Published online Jan 27, 2025. doi: 10.4240/wjgs.v17.i1.98263
Revised: October 16, 2024
Accepted: November 11, 2024
Published online: January 27, 2025
Processing time: 188 Days and 4.4 Hours
Endoscopy allows for the direct observation of primary tumor characteristics and responses after neoadjuvant treatment. However, reports on endoscopic evalua
To examine the predictive value of endoscopic findings of primary tumors for responses to neoadjuvant immunotherapy.
This retrospective study, conducted at a tertiary center in China, evaluated 74 patients with colorectal cancer, including 17 with deficient mismatch repair (dMMR) and 15 with proficient mismatch repair (pMMR) tumors. Patients un
In the pMMR group (n = 57 evaluable patients), endoscopy identified 11/17 patients who achieved a complete response (CR), while misidentifying 1/40 patients with residual disease as CR (64.7% vs 2.5%, P < 0.01). Con
Endoscopic evidence of CR or PR was well correlated with postoperative pathological outcomes in the pMMR cohort. Despite endoscopic indications of tumor residue, a complete pathological response post-surgery was possible in the dMMR cohort.
Core Tip: Endoscopy demonstrated strong predictive value for postoperative pathological outcomes in the proficient mismatch repair (MMR) cohort. Despite endoscopic indications of tumor residue in the deficient MMR cohort, a complete pathological response post-surgery was still possible. This study highlights the significant role of endoscopy in predicting responses to neoadjuvant immunotherapy, particularly in both proficient MMR and deficient MMR patient groups, providing valuable information for clinical decision-making.
- Citation: Li YG, Han CC, Zhuang M, Zhao W, Hu G, Qiu WL, Wang XS, Tang JQ. Evaluating the predictive value of endoscopic findings for residual colorectal cancer following neoadjuvant combination immunotherapy. World J Gastrointest Surg 2025; 17(1): 98263
- URL: https://www.wjgnet.com/1948-9366/full/v17/i1/98263.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i1.98263
In recent years, neoadjuvant immunotherapy has emerged as a crucial area of research in the treatment of colorectal cancer (CRC). Approximately 90% of patients with deficient mismatch repair (MMR) (dMMR) or high microsatellite instability (MSI-H) who undergo neoadjuvant immunotherapy can achieve a pathological complete response (pCR)[1-3]. Similarly, around 30% of patients with proficient MMR (pMMR) or microsatellite stability who receive a combination of radiotherapy and targeted therapy also reach pCR[4,5]. The ability to accurately screen these patients before surgery could help avoid the risks associated with colorectal surgery, such as anastomotic leakage and urinary and bowel dysfunction. Endoscopy offers a convenient method for directly observing the characteristics of the primary tumor and its clinical response after neoadjuvant treatment[6-8].
Currently, the clinical evaluation standard for ypT0N0 in patients with rectal cancer post-neoadjuvant therapy still follows the criteria proposed by Brazilian scholar Habr-Gama et al[9] in 2010. These criteria include post-treatment observations of the mucosa at the primary lesion site appearing pale and stiff, with capillary dilation and the absence of nodules upon palpation. Cercek et al[3] conducted regular endoscopic evaluations on 12 patients with CRC undergoing treatment with programmed cell death protein-1 inhibitors, marking the first assessment of endoscopic response to neoadjuvant immunotherapy, closely aligning with standards set by Habr-Gama et al[9]. However, there is a scarcity of reports on endoscopic evaluations following neoadjuvant therapy combined with immunotherapy. Thus, the objective of the current study was to explore the rate of endoscopic examination for residual CRC after neoadjuvant therapy combined with immunotherapy. Our findings could provide crucial insights and aid in refining treatment strategies for patients with CRC.
In this study, we performed a retrospective evaluation of a cohort of patients with CRC who were treated with immune checkpoint inhibitors (ICIs). Data for all relevant cases from February 2015 to January 2024 were extracted from a prospectively maintained institutional database and tumor registry. Eligible participants were required to meet the following criteria: (1) Adenocarcinoma confirmed by colonoscopy; (2) Preoperative treatment with ICIs; (3) Completion of surgery for the primary lesion; and (4) Availability of pretreatment imaging data and MMR status determined by immunohistochemistry. The exclusion criteria were as follows: Patients who (1) Received any other type of antitumor or experimental treatment; (2) Had previously received ICI therapy; and (3) Were diagnosed with other malignant tumors within the past 5 years.
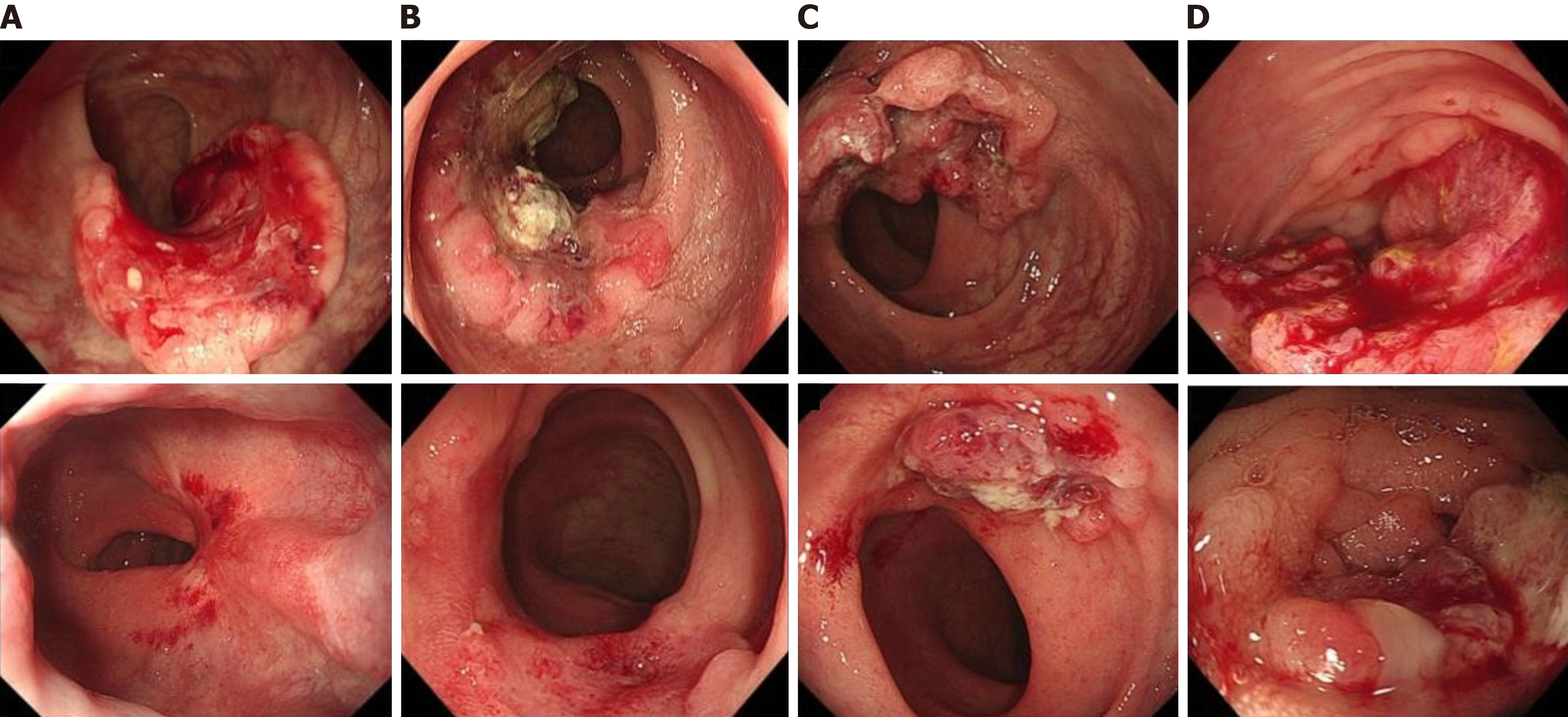
Endoscopic examinations were typically conducted pre-treatment to establish a baseline and immediately prior to surgery after neoadjuvant combination immunotherapy, requiring each patient to undergo at least two endoscopic assessments. Endoscopic responses were evaluated using images and descriptions from endoscopic reports, with the assessments performed by professional endoscopists who were blinded to the outcomes of the neoadjuvant therapy. Evaluation criteria were based on the standardized response criteria by Cercek et al[3], classifying each endoscopic response into one of five categories: CR, near CR, partial response (PR), stable disease (SD), or progressive disease. A CR was characterized by flat, white scars with or without capillary dilation and the absence of any residual ulcers or nodules. A near CR was identified by minor mucosal irregularities, such as small nodules or superficial ulcers. PR and SD statuses were defined as a reduction in tumor volume and no change in tumor presence, respectively, while a progressive disease indicated an increase in tumor volume. Representative images are provided in Figure 1.
The dataset encompassed basic patient demographics, including sex, age, body mass index, tumor location, preoperative imaging, colonoscopy pathology, carcinoembryonic antigen levels, and MMR status. It also included time intervals between different treatment stages and postoperative pathological outcomes, such as pathological tumor-node-metastasis staging and tumor regression grade[10].
Statistical analysis was conducted using IBM SPSS Statistics version 26 (IBM Corp., Armonk, NY, United States), focusing on the sensitivity, specificity, positive predictive value (PPV), negative predictive value, and accuracy of endoscopic evaluations. Categorical data were analyzed using Pearson’s χ2-tests or Fisher’s exact test, as appropriate, with statistical significance defined as P < 0.05. Normality tests were conducted to confirm the distribution of the data. All tests performed were two-sided.
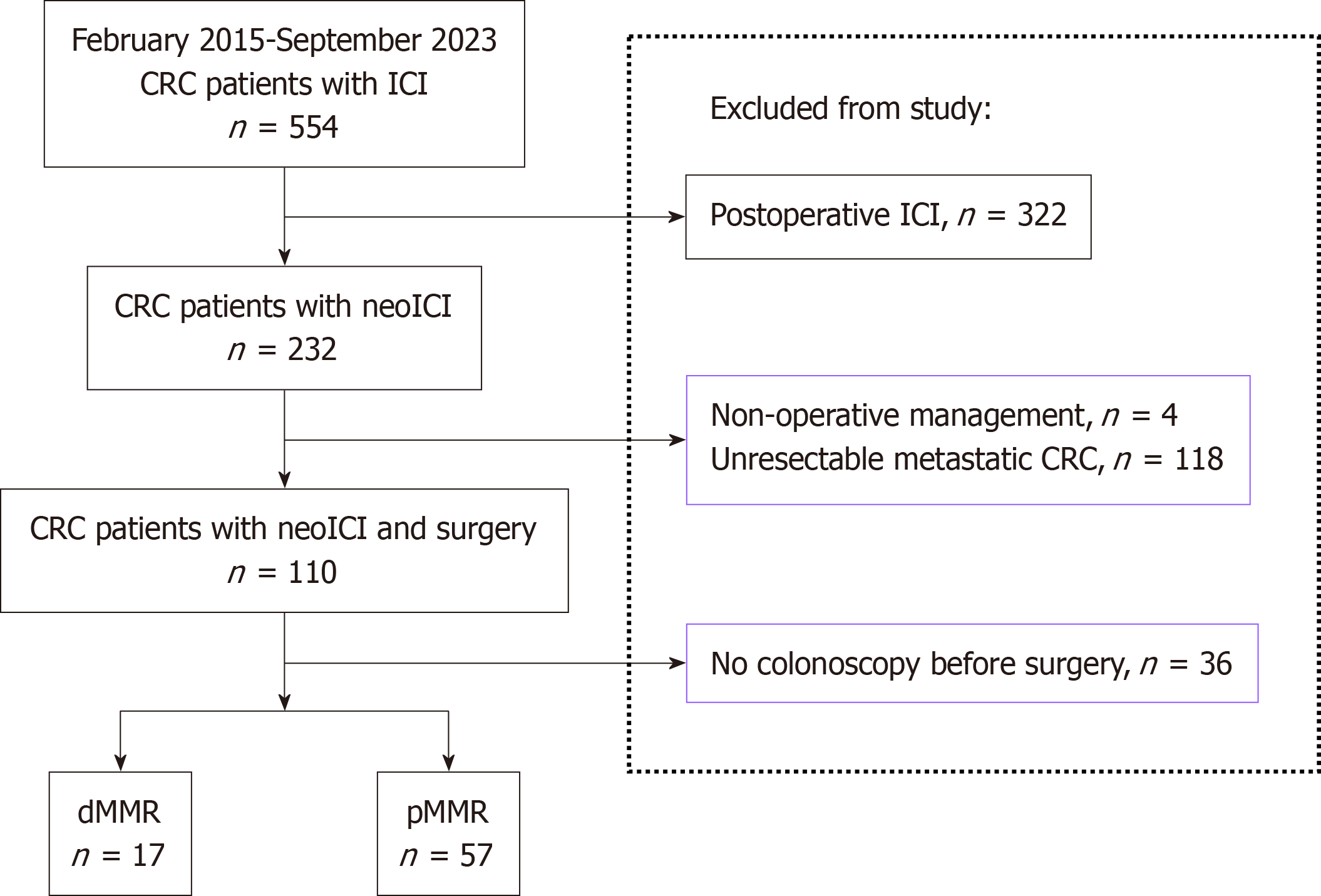
The study cohort comprised 74 patients with CRC who fulfilled the inclusion criteria from February 2015 to January 2024, including 57 patients with pMMR and 17 with dMMR. All participants underwent endoscopic examinations before and after receiving neoadjuvant combination immunotherapy. Figure 2 presents a flowchart of the study. Within the pMMR group, histopathological analysis of the resected specimens revealed that 40 patients (70.2%) had residual lesions, while 17 patients (29.8%) achieved a CR. In the dMMR group, 14/17 patients (82.4%) exhibited a pCR, with the remaining 3/17 patients (17.6%) presenting with residual disease post-surgery. The baseline characteristics before treatment and the postoperative pathological outcomes for the 74 patients are detailed in Table 1.
| Characteristics | pMMR (n = 57) | dMMR (n = 17) |
| Age (years) | 59 (51-66.5) | 43 (37-63.5) |
| Sex (male) | 37 (64.9) | 10 (58.8) |
| BMI (kg/m2) | 23.4 (21.0-25.7) | 23.5 (21.8-28.5) |
| Primary tumor location | ||
| Ascending colon | 5 (8.8) | 7 (41.2) |
| Sigmoid colon | 10 (17.5) | 3 (17.6) |
| Rectum | 42 (73.7) | 7 (41.2) |
| Clinical TNM stage | ||
| II | 10 (17.5) | 1 (5.9) |
| III | 36 (63.2) | 14 (82.4) |
| IV | 11 (19.3) | 2 (11.8) |
| Clinical T-stage | ||
| T3 | 35 (61.4) | 9 (52.9) |
| T4 | 22 (38.6) | 8 (47.1) |
| Clinical N-stage | ||
| Negative | 12 (21.1) | 1 (5.9) |
| Positive | 45 (78.9) | 16 (94.1) |
| CEA elevation (≥ 5 μg/L) | 26 (45.6) | 3 (17.6) |
| Histological appearance | ||
| Well differentiated | 2 (3.5) | 0 |
| Moderately differentiated | 9 (15.8) | 2 (11.8) |
| Poorly differentiated | 46 (80.7) | 15 (88.2) |
| Neoadjuvant immunotherapy | ||
| PD-1 inhibitor | 39 (68.4) | 12 (70.6) |
| PD-L1 inhibitor | 6 (10.5) | 1 (5.9) |
| PD-1 + CTLA-4 inhibitors | 12 (21.1) | 4 (23.5) |
| Previous treatment | ||
| NAC | 39 (68.4) | 9 (52.9) |
| Target therapy | 15 (26.3) | 2 (11.8) |
| NRT | 26 (65.6) | 2 (11.8) |
| Days from diagnosis to initiation of any therapy | 28 (17-41.5) | 31 (14-52) |
| Days from initiation of any therapy to completion of all therapy | 97 (76-122) | 90 (42.5-150) |
| Days from completion of all therapy to surgery | 50 (31.5-63) | 42 (35-68) |
| pCR rate | 17 (29.8) | 14 (82.4) |
| TRG | ||
| 1 | 7 (12.3) | 1 (5.9) |
| 2 | 23 (40.4) | 0 |
| 3 | 10 (17.5) | 3 (17.6) |
| 4 | 17 (29.8) | 13 (76.5) |
| Pathological T stage | ||
| ypT0 | 18 (31.6) | 13 (76.5) |
| ypT1 | 4 (7.0) | 1 (5.9) |
| ypT2 | 11(19.3) | 2(11.8) |
| ypT3 | 16 (28.3) | 0 |
| ypT4 | 8 (14.0) | 1 (5.9) |
| Pathological N stage | ||
| ypN0 | 42 (73.7) | 16 (94.1) |
| ypN1 | 9 (15.8) | 1 (5.9) |
| ypN2 | 6 (10.5) | 0 |
| Vascular invasion | 6 (10.5) | 1 (5.9) |
| Perineural invasion | 13 (22.8) | 1 (5.9) |
In the cohort of 57 evaluable pMMR patients, 17 achieved a pCR. Of these, 11/17 were identified as CR under endoscopy, compared with only 1/40 patients with residual tumors (64.7% vs 2.5%, P < 0.01). Additionally, 22/40 patients with residual tumors and 1/17 with CR were classified as partial responders based on endoscopic assessment (55.0% vs 5.9%, P < 0.01). Among patients with dMMR tumors, 9/17 patients with CR and 2/3 with residual tumors were considered as partial responders based on endoscopic assessment (64.3% vs 66.7%, P = 0.73) (Tables 2 and 3).
| Total (n = 57) | Residual disease (n = 40) | Complete response (n = 17) | P value | |
| CR | 12 (21.1) | 1 (2.5) | 11 (64.7) | < 0.01 |
| NCR | 17 (29.8) | 12 (30.0) | 5 (29.4) | 0.96 |
| PR | 23 (40.4) | 22 (55.0) | 1 (5.9) | < 0.01 |
| SD | 5 (8.8) | 5 (12.5) | 0 (0) | 0.31 |
| Total (n = 17) | Residual disease (n = 3) | Complete response (n = 14) | P value | |
| CR | 2 (11.8) | 0 (0) | 2 (14.3) | 0.67 |
| NCR | 4 (23.5) | 1 (33.3) | 3 (21.4) | 0.58 |
| PR | 11 (64.7) | 2 (66.7) | 9 (64.3) | 0.73 |
In the group comprising 57 patients with pMMR, the sensitivity, specificity, PPV, negative predictive value, and accuracy of endoscopic diagnosis for pCR were 64.7%, 97.5%, 91.7%, 86.7%, and 87.7%, respectively. Meanwhile, among the 17 patients with dMMR, the sensitivity, PPV, and accuracy of endoscopic diagnosis for pCR were 100%, 82.4%, and 82.4%, respectively (Table 4).
| pMMR (n = 57) | dMMR (n = 17) | |
| Sensitivity | 64.7 | 100 |
| Specificity | 97.5 | 0 |
| PPV | 91.7 | 82.4 |
| NPV | 86.7 | 0 |
| Accuracy | 87.7 | 82.4 |
To identify endoscopic features of post-neoadjuvant combination immunotherapy that could inform clinical decision-making, we analyzed the endoscopic characteristics of 74 patients. In patients with pMMR tumors, tumor focal retraction (96.5%) and visible residual tumors (86.0%) were the most frequently observed endoscopic signs. Likewise, in patients with dMMR tumors, tumor focal retraction (100%) and visible residual tumors (88.2%) were predominant features. Eccentric stenosis was a less common finding, observed in 38.6% and 64.7% of patients with pMMR and dMMR tumors, respectively (Table 5).
| pMMR, present | pMMR, absent | dMMR, present | dMMR, absent | |
| Ulceration | 38 (66.7) | 19 (33.3) | 13 (76.4) | 4 (23.6) |
| Easy bleeding | 42 (73.7) | 15 (26.3) | 13 (76.5) | 4 (23.5) |
| Focal retraction | 55 (96.5) | 2 (3.5) | 17 (100) | 0 (0) |
| Residual mass | 49 (86.0) | 8 (14.0) | 15 (88.2) | 2 (11.8) |
| Eccentric stenosis | 22 (38.6) | 35 (61.4) | 11 (64.7) | 6 (35.3) |
In the current study, endoscopic signs of CR or PR correlated with postoperative pathological outcomes in the pMMR cohort. This relationship may assist clinicians in making informed treatment decisions following neoadjuvant com
To monitor treatment response effectively, magnetic resonance imaging and endorectal ultrasound are current methods that are widely utilized to assess the efficacy of neoadjuvant therapy in rectal cancer. Supporting this, a meta-analysis encompassing 46 studies revealed that the combined sensitivity and specificity of magnetic resonance imaging and endorectal ultrasound for predicting CR were 95% and 31%, and 97% and 30%, respectively[11]. Further emphasizing the importance of imaging techniques, Rengo et al[12] employed T2-weighted imaging to quantitatively measure the relative composition of fibrous tissue, achieving a sensitivity of 78.26% and specificity of 97.62% in predicting ypT0N0. Building on this, in our study, which focuses on the same population undergoing immunotherapy, we adopted the classification method of Cercek et al[3], using endoscopy to determine the sensitivity (64.7%) and specificity (97.5%) for predicting CR post-neoadjuvant therapy combined with immunotherapy in the pMMR population. Notably, although endoscopy presents advantages such as convenience and speed, combining endoscopy with advancements in artificial intelligence, machine learning, radiomics, and other fields could enhance its sensitivity in predicting CR.
The phenomenon of pseudo-progression or pseudo-residuals following immunotherapy has garnered considerable attention in the clinical domain[13-15]. Xie et al[16] revealed that among 13 patients treated with novel ICIs, radiological assessments indicated disease progression in 3 patients, SD in 1 patient, PR in 7 patients, and CR in 2 patients. Notably, subsequent evaluations confirmed pCR in these patients, leading to pseudo-progression and pseudo-residual rates of 23.1% (n = 3) and 76.9% (n = 10), respectively. This highlights that pseudo-progression and pseudo-residuals represent distinct and prevalent response patterns in patients with MSI-H/dMMR CRC undergoing novel ICI therapy. Confirming these findings, our study indicates that these phenomena are relatively common in patients receiving neoadjuvant therapy combined with immunotherapy, particularly in those with MSI-H/dMMR CRC. This observation highlights the need for caution when assessing the efficacy of neoadjuvant combination immunotherapy to avoid premature or overly aggressive clinical interventions. Notably, for pMMR cohorts, endoscopic evaluation may have limitations, necessitating the use of additional diagnostic methods. However, for dMMR patients, the concordance between endoscopic evaluation and postoperative pathology tends to be high.
The timing of post-treatment assessment is a critical factor in evaluating the efficacy of neoadjuvant chemoradiotherapy (nCRT) between 6 to 8 weeks post-treatment[17,18]. In an effort to improve outcomes, Habr-Gama et al[9] proposed extending this assessment period to 10 weeks following an enhanced nCRT regimen, observing that patients demonstrated improved rates of complete CR and favorable long-term prognoses related to the tumor. However, the GRECCAR-6 clinical trial, which allocated patients with progressive mid-low rectal cancer to post-nCRT intervals of 7 and 11 weeks, did not detect a statistically significant difference in the rates of pCR between the two groups. Addi
This single-center retrospective study has several limitations. Firstly, despite the consideration of endoscopy-based efficacy assessments conducted by professional endoscopists, a degree of heterogeneity exists. Secondly, the inclusion of patients with both colon and rectal cancers, including advanced stages, along with variations in preoperative treatment plans and cycles, may have influenced the outcomes. Lastly, the relatively small sample size of this study limits the generalizability of the findings. Future multicenter prospective studies with larger sample sizes are warranted to validate these observations.
In the pMMR cohort, endoscopic signs of either CR or PR were strongly associated with postoperative pathological results. Conversely, pCRs may be achieved in the dMMR cohort post-surgery, even when endoscopic evaluation suggests the presence of a residual tumor. Therefore, it is crucial to perform regular endoscopic assessments following neo
The authors express their gratitude to the team members for their diligent efforts in data collection.
| 1. | Chalabi M, Fanchi LF, Dijkstra KK, Van den Berg JG, Aalbers AG, Sikorska K, Lopez-Yurda M, Grootscholten C, Beets GL, Snaebjornsson P, Maas M, Mertz M, Veninga V, Bounova G, Broeks A, Beets-Tan RG, de Wijkerslooth TR, van Lent AU, Marsman HA, Nuijten E, Kok NF, Kuiper M, Verbeek WH, Kok M, Van Leerdam ME, Schumacher TN, Voest EE, Haanen JB. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. 2020;26:566-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 897] [Article Influence: 179.4] [Reference Citation Analysis (0)] |
| 2. | Chalabi M, Verschoor Y, van den Berg J, Sikorska K, Beets G, Lent A, Grootscholten M, Aalbers A, Buller N, Marsman H, Hendriks E, Burger P, Aukema T, Oosterling S, Beets-tan R, Schumacher T, van Leerdam M, Voest E, Haanen J. LBA7 Neoadjuvant immune checkpoint inhibition in locally advanced MMR-deficient colon cancer: The NICHE-2 study. Ann Oncol. 2022;33:S1389. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 136] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 3. | Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, El Dika IH, Segal N, Shcherba M, Sugarman R, Stadler Z, Yaeger R, Smith JJ, Rousseau B, Argiles G, Patel M, Desai A, Saltz LB, Widmar M, Iyer K, Zhang J, Gianino N, Crane C, Romesser PB, Pappou EP, Paty P, Garcia-Aguilar J, Gonen M, Gollub M, Weiser MR, Schalper KA, Diaz LA Jr. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N Engl J Med. 2022;386:2363-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 989] [Cited by in RCA: 904] [Article Influence: 301.3] [Reference Citation Analysis (0)] |
| 4. | Bando H, Tsukada Y, Inamori K, Togashi Y, Koyama S, Kotani D, Fukuoka S, Yuki S, Komatsu Y, Homma S, Taketomi A, Uemura M, Kato T, Fukui M, Wakabayashi M, Nakamura N, Kojima M, Kawachi H, Kirsch R, Yoshida T, Suzuki Y, Sato A, Nishikawa H, Ito M, Yoshino T. Preoperative Chemoradiotherapy plus Nivolumab before Surgery in Patients with Microsatellite Stable and Microsatellite Instability-High Locally Advanced Rectal Cancer. Clin Cancer Res. 2022;28:1136-1146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 99] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 5. | Kalinsky K, Thomas A, Cescon DW. On the Road to Precision: Understanding the Biology Driving Genomic Assays. J Clin Oncol. 2021;39:100-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Stijns RCH, Leijtens J, de Graaf E, Bach SP, Beets G, Bremers AJA, Beets-Tan RGH, de Wilt JHW. Endoscopy and MRI for restaging early rectal cancer after neoadjuvant treatment. Colorectal Dis. 2023;25:211-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Felder SI, Feuerlein S, Parsee A, Imanirad I, Sanchez J, Dessureault S, Kim R, Hoffe S, Frakes J, Costello J. Endoscopic and MRI response evaluation following neoadjuvant treatment for rectal cancer: a pictorial review with matched MRI, endoscopic, and pathologic examples. Abdom Radiol (NY). 2021;46:1783-1804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 8. | Takao M, Yamazaki K, Boku N, Ikehara H, Hamauchi S, Tsushima T, Taniguchi H, Todaka A, Machida N, Fukutomi A, Onozawa Y, Yasui H. Endoscopic evaluation of primary tumor response in patients with metastatic colorectal cancer treated by systemic chemotherapy. Int J Clin Oncol. 2013;18:864-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Habr-Gama A, Perez RO, Wynn G, Marks J, Kessler H, Gama-Rodrigues J. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: characterization of clinical and endoscopic findings for standardization. Dis Colon Rectum. 2010;53:1692-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 324] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 10. | Trakarnsanga A, Gönen M, Shia J, Nash GM, Temple LK, Guillem JG, Paty PB, Goodman KA, Wu A, Gollub M, Segal N, Saltz L, Garcia-Aguilar J, Weiser MR. Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment. J Natl Cancer Inst. 2014;106:dju248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 194] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 11. | de Jong EA, ten Berge JC, Dwarkasing RS, Rijkers AP, van Eijck CH. The accuracy of MRI, endorectal ultrasonography, and computed tomography in predicting the response of locally advanced rectal cancer after preoperative therapy: A metaanalysis. Surgery. 2016;159:688-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Rengo M, Picchia S, Marzi S, Bellini D, Caruso D, Caterino M, Ciolina M, De Santis D, Musio D, Tombolini V, Laghi A. Magnetic resonance tumor regression grade (MR-TRG) to assess pathological complete response following neoadjuvant radiochemotherapy in locally advanced rectal cancer. Oncotarget. 2017;8:114746-114755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Chae YK, Wang S, Nimeiri H, Kalyan A, Giles FJ. Pseudoprogression in microsatellite instability-high colorectal cancer during treatment with combination T cell mediated immunotherapy: a case report and literature review. Oncotarget. 2017;8:57889-57897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Parseghian CM, Patnana M, Bhosale P, Hess KR, Shih YT, Kim B, Kopetz S, Overman MJ, Varadhachary GR, Javle M, Naing A, Piha-Paul S, Hong D, Le H, Subbiah V, Pant S. Evaluating for Pseudoprogression in Colorectal and Pancreatic Tumors Treated With Immunotherapy. J Immunother. 2018;41:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Punt CJA, Huiskens J, van Gulik T, Engelbrecht M. Pseudoprogression on bevacizumab treatment: tumor-dynamics in the modern era of systemic treatment for metastatic colorectal cancer. Acta Oncol. 2018;57:681-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Xie Y, Lin J, Zhang N, Wang X, Wang P, Peng S, Li J, Wu Y, Huang Y, Zhuang Z, Shen D, Zhu M, Liu X, Liu G, Meng X, Huang M, Yu H, Luo Y. Prevalent Pseudoprogression and Pseudoresidue in Patients With Rectal Cancer Treated With Neoadjuvant Immune Checkpoint Inhibitors. J Natl Compr Canc Netw. 2023;21:133-142.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 17. | Hupkens BJP, Maas M, Martens MH, van der Sande ME, Lambregts DMJ, Breukink SO, Melenhorst J, Houwers JB, Hoff C, Sosef MN, Leijtens JWA, Berbee M, Beets-Tan RGH, Beets GL. Organ Preservation in Rectal Cancer After Chemoradiation: Should We Extend the Observation Period in Patients with a Clinical Near-Complete Response? Ann Surg Oncol. 2018;25:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 18. | Petrelli F, Sgroi G, Sarti E, Barni S. Increasing the Interval Between Neoadjuvant Chemoradiotherapy and Surgery in Rectal Cancer: A Meta-analysis of Published Studies. Ann Surg. 2016;263:458-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 185] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 19. | Lefèvre JH, Mineur L, Cachanado M, Denost Q, Rouanet P, de Chaisemartin C, Meunier B, Mehrdad J, Cotte E, Desrame J, Karoui M, Benoist S, Kirzin S, Berger A, Panis Y, Piessen G, Saudemont A, Prudhomme M, Peschaud F, Dubois A, Loriau J, Tuech JJ, Meurette G, Lupinacci R, Goasguen N, Creavin B, Simon T, Parc Y; The French Research Group of Rectal Cancer Surgery (GRECCAR). Does A Longer Waiting Period After Neoadjuvant Radio-chemotherapy Improve the Oncological Prognosis of Rectal Cancer?: Three Years' Follow-up Results of the Greccar-6 Randomized Multicenter Trial. Ann Surg. 2019;270:747-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |