Published online Jan 27, 2025. doi: 10.4240/wjgs.v17.i1.98283
Revised: October 3, 2024
Accepted: November 8, 2024
Published online: January 27, 2025
Processing time: 187 Days and 4.4 Hours
Hepatobiliary stone disease involves an intrahepatic bile duct stone that occurs above the confluence of the right and left hepatic ducts. One-step percutaneous transhepatic cholangioscopic lithotripsy (PTCSL) using the percutaneous trans
To explore the value of DynaCT biliary soft tissue reconstruction technology for the diagnosis and treatment of hepatolithiasis with bile duct stenosis, and to assess the feasibility and effectiveness of the PTOBF technique guided by DynaCT biliary soft tissue reconstruction technology.
The clinical data of 140 patients with complex biliary stenosis disease combined with bile duct stenosis who received PTOBF and were admitted to the First Affiliated Hospital of Guangzhou Medical University from January 2020 to December 2024 were collected. The patients were divided into two groups: DynaCT-PTOBF group (70 patients) and conventional PTOBF group (70 patients). These groups were compared in terms of the preoperative bile duct stenosis, location of the liver segment where the stone was located, intraoperative operative time, im
DynaCT biliary soft tissue reconstruction technology was successfully performed in 70 patients. The DynaCT-PTOBF group had a higher detection rate of target bile ducts where bile duct stones and biliary strictures were located than the PTOBF group. Compared with the PTOBF group, the DynaCT-PTOBF group was characterized by a significantly greater immediate stone removal rate (68.6% vs 50.0%, P = 0.025), greater immediate stenosis dilatation success rate (72.9% vs 55.7%, P = 0.034), greater final stenosis release rate (91.4% vs 75.7%, P = 0.012), shorter duration of intraoperative hemorrhage (3.14 ± 2.00 vs 26.5 ± 52.1, P = 0.039), and lower incidence of distant cholangitis (2.9% vs 11.4%, P = 0.49). There were no significant differences between the two groups in terms of the final stone removal rate, reoperation rate, or long-term complication incidence rate.
DynaCT biliary soft tissue reconstruction technology guiding the PTOBF technique in patients with hepatolithiasis with bile duct stenosis is feasible and accurate. It may be beneficial for optimizing the preoperative evaluation of the PTOBF technique.
Core Tip: DynaCT was used for the first time in patients with hepatolithiasis with biliary stenosis. Compared with computed tomography, DynaCT for biliary reconstruction results in higher-quality, three-dimensional biliary, blood vessel, and liver images. Based on DynaCT biliary model, one-step percutaneous transhepatic cholangioscopic lithotripsy has the potential to improve the stone clearance rate, shorten the stone clearance time, and reduce the reoperation rate.
- Citation: Ye YQ, Li PH, Ding ZW, Zhang SF, Li RQ, Cao YW. Application of DynaCT biliary soft tissue reconstruction technology in diagnosis and treatment of hepatolithiasis. World J Gastrointest Surg 2025; 17(1): 98283
- URL: https://www.wjgnet.com/1948-9366/full/v17/i1/98283.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i1.98283
Hepatobiliary stone disease involves an intrahepatic bile duct stone that occurs above the confluence of the right and left hepatic ducts. It is a common benign biliary disease in hepatobiliary surgery and is the main cause of death from nonneoplastic biliary diseases. It is highly prevalent in the Southwest, East Coast, Hong Kong, and Taiwan in China, with a prevalence of up to 6.1%[1,2]. An average of 24.3% of patients with hepatic bile duct stone disease have hepatic bile duct stenosis[3,4]. One-step percutaneous transhepatic cholangioscopic lithotripsy (PTCSL) using the percutaneous trans
Ultrasound is the preferred method for treating hepatolithiasis[5]; however, it is not effective in providing an overall picture of the biliary tree. Enhanced abdominal computed tomography (CT) has excellent resolution in the bile duct, hepatic arteries, and portal veins. Moreover, it can display high-density stones clearly, but this technology is not effective for showing structures such as negative calculi, small sediment-like stones, and bile duct strictures[6,7]. Magnetic resonance cholangiopancreatography (MRCP) is the most sensitive noninvasive method for visualizing the biliary tree and bile duct calculi[8,9]. However, it has several limitations. For example, previous studies have shown that its sensitivity in the diagnosis of small stones (≤ 3 mm) is only 64%[10]. Koshinaga et al[11] missed calculi in 29% of patients with small stones (3-5 mm). Moreover, MRCP cannot visualize the biliary tree in the presence of abnormal biliary pathology. In addition, blood, proteinaceous material, or air in the bile duct can mimic a stone, and sludge can simulate a stenosis[12]. T-tube cholangiography can show subtle lesions in the bile ducts clearly and detect small stones, but it provides two-dimensional images that are poor at visualizing bile duct stones and their adjacent spatial structures[13]. The lack of accurate imaging tools to guide percutaneous transhepatic cholangioscopic lithotripsy procedures is a majorly influential factor.
To address these challenges, our center introduced an innovative approach involving the use of DynaCT for biliary reconstruction and the successful use of DynaCT biliary soft tissue reconstruction technology images for the preoperative assessment and treatment of hepatolithiasis[14]. DynaCT is a type of cone beam CT (CBCT) attached to the Artis Zee DSA system produced by the SIEMENS Company in Germany, which can realize synchronized acquisition with a flat-panel detector during C-arm rotation[15]. This application of DynaCT biliary soft tissue reconstruction technology enables us to obtain optimal images of the bile duct and stones. First, DynaCT biliary soft tissue reconstruction technology has the ability to enhance three-dimensional (3D) and anatomy-assisted visualizations of the bile duct, which can increase the knowledge of the biliary anatomy, morphology, size, and distribution of the calculus. Second, it can generate more accurate structures of the vascular, liver, and biliary systems, which allows the surgeon to plan the surgery accordingly and tailor the procedure for each individual patient.
The aim of this study was to evaluate the effectiveness and clinical value of DynaCT biliary soft tissue reconstruction technology in the diagnosis and treatment of hepatolithiasis with bile duct stenosis.
At our institution, all patients with suspected hepatolithiasis undergo a standardized diagnostic work-up according to the current guidelines, and patients with a confirmed diagnosis of hepatolithiasis are treated by one-step PTCSL. This retrospective study included 140 patients with hepatolithiasis from January 2020 to December 2024. Among these patients, those undergoing PTOBF served as the control group, which included 70 patients whose procedures were guided by ultrasound. Their relevant surgical index data were recorded. Patients undergoing DynaCT-PTOBF served as the experimental group, which also included 70 patients. Intrahepatic stones were observed, and their locations were recorded by DynaCT biliary soft tissue reconstruction technology, which guided one-step PTCSL. Similarly to the PTOBF group, their relevant surgical index data were recorded. All procedures were completed successfully without complications, such as biliary leakage, biliary bleeding, or death.
Patients were included if they: (1) Were in a state with a biliary drain; (2) Had confirmed hepatic bile duct stones and needed to receive PTOBF; and (3) Were able to hold their breath for more than 6 s.
Patients were excluded if they: (1) Had a history of liver transplant; (2) Had poor basal vital signs or were unable to hold their breath for more than 6 s; (3) Had an allergy to iodine contrast agents; or (4) Had a mental disorder or coagulation disorder.
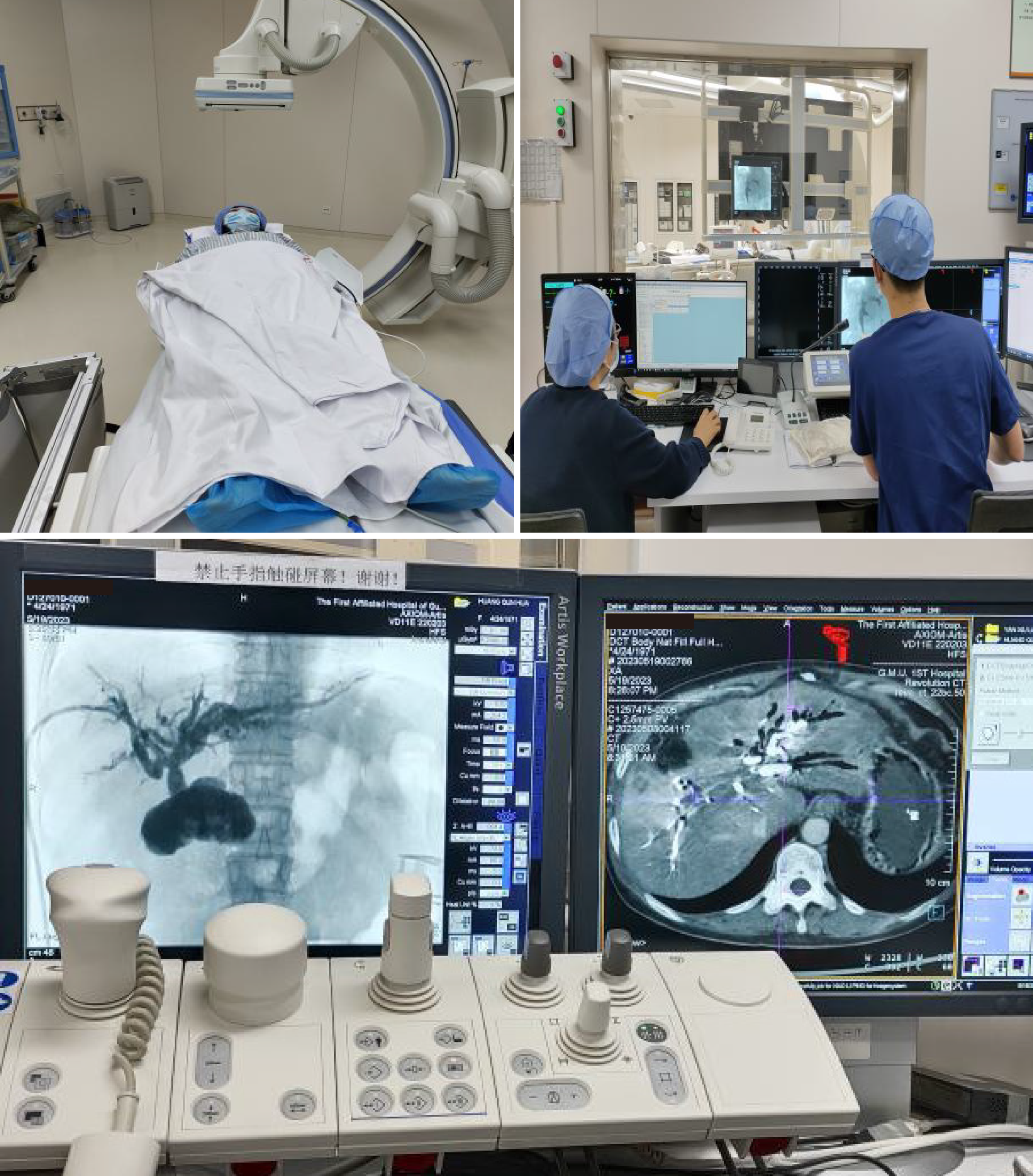
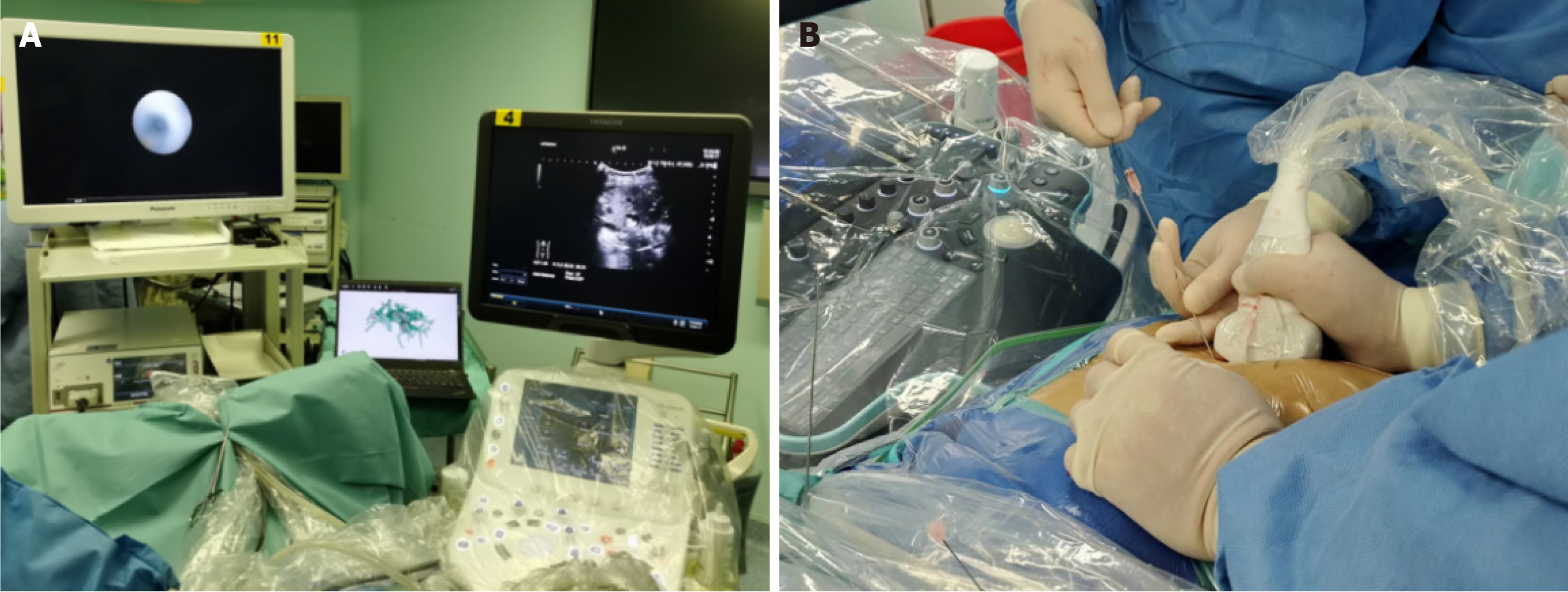
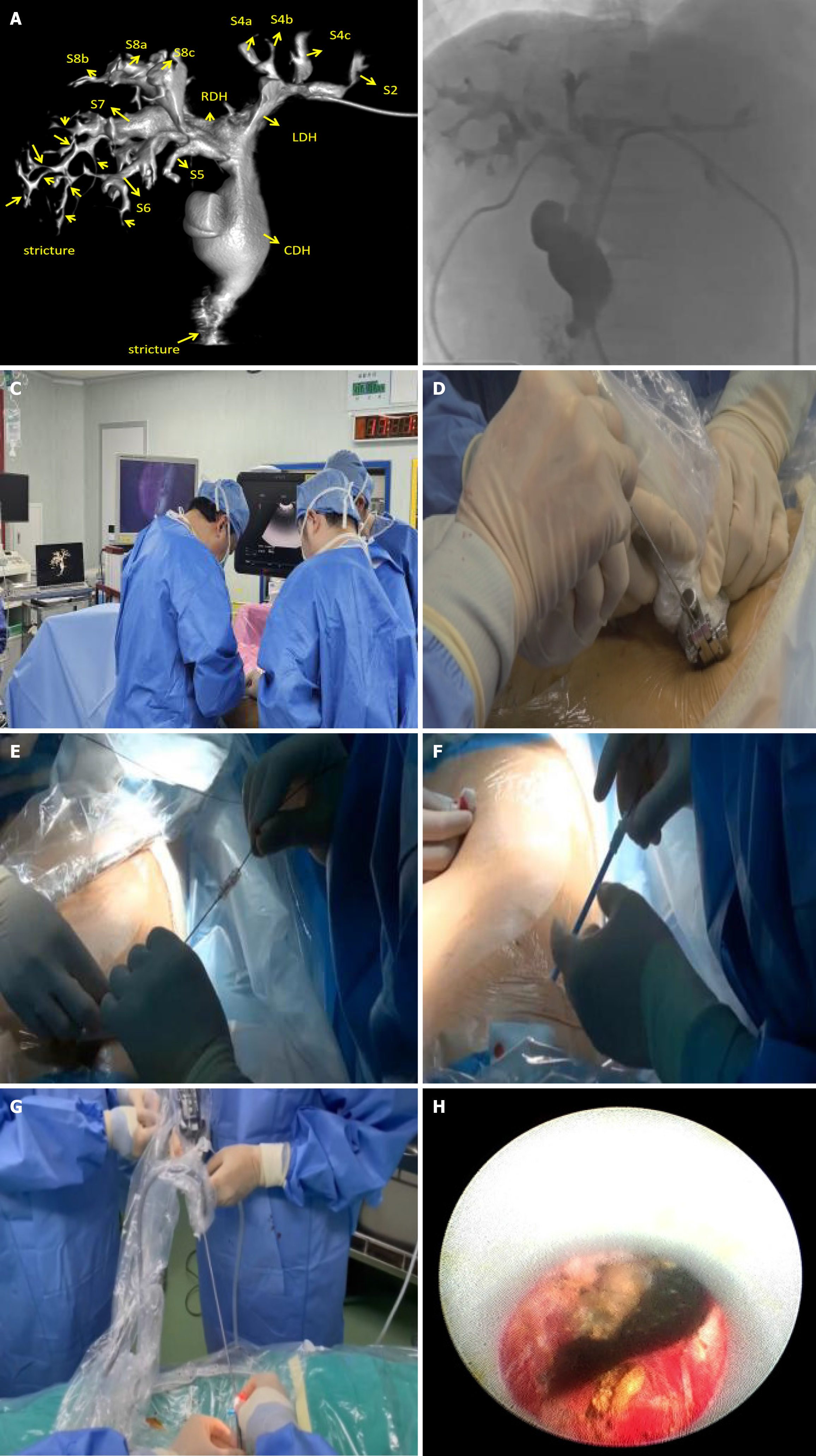
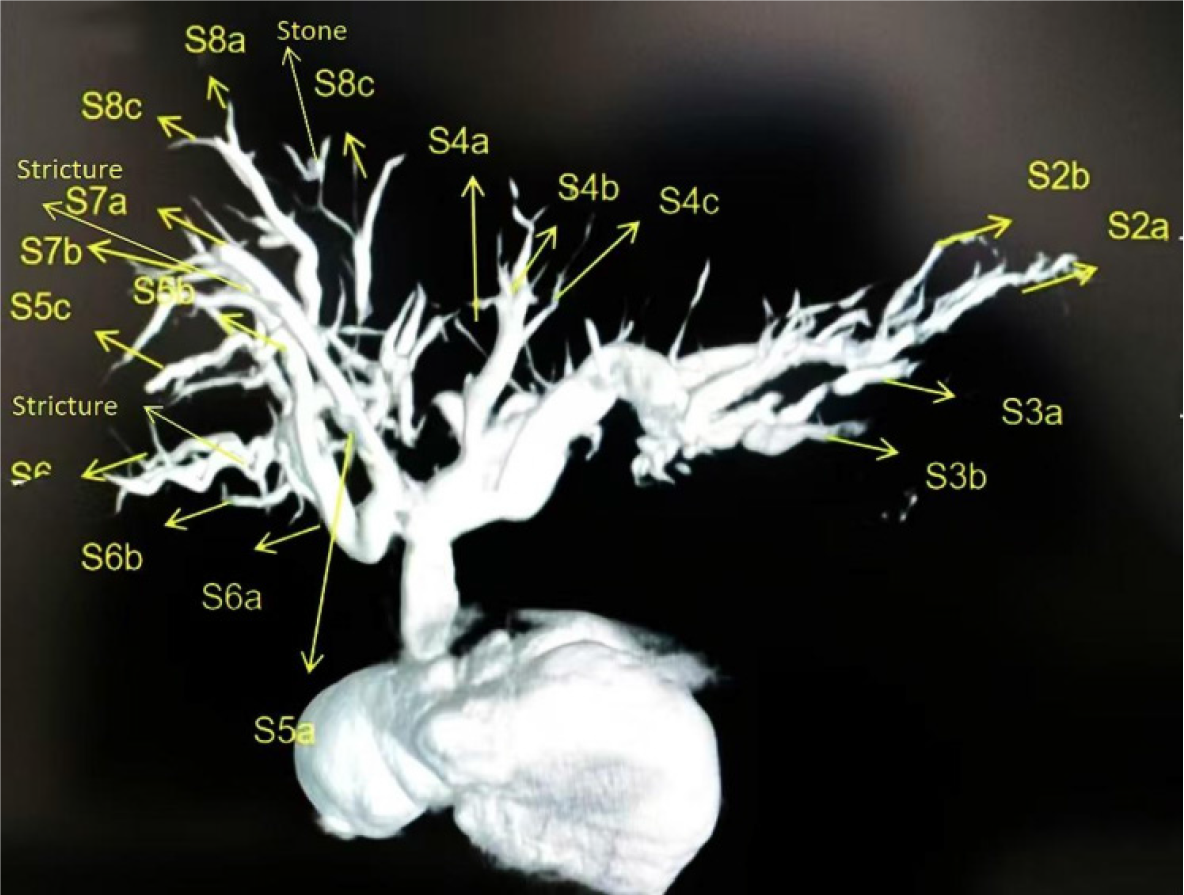
The DynaCT-PTOBF procedure consisted of: (1) DynaCT biliary soft tissue reconstruction: A C-arm CT machine was used to scan each patient, who was instructed to hold their breath for more than 6 s while laying flat. The digital subtraction angiography system used was the SIEMENS Artis Zee III ceiling, and the scanning sequence was the 6 s DCT Body. High-pressure syringes were used to manually inject a contrast agent (Visipaque®, 320 mg/mL, GE Healthcare, 2:3 ratio with saline) at a flow rate of 1 to 2 mL/s. The scanning time was 6 s, and the pressure limit was 100 psi. Finally, the 3D biliary system was reconstructed via multiplanar reconstruction (MPR) and volumetric reconstruction (VR) software systems (Figures 1 and 2); and (2) DynaCT-guided PTOBF: Based on a DynaCT biliary reconstruction model, ultrasound and other imaging examinations were used to determine the location and degree of the target bile duct stenosis and stones. Biliary puncture was performed with an 18-G needle and a 0.035-inch hydrophilic guidewire. Fistula dilation was completed via fascial dilators from 8 Fr to 14 Fr sequentially. Sheath insertion was done with a 14-Fr sheath. Fragmentation and clearance were performed with a 12-Fr rigid nephroscope. A 14-Fr drainage tube was employed (Figure 3).
SPSS 21.0 software was used to process the data, and data that conformed to a normal distribution are expressed as the mean ± SD; those that did not conform to a normal distribution are expressed as medians and interquartile ranges (25th and 75th percentiles), and categorical data are expressed as cases. Each parameter in the two study groups was compared via the t-test or the Wilcoxon rank-sum test. A P value < 0.05 was considered statistically significant.
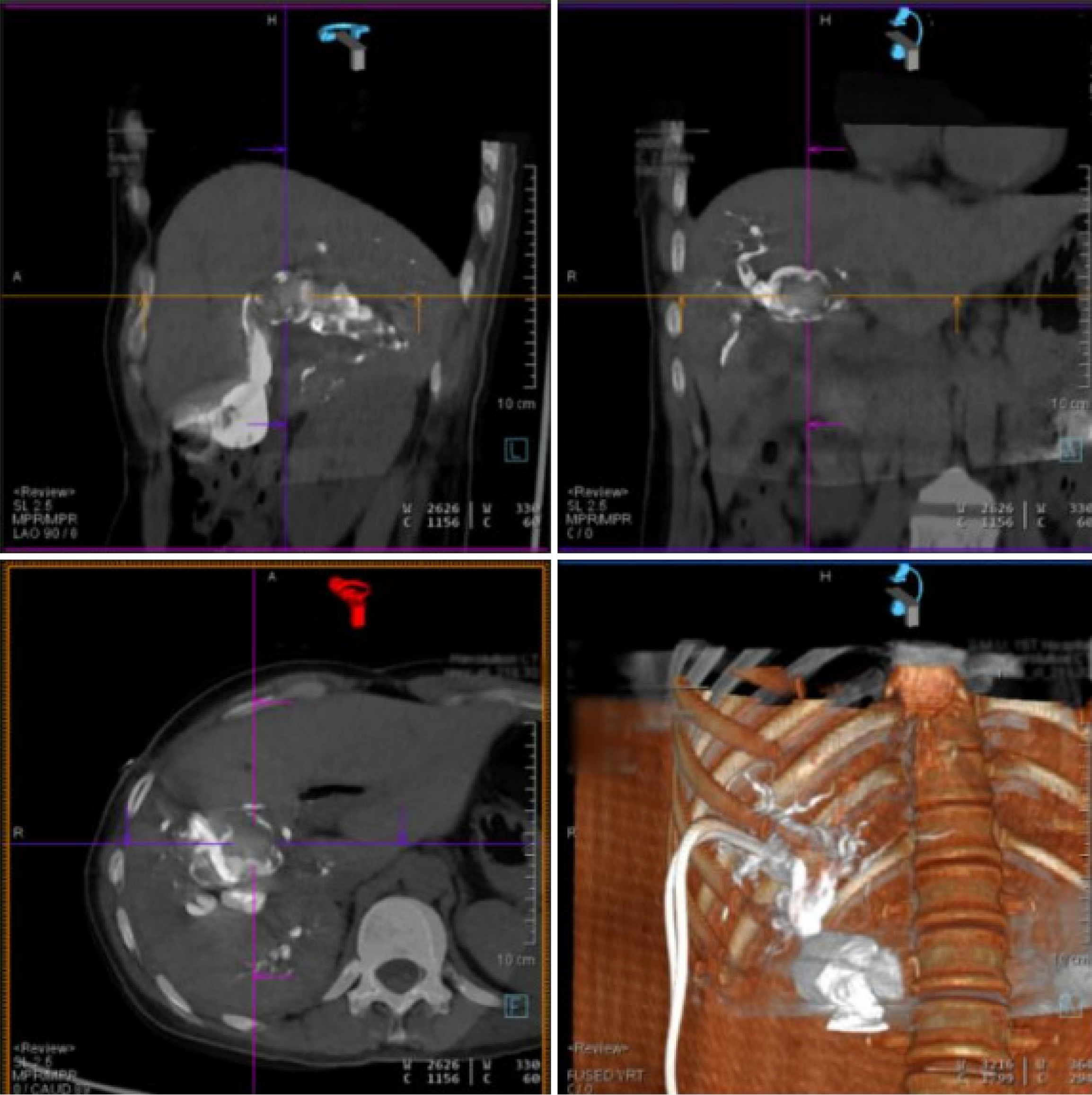
DynaCT allows a wider scanning area in a shorter period of time and generates higher-quality images of the biliary tract. The common hepatic duct, the right and left hepatic ducts, and the lobular bile ducts are easily visualized, and the terminal branching bile ducts can even be reconstructed. The width of the bile duct at the stenosis and the diameter of the dilatation before and after the stenosis can be measured accurately with DynaCT's VR and MPR software systems, endoscopic simulation, and other functions (Figures 4 and 5).
From January 2020 to December 2024, 140 patients were eligible for and included in the study. Seventy of them underwent DynaCT-PTOBF, whereas the remaining 70 underwent PTOBF. There were no significant differences between the two groups with respect to demographic characteristics, including age or sex. The DynaCT-PTOBF group had a higher detection rate of target bile ducts where bile duct stones and biliary strictures were located than the PTOBF group (Table 1).
| Variable | DynaCT-PTOBF (n = 70) | PTOBF (n = 70) | P value |
| Age (years) | 52.67 ± 14.35 | 50.5 ± 17.3 | 0.423 |
| Sex, n (%) | 0.716 | ||
| Male | 31 | 24 | |
| Female | 39 | 46 | |
| TBIL (mmol/L) | 28.39 ± 27.91 | 33.9 ± 32.9 | 0.291 |
| DBIL (mmol/L) | 13.3 ± 18.1 | 13.3 ± 18.1 | 0.366 |
| ALT (U/L) | 42.42 ± 49.30 | 59.9 ± 61.7 | 0.227 |
| Γ-GGT (U/L) | 139.97 ± 165.23 | 210.6 ± 262.2 | 0.059 |
| ALB (G/L) | 35.47 ± 4.08 | 39.3 ± 6.4 | 0.052 |
| PT (S) | 13.82 ± 1.13 | 14.2 ± 4.5 | 0.486 |
| Child-Pugh score, n (%) | 0.116 | ||
| Grade A | 62 (88.6) | 67 (95.7) | |
| Grade B | 8 (11.4) | 3 (4.3) | |
| Location of stone, n (%) | |||
| S1 | 6 (8.6) | 1 (1.4) | 0.053 |
| S2 | 12 (17.14) | 9 (12.9) | 0.478 |
| S3 | 14 (20.0) | 11 (15.7) | 0.508 |
| S4 | 15 (21.4) | 17 (24.3) | 0.687 |
| S5 | 15 (21.4) | 4 (5.7) | 0.007 |
| S6 | 13 (18.6) | 12 (17.1) | 0.825 |
| S7 | 11 (15.7) | 8 (11.4) | 0.459 |
| S8 | 10 (14.3) | 12 (17.1) | 0.642 |
| Common bile duct, n (%) | 21 (30.0) | 23 (32.9) | 0.716 |
| Location of bile duct stenosis, n (%) | |||
| S1 | 2 (2.86) | 0 | 0.154 |
| S2 | 14 (20.0) | 1 (1.4) | 0.000 |
| S3 | 13 (18.6) | 8 (11.4) | 0.237 |
| S4 | 11 (15.7) | 14 (20.0) | 0.508 |
| S5 | 16 (22.9) | 4 (5.7) | 0.004 |
| S6 | 12 (17.1) | 11 (15.7) | 0.820 |
| S7 | 12 (17.1) | 6 (8.6) | 0.130 |
| S8 | 6 (8.6) | 7 (10.0) | 0.771 |
| Biliary-intestinal anastomotic stenosis, n (%) | 15 (21.4) | 12 (17.1) | 0.520 |
| Common bile duct, n (%) | 13 (18.6) | 25 (35.7) | 0.023 |
Compared with the PTOBF group, the DynaCT-CT fusion group was characterized by the following significant observations: A greater rate of immediate stone removal (68.6% vs 50.0%, P = 0.025), greater immediate stenosis dilatation success rate (72.9% vs 55.7%, P = 0.034), greater final stenosis release rate (91.4% vs 75.7%, P = 0.012), shorter duration of intraoperative hemorrhage (3.14 ± 2.00 vs 26.5 ± 52.1, P = 0.039), and lower incidence of distant cholangitis ( 2.9% vs 11.4%, P = 0.49). The mean follow-up duration was 339.5 ± 316.2 d. There was no significant difference between the two groups in the final stone removal rate, reoperation rate, or incidence of long-term complications (P > 0.05) (Table 2).
| Variable | DynaCT-PTOBF (n = 70) | PTOBF (n = 70) | P value |
| Total surgical time (min) | 55.8 ± 24.0 | 55.2 ± 34.0 | 0.900 |
| Intraoperative haemorrhage (mL) | 3.14 ± 2.00 | 26.5 ± 52.1 | 0.039 |
| Immediate stone removal rate | 48 (68.6) | 35 (50.0) | 0.025 |
| Immediate stenosis dilatation success rate | 51 (72.9) | 39 (55.7) | 0.034 |
| Short-term complications | 11 (12.9) | 6 (8.6) | 0.196 |
| Haemorrhage | 2 (2.9) | 1 (1.4) | 0.559 |
| Pulmonary infection | 1 (1.4) | 2 (2.9) | 0.559 |
| Cholangitis | 6 (5.7) | 2 (2.9) | 0.145 |
| Pleural effusion | 2 (2.9) | 1 (1.4) | 0.559 |
| Follow-up time (d) | 209.2 ± 20.7 | 498.0 ± 373.5 | 0.000 |
| Final stone extraction rate | 60 (85.7) | 54 (77.1) | 0.192 |
| Final stenosis release rate | 64 (91.4) | 53 (75.7) | 0.012 |
| Number of operations | 3.0 ± 1.4 | 3.1 ± 1.5 | 0.056 |
| Stone recurrence rate | 3 (4.3) | 11 (15.7) | 0.024 |
| Reoperation rate | 2 (2.9) | 7 (10.0) | 0.085 |
| Long-term complications | 3 (4.3) | 9 (12.9) | 0.070 |
| Distant cholangitis | 2 (2.9) | 8 (11.4) | 0.049 |
| Liver failure | 1 (1.4) | 1 (1.4) | 1.00 |
DynaCT is a type of CBCT attached to the Artis Zee DSA system produced by SIEMENS Company in Germany and has been widely applied in the diagnosis and treatment of different diseases, such as vascular, cardiothoracic, and neurosurgical conditions[16,17]. However, its application in hepatobiliary surgery is rare. Wallace et al[18] reported that CBCT can provide images of accurate biliary anatomy and calculate the volume of the liver, especially in complex states. Raj et al[19] reported that, compared with conventional 2D cholangiography, CBCT assists in the 3D reconstruction of cholangiography images with high spatial resolution. In this study, we applied a newly developed DynaCT technology for the diagnosis and treatment of hepatolithiasis. This technique can show the distal end of the bile duct, biliary stenosis, and stones clearly, which allows the acquisition of biliary information from any angle. The utility of MPR or VR in DynaCT is that it displays complex and abnormal anatomic structures, such as multiple intrahepatic biliary strictures after liver transplantation and primary sclerosing cholangitis.
The DynaCT biliary soft tissue reconstruction model can guide precise surgical punctures. During one-step PTCSL procedures, surgeons pinpoint the locations of the calculi intraoperatively, puncture the target bile ducts, and remove the stones via real-time ultrasound combined with preoperative CT or magnetic resonance[20]. Traditional two-dimensional images are sometimes difficult to identify stones from multiple angles, especially when the bile ducts have numerous bifurcation openings with distortion, irregularity, and stenosis. The use of C-arm rotational acquisition bile duct reconstruction technology can help surgeons to obtain the 3D spatial structure of the bile duct more effectively[21]. Kapoor et al[22] reported that CBCT combined with previous CT images clearly visualize the stenotic bile ducts, assisting surgeons in planning bile-intestinal anastomosis pathways.
To obtain information on the patency of the bile ducts and the location of biliary strictures, DynaCT cholangiography can also be performed in the hybrid operating room. Surgeons can employ a rigid choledochoscope to treat the stenosed bile duct and leave an 18-F drainage tube in the distal end of the stenosed bile ducts for 6-9 mo[23,24]. In this study, the DynaCT-CT group was characterized by several significant observations. Direct biliary imaging via DynaCT revealed more stones in hepatic segments III (grade 3) and even IV (grade 4) and negative stones. The immediate stone removal, immediate stenosis dilatation success, and final stenosis release rates in the DynaCT-PTOBF group were significantly greater than those in the PTOBF group (P < 0.05). Having accurate information about the location and size of hepatobiliary stenoses and stones may help surgeons to resolve strictures more quickly, which may further reduce the stone recurrence and reoperation rates.
There were several limitations in this study. It was a single-center study including a limited number of patients; hence, there was some inevitable selection bias between the groups that may have impacted the results.
In conclusion, this study showed that the application of DynaCT biliary soft tissue reconstruction technology can further optimize the diagnosis of hepatobiliary stones and strictures. This technology has shown unique advantages in one-step PTCSL, including a greater immediate stone removal rate, greater immediate stenosis dilatation success rate, greater final stenosis release rate, shorter duration of intraoperative hemorrhage, and lower incidence of distant cholangitis. These findings suggest that DynaCT-CT fusion can be an ideal option to guide one-step PTCSL for patients who are intolerant to conventional surgery or who have postoperative remnant calculi.
| 1. | Pereira-Lima JC, Jakobs R, Winter UH, Benz C, Martin WR, Adamek HE, Riemann JF. Long-term results (7 to 10 years) of endoscopic papillotomy for choledocholithiasis. Multivariate analysis of prognostic factors for the recurrence of biliary symptoms. Gastrointest Endosc. 1998;48:457-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 140] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 2. | Ueno N, Ozawa Y, Aizawa T. Prognostic factors for recurrence of bile duct stones after endoscopic treatment by sphincter dilation. Gastrointest Endosc. 2003;58:336-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Lee SK, Seo DW, Myung SJ, Park ET, Lim BC, Kim HJ, Yoo KS, Park HJ, Joo YH, Kim MH, Min YI. Percutaneous transhepatic cholangioscopic treatment for hepatolithiasis: an evaluation of long-term results and risk factors for recurrence. Gastrointest Endosc. 2001;53:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 87] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Demidova-Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 1: normal and chronic wounds: biology, causes, and approaches to care. Adv Skin Wound Care. 2012;25:304-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 454] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 5. | Lin W, Chen M, Li B, Wang W, Li S, Lyu G. Intraoperative ultrasound-guided percutaneous hepatocholangiostomy applied in the cholangioscopic lithotripsy for hepatolithiasis and choledocholithiasis. Surg Endosc. 2023;37:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 6. | Mori T, Sugiyama M, Atomi Y. Gallstone disease: Management of intrahepatic stones. Best Pract Res Clin Gastroenterol. 2006;20:1117-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 7. | Sajjad Z, Oxtoby J, West D, Deakin M. Biliary imaging by spiral CT cholangiography--a retrospective analysis. Br J Radiol. 1999;72:149-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Kalloo AN, Kantsevoy SV. Gallstones and biliary disease. Prim Care. 2001;28:591-606, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Ali F, Aamir N, Hassan MK; Fakhar-E-Alam, Khan HU, Khan D. Comparison of MRCP and ERCP findings: A retrospective secondary data analysis. J Pak Med Assoc. 2022;72:284-286. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Ma S, Hu S, Gao F, Liang R. Endoscopy Lithotomy for Intrahepatic Gallstones: A Meta-Analysis. Surg Laparosc Endosc Percutan Tech. 2015;25:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Koshinaga T, Inoue M, Ohashi K, Sugito K, Ikeda T, Hagiwara N, Tomita R. Persistent biliary dilatation and stenosis in postoperative congenital choledochal cyst. J Hepatobiliary Pancreat Sci. 2011;18:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Erden A, Haliloğlu N, Genç Y, Erden I. Diagnostic value of T1-weighted gradient-echo in-phase images added to MRCP in differentiation of hepatolithiasis and intrahepatic pneumobilia. AJR Am J Roentgenol. 2014;202:74-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Vest M, Ciobanu C, Nyabera A, Williams J, Marck M, Landry I, Sumbly V, Iqbal S, Shah D, Nassar M, Nso N, Rizzo V. Biliary Anastomosis Using T-tube Versus No T-tube for Liver Transplantation in Adults: A Review of Literature. Cureus. 2022;14:e24253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Meister B, Rassweiler MC, Weiß C, Häcker A, Ritter M. Accuracy in detecting and measuring residual fragments with the Uro Dyna-CT. World J Urol. 2015;33:1039-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Aldosary G, Szanto J, Holmes O, Lavigne B, Althobaity W, Sheikh A, Foottit C, Vandervoort E. Geometric inaccuracy and co-registration errors for CT, DynaCT and MRI images used in robotic stereotactic radiosurgery treatment planning. Phys Med. 2020;69:212-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Toroi P, Kaasalainen T, Uusi-Simola J, Aho P, Mäkelä T, Kortesniemi M. Intraoperative CBCT imaging in endovascular abdomen aneurysm repair - Optimization of exposure parameters using a stent phantom. Phys Med. 2023;112:102634. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Baccellieri D, Apruzzi L, Ardita V, Bilman V, De Cobelli F, Melissano G, Chiesa R. Intraoperative completion cone-beam computed tomography for the assessment of residual lesions after primary treatment of proximal venous outflow obstructions. Phlebology. 2022;37:55-62. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Wallace MJ, Kuo MD, Glaiberman C, Binkert CA, Orth RC, Soulez G; Technology Assessment Committee of the Society of Interventional Radiology. Three-dimensional C-arm cone-beam CT: applications in the interventional suite. J Vasc Interv Radiol. 2009;20:S523-S537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Raj S, Irani FG, Tay KH, Tan BS. C-arm Cone Beam Computed Tomography: A New Tool in the Interventional Suite. Ann Acad Med Singap. 2013;42:585-592. [PubMed] |
| 20. | Cha SW. [Management of Intrahepatic Duct Stone]. Korean J Gastroenterol. 2018;71:247-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | van der Horst A, Lens E, Wognum S, de Jong R, van Hooft JE, van Tienhoven G, Bel A. Limited role for biliary stent as surrogate fiducial marker in pancreatic cancer: stent and intratumoral fiducials compared. Int J Radiat Oncol Biol Phys. 2014;89:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Kapoor BS, Mauri G, Lorenz JM. Management of Biliary Strictures: State-of-the-Art Review. Radiology. 2018;289:590-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 23. | Tao H, Wang P, Sun B, Zhou X, Xie J. One-step Percutaneous Transhepatic Cholangioscopy Combined With High-frequency Needle-knife Electrotomy in Biliary Strictures After Liver Transplantation. Surg Laparosc Endosc Percutan Tech. 2021;31:787-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Yang YL, Zhang C, Zhao G, Wu P, Ma YF, Zhang HW, Shi LJ, Li JY, Lin MJ, Yang SM, Lv Y. Choledochoscopic high-frequency needle-knife electrotomy as an effective treatment for intrahepatic biliary strictures. J Gastroenterol Hepatol. 2015;30:1438-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |