diagnosis and treatment of an adult patient with cystic lymphangioma of the ascending colon. One patient who came to our hospital with “dull pain in the left lower abdomen for 2 days” was initially misdiagnosed with a colon cyst according to endoscopy and then underwent endoscopic submucosal dissection. The final pathological results suggested cystic lymphangioma. One year later, no recurrence was found on re-examination via colonoscopy.
Cystic lymphangioma in the gastrointestinal tract rarely occurs in adults and is easily misdiagnosed or missed. Endoscopy, imaging, histology, and immunohistochemical staining are useful for diagnosis. Surgical resection is the preferred treatment.
Core Tip: We report an endoscopic diagnosis and treatment of cystic lymphangioma of the ascending colon in adults. Based on a literature review, we summarize the aetiology, symptoms, diagnosis, treatment, and prognosis of cystic lymphangioma. Because the lesion is located in the intestinal cavity, it is extremely rare, providing additional information and reference for future diagnosis and treatment of such cases.
- Citation: Qu LW, Li QX, Zhu WY, Kang M. Endoscopic submucosal dissection in the treatment of adult cystic lymphangioma: A case report. World J Gastrointest Surg 2025; 17(1): 98891
- URL: https://www.wjgnet.com/1948-9366/full/v17/i1/98891.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i1.98891
Cystic lymphangioma is a rare benign congenital lymphoid tissue malformation that develops during the embryonic period. It is also known as a hydrocystoma. Cystic lymphangioma is not a true tumour but a hamartoma. It is more common in children, nearly 80%-90% of cystic lymphangiomas occur in 2-year-old children, and they are commonly found in children’s heads, necks, and armpits[1]. Cystic lymphangiomas in the gastrointestinal tract are extremely rare, especially in adults. This article reports a case of cystic lymphangioma of the ascending colon in an adult diagnosed after endoscopic submucosal dissection resection.
A 45-year-old woman presented with “dull pain in the left lower abdomen for 2 days”.
Colonoscopy at the local hospital revealed an “uplifting lesion of the ascending colon”, and the patient was admitted to our hospital for further diagnosis and treatment.
The patient denied a previous medical history.
Her father and sister had a history of ulcerative colitis.
The physical examination revealed no positive signs.
No significant abnormal results were found in laboratory tests.
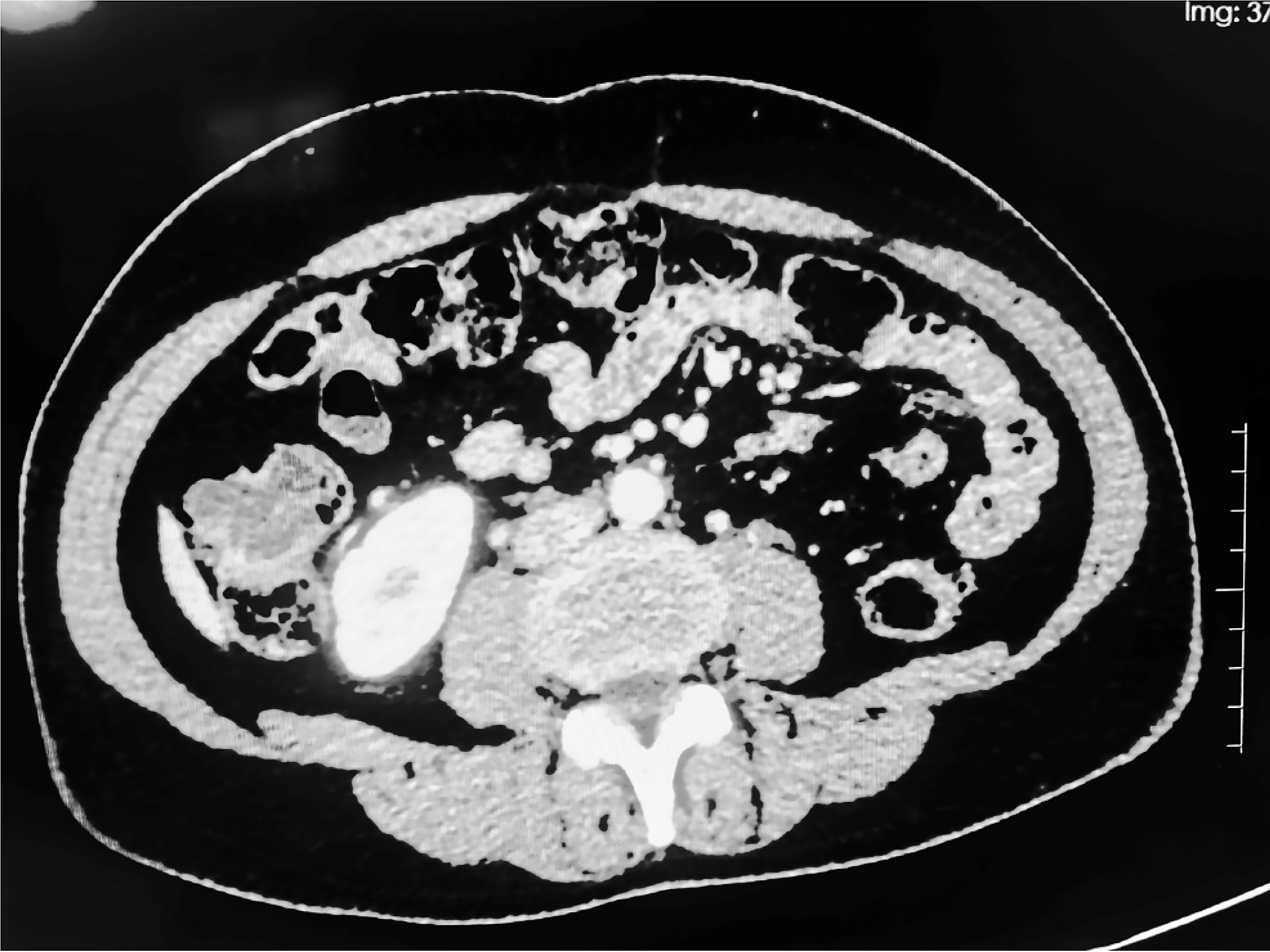
Electronic colonoscopy revealed an obvious bulge near the ascending colonic lumen, which occupied the entire lumen. The surface was dumbbell shaped, and the wall was translucent. The preliminary diagnosis was a large cyst of the colon. Computed tomography (CT) revealed localized cystic fluid low-density shadows in the ascending colon with irregular shapes and no obvious enhancement, with a maximum plane size of approximately 2.6 cm × 2.1 cm (Figure 1).
Based on the patient’s medical history and the findings of pathological and immunohistochemical examinations, the final diagnosis was cystic lymphangioma.
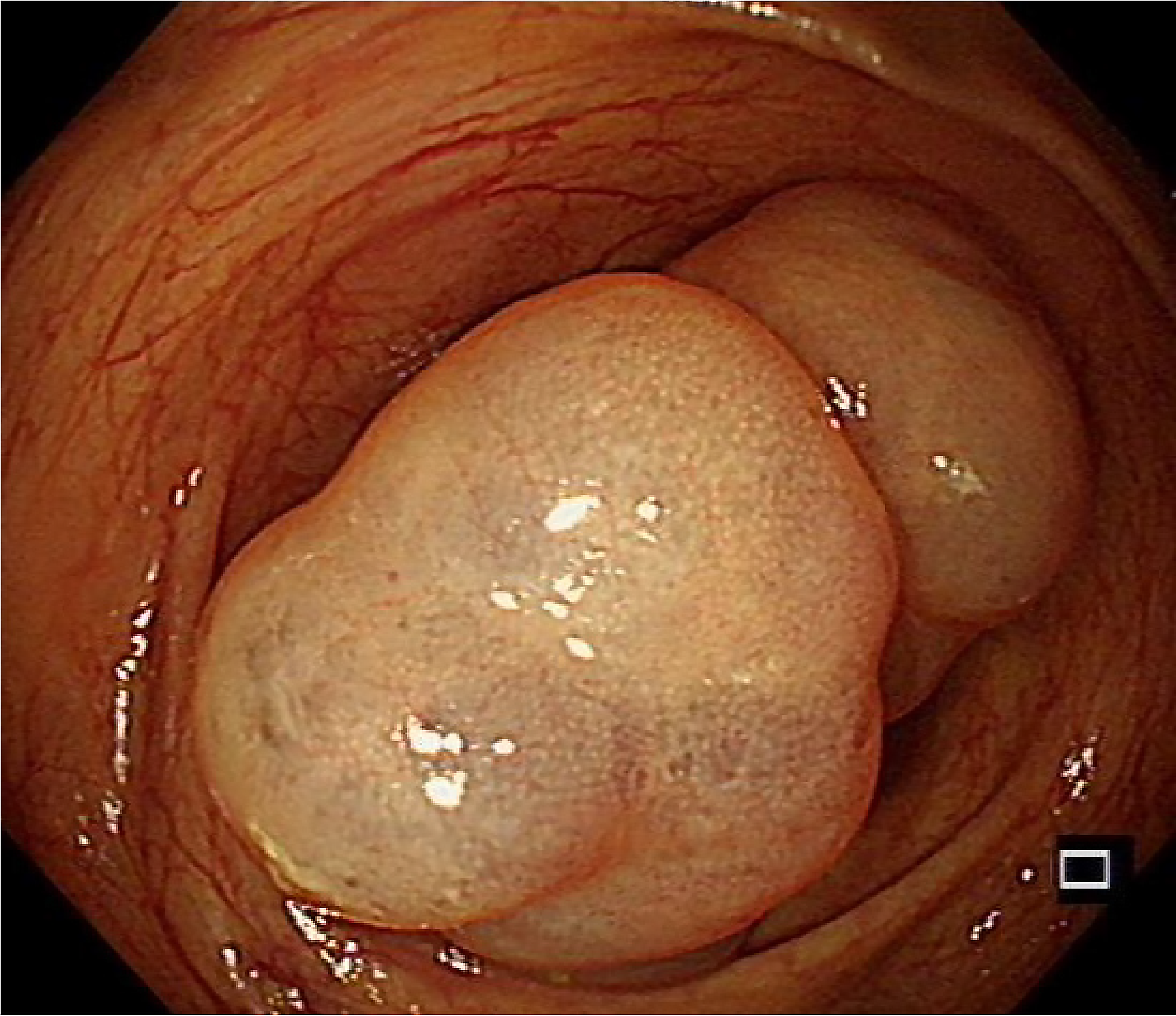
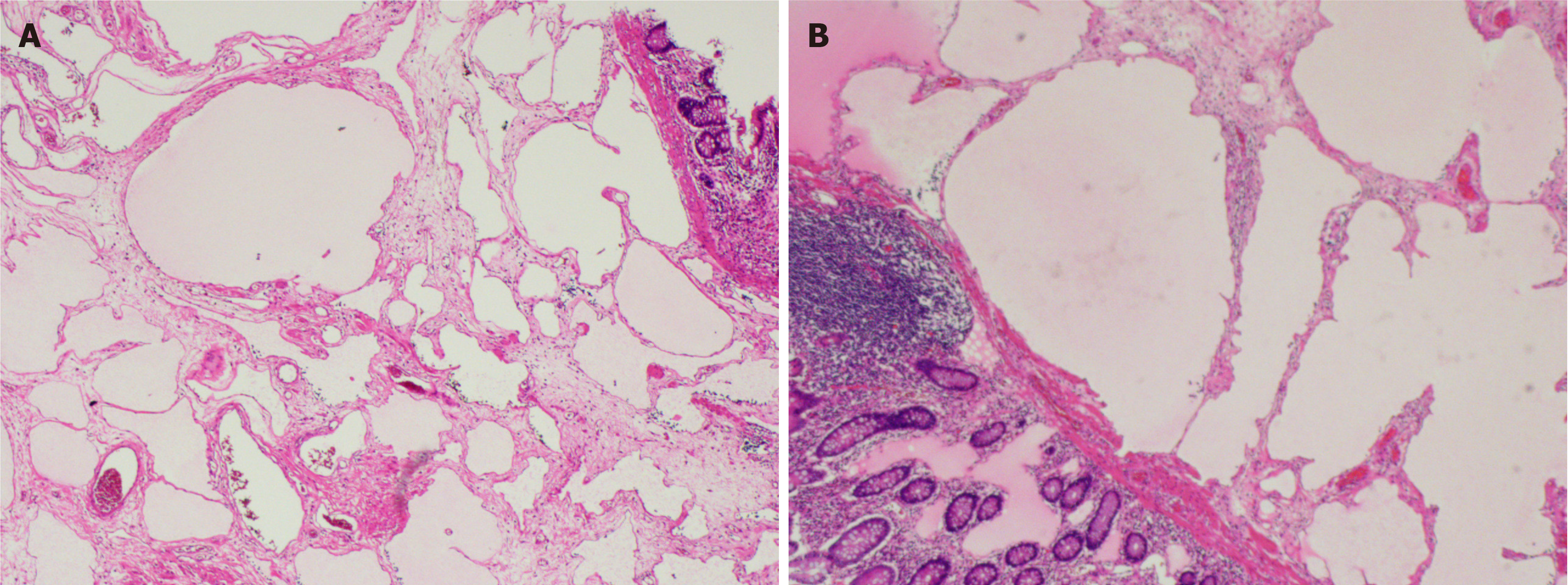
Endoscopic submucosal dissection was performed after excluding contraindications to surgery. Intraoperatively, there were two translucent lesions connected at the ascending colon (Figure 2). The overall size was approximately 3.5 cm × 3.0 cm, the surface mucosa was smooth, and there were mucosal bridges at the base. The mucosa and submucosa were incised intraoperatively, and submucosal dissection was performed at the incision site (Figure 3A-C). After complete resection, the lesion was sent for histopathology examination and the results indicated (Figure 4) that the size of the lesion was 3.2 cm × 2.6 cm × 1.3 cm and the section was a grey and white solid gel. Multiple lymphatic vessels of different sizes could be seen under a microscope, and the lining was lined with flat endothelial cells, which was consistent with cystic lymphangioma.
One year later, the patient was re-examined via colonoscopy and no recurrence was observed (Figure 3D).
Lymphangioma is classified by Wegner into three types: Cystic lymphangioma, capillary lymphangioma and cavernous lymphangioma[2]. Gangl et al[3] concluded through a literature review that gastrointestinal lymphangioma most frequently occurs in the colon, followed by the rectum and duodenum, and it rarely occurs in the oesophagus and stomach. Cystic lymphangioma is caused mainly by congenitally abnormal development of lymphatic vessels, which obstructs lymphatic drainage, and abnormal expansion of the lymphatic lumen leads to tumour-like enlargement of lymphatic vessels, which explains why it is more common in children and can also be caused by abdominal surgery, abdominal trauma, inflammation, lymphatic obstruction and other reasons. Studies have also shown that excessive production of bidirectional regulatory proteins by fibroblasts and lymphoendothelial cells around lymphatic vessels can cause cystic lymphangioma[4]. Cystic lymphangioma is a benign lesion with slow growth, low tension and certain morphological plasticity of early cysts, most of which have no symptoms. When the cyst is large, accompanying symptoms of an abdominal mass and mass compression may occur, or corresponding accompanying symptoms such as fever, abdominal pain and intestinal obstruction may occur due to cyst infection, bleeding, torsion and rupture. Additionally, the clinical symptoms are nonspecific[5].
Diagnostically, Romeo et al[6] suggested that appropriate diagnostic imaging protocols for patients with cystic lymphangioma should first include an American study of continuous magnetic resonance imaging (MRI) scans with contrast media to further characterize the lesion. MRI revealed low signal intensity on T1-weighted images and high signal intensity on T2-weighted images, in which the lesion wall was thin and presented with multiple strip-separated shadows, and mild separation enhancement was found after contrast agent injection[7]. The CT findings of gastrointestinal lymphangioma are similar: A low-attenuation oval mass with clear boundaries can be seen under the mucosa, the cyst is not enhanced after enhancement, and the cyst wall and septa may not be enhanced or may be enhanced only slightly[8]. When the cyst contains liquid contents, the CT value is similar to that of water when the cyst fluid is serous or chylous fluid, and the CT value can be negative when a lipid component is present. If the cyst contents are combined with bleeding or infection, the density of the cyst contents can be increased. Because MRI has higher contrast resolution than CT, MRI can better evaluate the internal lesion wall thickness and lesion content. However, in emergency situations, CT scans play a central role in diagnosis.
Cystic lymphangioma under endoscopy is characterized by a steep rise at the edge of the tumour and a slightly narrow base, which can cover the normal colon mucosa. It is smoother, glossier and more transparent than adenomas. Changes in shape due to changes in position or pressure are called the “pad sign”[9]. Endoscopic ultrasound can have value in the diagnosis of this disease. The lesion site is usually located in the uniform anechoic zone of the second to third layers and sometimes presents as a multilocular anechoic zone without separation[10,11]. Endoscopic ultrasound can identify the origin layer, echo intensity, presence or absence of a septum, and integrity of the submucosa. This helps to distinguish it from other submucosal masses (such as haemangiomas, lipomas, stromal tumours, leiomyomas, and schwannomas). Haemangioma and lipoma, in particular, share common characteristics with lymphangioma and are classified into type C (broad base sessile and lobed surface) and type D (pedicled, smooth, or granular surface). Under endoscopy, haemangiomas are full of blood. When cystic lymphangioma is secondary to haemorrhage, it is difficult to distinguish it from a haemangioma[12]. Moreover, attention should be given to differentiating these lesions from colon repeat cysts, benign polycystic mesothelioma, lymphangiectasia, and intestinal cysts. Ultrasonography of colon repeat cysts revealed that the cyst wall had an internal hyperechoic edge corresponding to the mucosa and submucosa and an external hypoechoic edge corresponding to the smooth muscle layer, which was called the “double wall” sign[13]. Intestinal cysts lack recognizable cyst walls on CT and MRI, and ultrasonography reveals hypoechoic cystic masses with occasional thin septa[14].
In terms of differential diagnosis, compared with CT, MRI can better distinguish these masses from other cyst-like masses before surgery; for example, there are different components (bleeding, protein, and myxoid substances) in the T2-weighted image sequence of the liquid plane[15,16]. In addition, for cyst-like lesions containing a certain amount of fat, such as dermoid cysts, MRI can easily identify dermoid cysts with high signals on both T1- and T2-weighted images[17]. The imaging value of cystic lymphangioma lies in the exclusion of malignant tumours and the accurate anatomic location of the tumour before surgery. These imaging methods have difficulty accurately preoperatively diagnosing cystic lymphangiomas and generally require postoperative pathology to confirm the diagnosis. Histopathologically, cystic lymphangiomas contain large lymphatic cysts with thin walls and flat endothelial cells. The cysts are often multilocular and contain pale yellow watery fluid, and they sometimes contain smooth and collagen bundles. In contrast, patients with lymphangiectasia usually lack an intact endothelial lining or a smooth muscle component[18]. Histologically, the walls of intestinal cysts lack muscular layers[14]. For atypical histology, there is an immunohistochemical response to the lymphatic endothelial markers D2-40 and vascular markers (CD31-, CD34-, viii-associated antigens and vascular endothelial growth factor receptor 3). Cystic lymphangioma has no immune response to calciotretinoin, which can rule out benign polycystic mesothelioma[19,20]. However, Bhutani et al[12] reported that cystic lymphangioma of the colon can be diagnosed only through classical endoscopic ultrasonographic manifestations (submucosal echoless and multiple septal cystic spaces).
Endoscopy has become an important means of diagnosing and treating gastrointestinal mucosal and submucosal masses and can be used to identify more asymptomatic masses. Surgical resection is the preferred treatment measure. Pedicled or semipedicled tumours with a maximum diameter of 2 cm or smaller can be completely resected under endoscopy, and for large tumours, partial resection or aspiration can be performed[9]. To date, the scope of endoscopic resection of lesions described in the literature is 2-3.5 cm[21]. Endoscopic therapy is characterized by less trauma, less pain, and faster recovery. In this case, the tumour was located in the submucosa and was completely removed via endoscopic submucosal dissection. Most patients with lymphangioma have a good prognosis after surgical resection, but when there is tumour bleeding and infection, the prognosis is poor if the treatment is not timely[22]. Only a single case was reported in this study, which has limitations. To further understand adult gastrointestinal lymphangiomas, especially the clinical manifestations and treatment outcomes of different cases, future studies should focus on larger case series or multicentre studies.
Cystic lymphangioma has an insidious onset and is rarely observed in the clinic. It has a relatively high incidence in children and has no typical symptoms. It is easy to misdiagnose or miss. Surgical excision is essential for the prevention of complications, histological diagnosis, and definite exclusion of malignancies. The aim of this case report and literature review was to improve endoscopists’ understanding of this disease and its diagnostic accuracy.
| 1. | Chen CW, Hsu SD, Lin CH, Cheng MF, Yu JC. Cystic lymphangioma of the jejunal mesentery in an adult: a case report. World J Gastroenterol. 2005;11:5084-5086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Roisman I, Manny J, Fields S, Shiloni E. Intra-abdominal lymphangioma. Br J Surg. 1989;76:485-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 128] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Gangl A, Polterauer P, Krepler R, Kumpan W. A further case of submucosal lymphangioma of the duodenum diagnosed during endoscopy. Endoscopy. 1980;12:188-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Yoshida N, Yamamoto S, Hamashima T, Okuno N, Okita N, Horikawa S, Hayashi M, Dang TC, Nguyen QL, Nishiyama K, Makino T, Ishii Y, Tomihara K, Shimizu T, Shibuya M, Noguchi M, Sasahara M. Dysregulation of Amphiregulin stimulates the pathogenesis of cystic lymphangioma. Proc Natl Acad Sci U S A. 2021;118:e2019580118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Bhavsar T, Saeed-Vafa D, Harbison S, Inniss S. Retroperitoneal cystic lymphangioma in an adult: A case report and review of the literature. World J Gastrointest Pathophysiol. 2010;1:171-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Romeo V, Maurea S, Mainenti PP, Camera L, Aprea G, Cozzolino I, Salvatore M. Correlative imaging of cystic lymphangiomas: ultrasound, CT and MRI comparison. Acta Radiol Open. 2015;4:2047981614564911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Choi WJ, Jeong WK, Kim Y, Kim J, Pyo JY, Oh YH. MR imaging of hepatic lymphangioma. Korean J Hepatol. 2012;18:101-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Zhu H, Wu ZY, Lin XZ, Shi B, Upadhyaya M, Chen K. Gastrointestinal tract lymphangiomas: findings at CT and endoscopic imaging with histopathologic correlation. Abdom Imaging. 2008;33:662-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Matsuda T, Matsutani T, Tsuchiya Y, Okihama Y, Egami K, Yoshioka M, Maeda S, Onda M. A clinical evaluation of lymphangioma of the large intestine: a case presentation of lymphangioma of the descending colon and a review of 279 Japanese cases. J Nippon Med Sch. 2001;68:262-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Hizawa K, Aoyagi K, Kurahara K, Suekane H, Kuwano Y, Nakamura S, Fujishima M. Gastrointestinal lymphangioma: endosonographic demonstration and endoscopic removal. Gastrointest Endosc. 1996;43:620-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Ishikawa N, Fuchigami T, Kikuchi Y, Kobayashi H, Sakai Y, Nakanishi M, Matsumoto T. EUS for gastric lymphangioma. Gastrointest Endosc. 2000;52:798-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Bhutani MS, Annangi S, Koduru P, Aggarwal A, Suzuki R. Diagnosis of cystic lymphangioma of the colon by endoscopic ultrasound: Biopsy is not needed! Endosc Ultrasound. 2016;5:335-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Gandhi D, Garg T, Shah J, Sawhney H, Crowder BJ, Nagar A. Gastrointestinal duplication cysts: what a radiologist needs to know. Abdom Radiol (NY). 2022;47:13-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 14. | Leland HA, Lee JT, Tan JH, Romine LE, Bansal V. Cystic lymphangioma of the lesser curvature of the stomach--case report. J Radiol Case Rep. 2011;5:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 15. | Jeung MY, Gasser B, Gangi A, Bogorin A, Charneau D, Wihlm JM, Dietemann JL, Roy C. Imaging of cystic masses of the mediastinum. Radiographics. 2002;22 Spec No:S79-S93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 233] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 16. | Yang DM, Jung DH, Kim H, Kang JH, Kim SH, Kim JH, Hwang HY. Retroperitoneal cystic masses: CT, clinical, and pathologic findings and literature review. Radiographics. 2004;24:1353-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 241] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 17. | Yoo E, Kim MJ, Kim KW, Chung JJ, Kim SH, Choi JY. A case of mesenteric cystic lymphangioma: fat saturation and chemical shift MR imaging. J Magn Reson Imaging. 2006;23:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Lawless ME, Lloyd KA, Swanson PE, Upton MP, Yeh MM. Lymphangiomatous Lesions of the Gastrointestinal Tract: A Clinicopathologic Study and Comparison Between Adults and Children. Am J Clin Pathol. 2015;144:563-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Mehmedovic Z, Mehmedovic M, Custovic MK, Sadikovic A, Mekic N. A rare case of giant mesenteric cystic lymphangioma of the small bowel in an adult: A case presentation and literature review. Acta Gastroenterol Belg. 2016;79:491-493. [PubMed] |
| 20. | Suthiwartnarueput W, Kiatipunsodsai S, Kwankua A, Chaumrattanakul U. Lymphangioma of the small bowel mesentery: a case report and review of the literature. World J Gastroenterol. 2012;18:6328-6332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Badipatla KR, Chandrala C, Ayyadurai P, Biyyam M, Sapkota B, Niazi M, Nayudu SK. Cystic Lymphangioma of the Colon: Endoscopic Removal beyond the Frontiers of Size. Case Rep Gastroenterol. 2017;11:178-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Lepre L, Costa G, Baldini D, Cortese F, Saputelli A, Gioffre A, Fransvea P. Emergency presentation of cystic lymphangioma of the colon: A case report and literature review. Int J Surg Case Rep. 2016;24:162-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |