Published online Jan 27, 2025. doi: 10.4240/wjgs.v17.i1.101793
Revised: November 11, 2024
Accepted: December 2, 2024
Published online: January 27, 2025
Processing time: 91 Days and 9.2 Hours
Improving the intraoperative and postoperative performance of laparoscopic hepatectomy was quite a challenge for liver surgeons.
To determine the benefits of indocyanine green (ICG) fluorescence imaging in patients with hepatocellular carcinoma (HCC) who underwent laparoscopic hepatectomy during and after surgery.
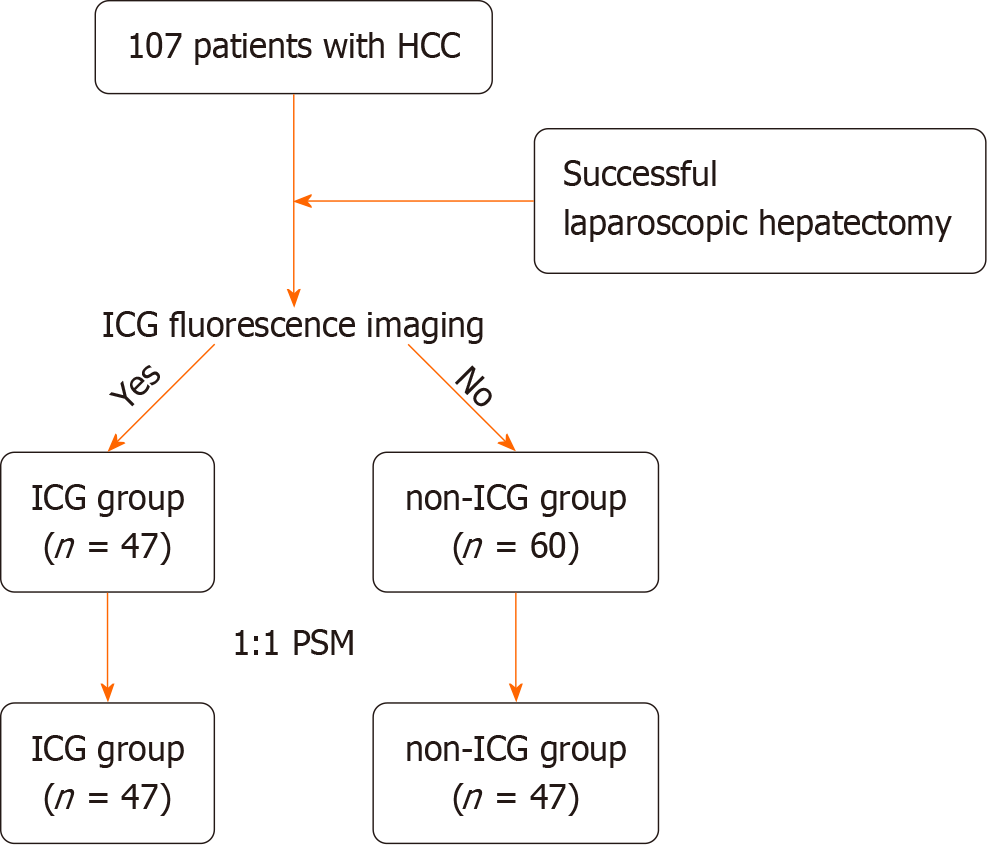
We retrospectively collected the clinicopathological data of 107 patients who successfully underwent laparoscopic hepatectomy at Zhongshan Hospital (Xiamen), Fudan University from June 2022 to June 2023. Whether using the ICG fluorescence imaging technique, we divided them into the ICG and non-ICG groups. To eliminate statistical bias, a 1:1 propensity score matching analysis was conducted. The comparison of perioperative outcomes, including inflammation-related markers and progression-free survival, was analyzed statistically.
Intraoperatively, the ICG group exhibited lower blood loss, a shorter surgical time, lower hepatic inflow occlusion (HIO) frequency, and a shorter total HIO time. Postoperatively, the participation of ICG resulted in a shorter duration of hospitalization (6.5 vs 7.6 days, P = 0.03) and postoperative inflammatory response attenuation (lower neutrophil-lymphocyte ratio on the first day after surgery and platelet-lymphocyte ratio on the third day, P < 0.05). Although the differences were not significant, the levels of all inflammation-related markers were lower in the ICG group. The rates of postoperative complications and the survival analyses, including progression-free and overall survivals showed no significant difference between the groups.
The involvement of ICG fluorescence imaging may lead to improved perioperative outcomes, especially postoperative inflammatory response attenuation, and ultimately improve HCC patients’ recovery after surgery.
Core Tip: This study aimed to determine the benefits of indocyanine green (ICG) fluorescence imaging in patients with hepatocellular carcinoma (HCC) who underwent laparoscopic hepatectomy. Compared with solitary laparoscopic hepatectomy, laparoscopic hepatectomy using ICG fluorescence imaging resulted in lower blood loss, a shorter surgical time, lower hepatic inflow occlusion (HIO) frequency, and a shorter total HIO time during surgery. Postoperatively, the participation of ICG resulted in a shorter duration of hospitalization and postoperative inflammatory response attenuation. The involvement of ICG fluorescence imaging may lead to better perioperative outcomes, especially postoperative inflammatory response attenuation, and ultimately improve HCC patients’ recovery after surgery.
- Citation: Wu WX, Huang MB, Wang MX, Chen LH, Hu B, Ding ZB. Laparoscopic hepatectomy using indocyanine green attenuates postoperative inflammatory response for hepatocellular carcinoma: A propensity score matching analysis. World J Gastrointest Surg 2025; 17(1): 101793
- URL: https://www.wjgnet.com/1948-9366/full/v17/i1/101793.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i1.101793
Hepatocellular carcinoma (HCC) has been one of the top five leading causes of cancer-related deaths in China over the past half-decade[1]. Although various medical treatments have been introduced[2], surgery remains the primary option for curative treatment. Laparoscopic hepatectomy has been proven to be superior to open surgery in causing less trauma and enabling faster recovery after surgery, and it is also non-inferior in survival outcomes[3,4]. Meanwhile, intraoperative ultrasonography has been introduced and considered as a useful tool for intraoperative assessment and identification of liver tumors[5]. Kose et al[6] found that intraoperative ultrasonography can detect HCC with a detection rate of 89% for superficial lesions and 94% for lesions deep in the liver.
Nevertheless, laparoscopy combined with intraoperative ultrasonography did not stop surgeons’ pursuit of achieving high surgical standards. Indocyanine green (ICG), one of the United States Food and Drug Administration -approved fluorescent probes for tumor imaging in clinical use[7], has been introduced for laparoscopic hepatectomy to enable more rapid and accurate intraoperative identification and localization of tumors, eventually achieving a high surgical standard[8]. Extensive literature has depicted the utility of ICG in laparoscopic hepatectomy and researchers have explored its potential positive impact compared with solitary laparoscopic surgery[9-12].
Enhanced recovery after surgery is a feasible and advantageous protocol for achieving improved perioperative outcomes[13,14]. Previous studies[15,16] have confirmed that inflammatory markers are related to postoperative reco
In this study, we aimed to investigate the value of ICG fluorescence imaging technique on the perioperative outcomes in HCC patients who underwent laparoscopic hepatectomy, particularly postoperative inflammatory responses.
This study retrospectively analyzed the general clinicopathological data of 107 patients who underwent laparoscopic hepatectomies performed by the same medical team at the Department of Liver Surgery, Zhongshan Hospital (Xiamen), Fudan University between June 2022 and June 2023. The inclusion criteria were as follows[17]: Fulfillment of the diagnostic criteria stated in the Guidelines for the Diagnosis and Treatment of Primary Liver Cancer (2019 Edition)[18], preoperative Child-Pugh scores of A or B[19], laparoscopic radical hepatectomy was indicated and successfully perfor
The following clinical and pathological data were collected and assessed. Preoperatively, sex, age, hepatitis B test results, albumin (Alb), total bilirubin (TBil), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and inflammation-related markers [including C-reactive protein (CRP) level, neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), lymphocyte-monocyte ratio (LMR), and systemic immune inflammation index]. Intraoperatively, blood loss, surgical time, surgical margins, and operations of HIO were recorded. Postoperatively, prothrombin time (PT), TBil level, inflammation-related markers, surgical complications[21], duration of hospitalization, transcatheter arterial chemoembolization (TACE) therapy, and follow-up findings [including recurrence and progression-free survival (PFS)] were evaluated. Notably, the recorded values for postoperative PT, TBil, ALT, AST, and CRP were the highest.
The Ethics Committee of Zhongshan Hospital (Xiamen), Fudan University (No. B2024-047R) reviewed and approved this study.
The investigators involved in this study were uniformly trained to conduct face-to-face, outpatient, and telephone follow-up visits. The visits were introduced every 3 months and generally included a physical examination, laboratory blood tests, abdominal ultrasonography, and semi-annual abdominal enhanced magnetic resonance imaging (MRI). All patients were followed up for at least 1 year of follow-up except for those who died.
The injectable ICG (25 mg) was obtained from Dandong Yichuang Pharmaceutical Co., Ltd. The standard dosage was 0.5 mg/kg. Routine intravenous injections were administered 72-96 hours before surgery, for patients with severe cirrhosis, the duration was modified to 7-14 days[22]. The surgical fluorescence imaging system was purchased from Nanjing Nuoyuan Medical Devices Co., Ltd, and was equipped with normal and fluorescent modes. A harmonic scalpel (Ethicon Endo-surgery, Cincinnati, OH, United States) was used for liver parenchymal transection.
All surgical procedures were performed by the same medical team experienced in laparoscopic hepatectomy. After general anesthesia, the patient was usually placed in the French position with the primary surgeon on the right side, and four or five trocar ports were applied. The pneumoperitoneum was maintained at 12-15 mmHg. The ICG fluorescence imaging display mode was switched on by an assistant via a button on the arm of the laparoscope. The surgeon conducted laparoscopic exploration to ensure the absence of abdominal metastasis and confirm the size, location, and invasion of the tumor(s) before the primary operations.
In the non-ICG group, the pre-incision line was determined via visual examination and preoperative medical imaging (routinely via abdominal contrast-enhanced MRI). As for the ICG group, the additional use of fluorescence imaging also played a role in pre-incision line determination. Upon observing fluorescence, the surgeon could precisely determine the width and depth of surgical resection, thus ensuring optimal surgical margins and minimal collateral damage. During the incision, anesthetists attempted to maintain the central venous pressure at < 5 cm H2O and controlled intravenous fluid infusion to reduce bleeding from the liver. The surgeon used hemostatic clips, electrocoagulation, and sutures for hemostasis. The liver specimens were retrieved through an enlarged abdominal incision. In the ICG group, fluorescence imaging was used to recheck the incision surface and margins after the tumor(s) was excised and removed. In each case, an abdominal drain was routinely placed in the upper right quadrant.
Continuous variables are presented as median ± SD, and categorical variables are expressed as n (%). Categorical variables were compared using the χ2 or Fisher’s exact test, whereas continuous variables were compared using the t-test. This study used a 1:1 propensity score matching (PSM) analysis[23-25] to minimize bias. The Cox regression analysis was used to determine the survival. The primary endpoint was recurrence. Thus, the statistical indicator was PFS. All data were analyzed using SPSS (version.25.0 for Windows SPSS Inc., Chicago, IL, United States) and R software version 4.1.2 (https://www.r-project.org/). Differences were considered statistically significant at P values of < 0.05.
Based on the presence or absence of ICG fluorescence, the patients were categorized into the ICG (n = 47) or the non-ICG
| Variables | All patients (n = 107) | Propensity-matched patients (n = 94) | ||||||
| Total (n = 107) | ICG (n = 47) | Non-ICG (n = 60) | P value | Total (n = 94) | ICG (n = 47) | Non-ICG (n = 47) | P value | |
| Sex | 0.235 | 0.789 | ||||||
| Male | 83 (77.6) | 39 (83.0) | 44 (73.3) | 77 (81.9) | 39 (83.0) | 38 (80.9) | ||
| Female | 24 (22.4) | 8 (17.0) | 16 (26.7) | 17 (18.1) | 8 (17.0) | 9 (19.1) | ||
| Age, years | 58.4 (11.1) | 55.2 (12.2) | 61.0 (9.5) | 0.007 | 57.5 (11.1) | 55.2 (12.2) | 59.7 (9.4) | 0.059 |
| Hepatitis serology | 0.045 | 0.313 | ||||||
| None | 28 (26.2) | 8 (17.0) | 20 (33.3) | 20 (21.3) | 8 (17.0) | 12 (25.5) | ||
| HBV | 79 (73.8) | 39 (83.0) | 40 (66.7) | 74 (78.7) | 39 (83.0) | 35 (74.5) | ||
| PRE-OP Alb, g/L | 43.0 (3.7) | 43.0 (3.5) | 43.0 (3.9) | 0.959 | 43.0 (3.7) | 43.0 (3.5) | 42.9 (3.9) | 0.934 |
| PRE-OP TBil, μmol/L | 11.6 (6.5) | 12.2 (7.7) | 11.1 (5.4) | 0.401 | 11.9 (6.8) | 12.2 (7.7) | 11.6 (5.7) | 0.650 |
| PRE-OP ALT, U/L | 26.3 (14.9) | 27.6 (17.5) | 25.3 (12.5) | 0.437 | 26.3 (15.4) | 27.6 (17.5) | 25.1 (12.9) | 0.443 |
| PRE-OP AST, U/L | 24.7 (8.3) | 23.4 (6.6) | 25.7 (9.3) | 0.455 | 23.7 (7.0) | 23.4 (6.6) | 24.0 (7.5) | 0.715 |
| Tumor location | 0.2 | 0.272 | ||||||
| caudal lobe | 2 (1.9) | 1 (2.1) | 1 (1.7) | 2 (2.1) | 1 (2.1) | 1 (2.1) | ||
| Left lateral lobe | 8 (7.5) | 6 (12.8) | 2 (3.3) | 8 (8.5) | 6 (12.8) | 2 (4.3) | ||
| Left medial lobe | 15 (14.0) | 9 (19.1) | 6 (10.0) | 14 (14.9) | 9 (19.1) | 5 (10.6) | ||
| Right anterior lobe | 42 (39.3) | 16 (34.0) | 26 (43.3) | 41 (43.6) | 16 (34.0) | 25 (53.2) | ||
| Right posterior lobe | 40 (37.4) | 15 (31.9) | 25 (41.7) | 29 (30.9) | 15 (31.9) | 14 (29.8) | ||
| Tumor size, cm | 3.1 (1.6) | 2.9 (1.7) | 3.3 (1.5) | 0.256 | 3.1 (1.6) | 2.9 (1.7) | 3.3 (1.6) | 0.234 |
| Tumor amount | 0.282 | 0.212 | ||||||
| Single | 97 (90.7) | 41 (87.2) | 56 (93.3) | 85 (90.4) | 41 (87.2) | 44 (93.6) | ||
| Multiple | 10 (9.3) | 6 (12.8) | 4 (6.7) | 9 (9.6) | 6 (12.8) | 3 (6.4) | ||
| Histological grading | 0.341 | 0.460 | ||||||
| I-II | 96 (89.7) | 44 (93.6) | 52 (86.7) | 86 (91.5) | 44 (93.6) | 42 (89.4) | ||
| III | 11 (10.3) | 3 (6.4) | 8 (13.3) | 8 (8.5) | 3 (6.4) | 5 (10.6) | ||
| Degree of hepatic fibrosis | 0.426 | 0.408 | ||||||
| 0-III | 57 (53.3) | 23 (48.9) | 34 (56.7) | 50 (53.2) | 23 (48.9) | 27 (57.4) | ||
| IV | 50 (46.7) | 24 (51.1) | 26 (43.3) | 44 (46.8) | 24 (51.1) | 20 (42.6) | ||
| MVI | 0.146 | 0.298 | ||||||
| M0 | 63 (58.9) | 24 (51.1) | 39 (65.0) | 53 (56.4) | 24 (51.1) | 29 (61.7) | ||
| M1-2 | 44 (41.1) | 23 (48.9) | 21 (35.0) | 41 (43.6) | 23 (48.9) | 18 (38.3) | ||
All surgeries were successfully performed without the need for conversion to open surgery. The intraoperative data are presented in Table 2. Although surgical margins were not statistically different between the groups the ICG group demonstrated lower blood loss (85.5 ± 59.6 vs 163.2 ± 136.5 mL, P = 0.001), shorter surgical time (128.2 ± 43.6 vs 177.0 ± 68.9 minutes, P < 0.001), and lower HIO frequency (times, P = 0.005; total time, P = 0.007).
| Variables | Total (n = 94) | ICG (n = 47) | Non-ICG (n = 47) | P value |
| Blood loss, mL | 124.4 (111.8) | 85.5 (59.6) | 163.2 (136.5) | 0.001 |
| Surgery time, minute | 152.6 (62.4) | 128.2 (43.6) | 177.0 (68.9) | < 0.001 |
| HIO times | 0.005 | |||
| 0 | 5 (5.3) | 4 (8.5) | 1 (2.1) | |
| 1 | 30 (31.9) | 20 (42.6) | 10 (21.3) | |
| 2 | 40 (42.6) | 20 (42.6) | 20 (42.6) | |
| 3 | 17 (18.1) | 2 (4.3) | 15 (31.9) | |
| 4 | 2 (2.1) | 1 (2.1) | 1 (2.1) | |
| Total HIO time, minute | 28.9 (13.6) | 25.1 (13.2) | 32.7 (13.2) | 0.007 |
| Margin, mm | 8.6 (6.9) | 8.8 (6.4) | 8.3 (7.5) | 0.724 |
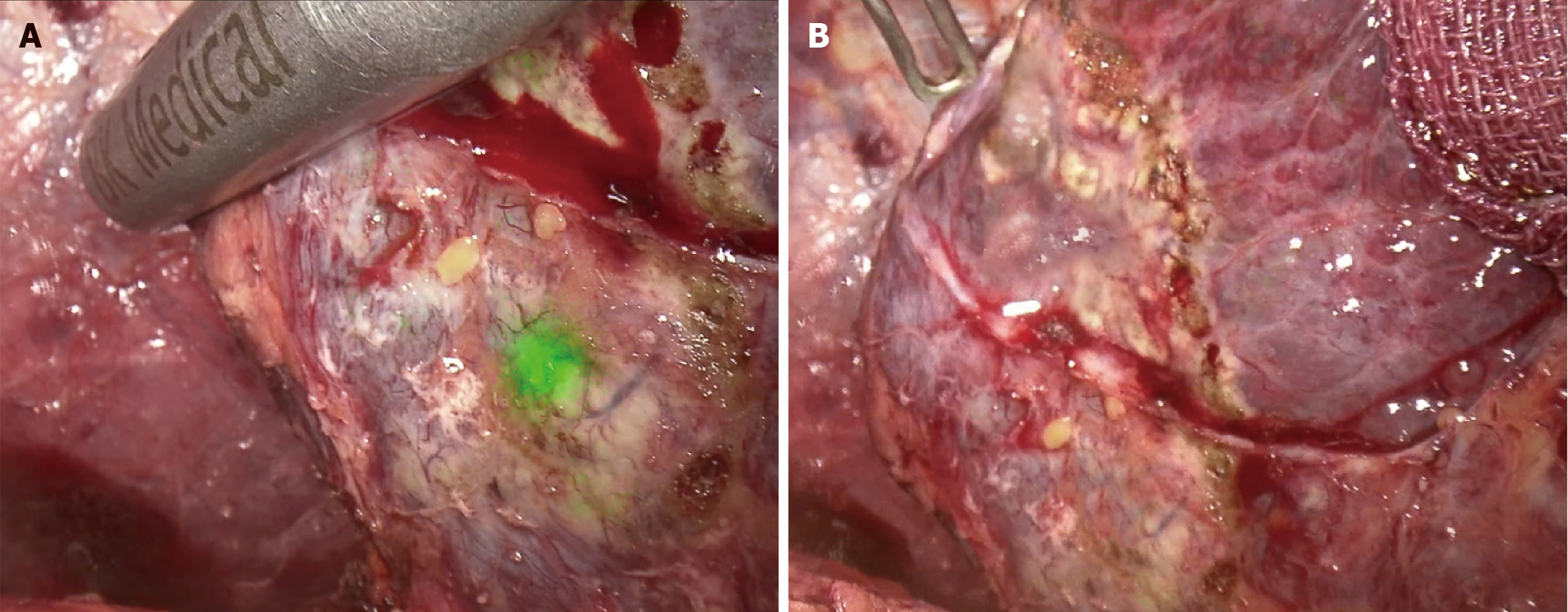
Preoperative imaging revealed 53 tumors in the non-ICG group, all of which were confirmed using ultrasonography and laparoscopy and were resected during surgery. Fifty-two tumors in the ICG group were detected by preoperative imaging, intraoperative ultrasonography, and laparoscopy. In addition, ICG fluorescence helped identify two additional non-eye-or-ultrasonic-detectable lesions (Figure 2), both of which were confirmed as HCC through pathologic examinations; the maximum diameter of the smaller of the two lesions was just 8 mm.
Table 3 presents some postoperative findings. Routine blood test results including PT, TBil, ALT, and AST levels were not significantly different between the groups. The decision for TACE therapy was made for patients with an intermediate risk (a single tumor of > 5 cm without microvascular invasion) or a high risk (a single tumor with microvascular invasion, or two or three tumors) of recurrence[18,26], and this medical decision was not significantly different between the groups.
| Variables | Total (n = 94) | ICG (n = 47) | Non-ICG (n = 47) | P value |
| POST-OP PT1, second | 13.9 (1.1) | 13.9 (0.9) | 13.9 (1.2) | 0.829 |
| POST-OP TBil1, μmol/L | 26.4 (13.5) | 27.5 (14.2) | 25.2 (12.7) | 0.418 |
| POST-OP ALT1, U/L | 272.1 (236.1) | 247.5 (159.0) | 296.7 (290.3) | 0.311 |
| POST-OP AST1, U/L | 238.0 (255.1) | 200.0 (129.7) | 275.9 (334.4) | 0.151 |
| POST-OP hospitalization, days | 7.1 (2.6) | 6.5 (1.2) | 7.6 (3.4) | 0.030 |
| Clavien-Dindo complications | 0.399 | |||
| None or grade I | 88 (93.6) | 45 (95.7) | 43 (91.5) | |
| Grade II | 6 (6.4) | 2 (4.3) | 4 (8.5) | |
| POST-OP TACE | 0.674 | |||
| Negative | 56 (59.6) | 29 (61.7) | 27 (57.4) | |
| Positive | 38 (40.4) | 18 (38.3) | 20 (42.6) | |
| Recurrence | 1.000 | |||
| Negative | 88 (93.6) | 44 (93.6) | 44 (93.6) | |
| Positive | 6 (6.4) | 3 (6.4) | 3 (6.4) |
The rate of postoperative complications (Clavien-Dindo grade II) tended to be lower in the ICG group (2% vs 4%), although the difference was not significant (P = 0.399). No grade III or higher complications were observed in both groups. After surgery, compared with patients in the non-ICG group, those in the ICG group were discharged approximately a day earlier (6.5 days vs 7.6 days, P = 0.030).
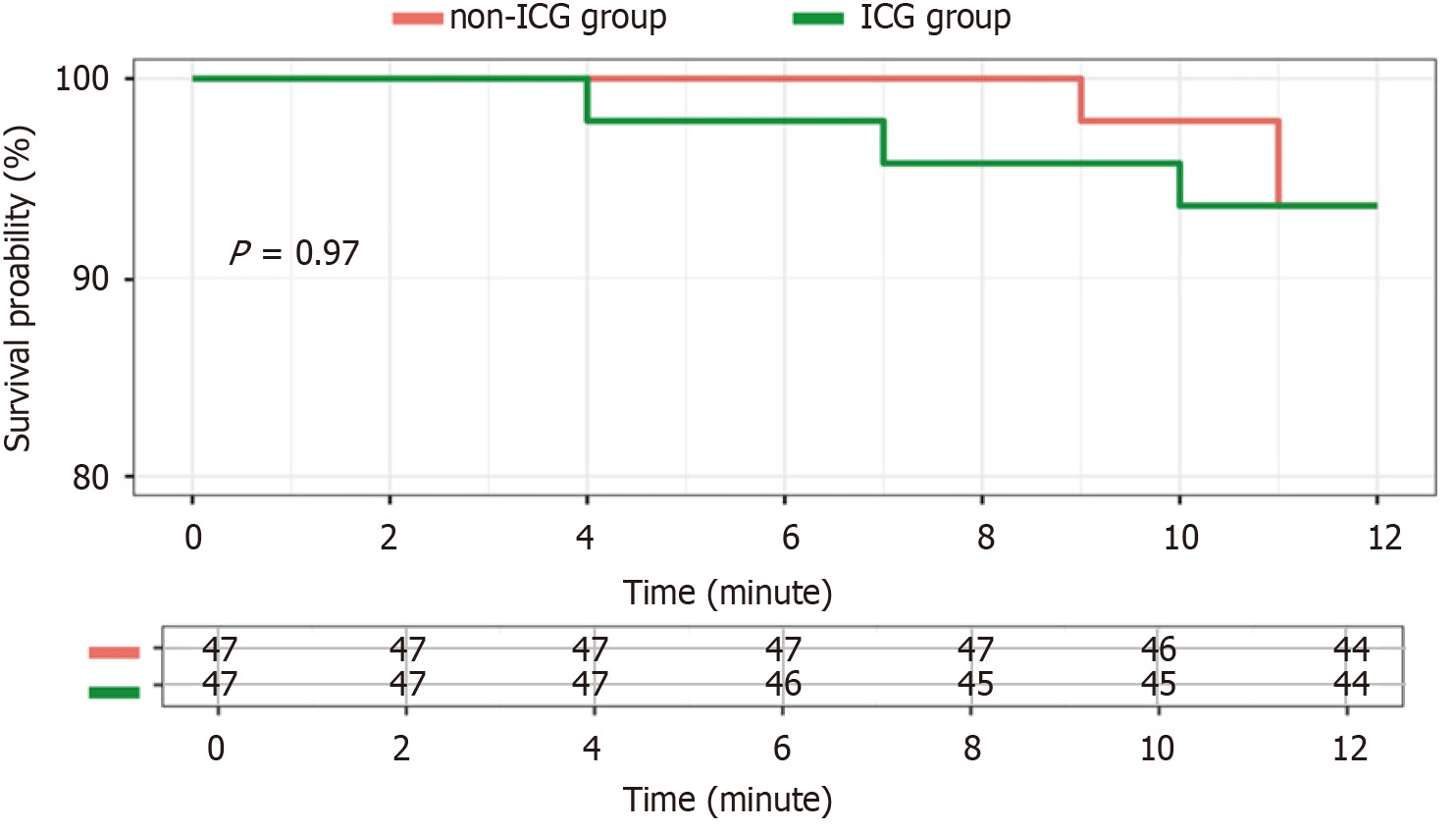
In the long-term analysis, the two groups showed no significant difference in tumor recurrence (6.4% vs 6.4%, P = 1.000), and the PFS (Figure 3) was similar. Only one patient died during the follow-up period (the non-ICG group, in the eighteenth month after surgery) due to coronavirus disease 2019. Therefore, the overall survival was not significantly different between the groups.
None of the patients showed noteworthy differences in the preoperative levels of inflammation-related markers (Table 4). The postoperative levels of inflammation-related markers were similar between the two groups. The levels of all serum inflammation-related markers were significantly elevated after surgery. All markers, except LMR, tended to decline on the third day after surgery. The postoperative test results showed that patients in the ICG group had a lower PLR on the first day (134.3 ± 53.6 vs 162.1 ± 77.6, P = 0.046) and lower NLR on the third day (4.5 ± 2.9 vs 6.3 ± 4.9 P = 0.033). Almost all postoperative marker levels were significantly lower in the ICG group although no significant difference was observed.
| Variables | Total (n = 94) | ICG (n = 47) | Non-ICG (n = 47) | P value |
| PRE-OP inflammation-related markers | ||||
| CRP | 1.8 (3.5) | 1.8 (4.4) | 1.7 (2.2) | 0.918 |
| NLR | 1.9 (0.8) | 1.9 (0.9) | 1.9 (0.8) | 0.990 |
| PLR | 98.7 (35.5) | 95.5 (33.2) | 101.8 (37.8) | 0.395 |
| LMR | 4.0 (1.5) | 4.0 (1.3) | 3.9 (1.8) | 0.701 |
| SII | 345.2 (200.0) | 332.6 (196.9) | 357.8 (204.4) | 0.544 |
| POST-OP inflammation-related markers | ||||
| CRP1 | 70.5 (49.8) | 61.1 (49.4) | 80.0 (49.0) | 0.066 |
| D1 NLR | 8.5 (4.7) | 8.2 (4.1) | 8.8 (5.3) | 0.536 |
| D1 PLR | 148.2 (67.8) | 134.3 (53.6) | 162.1 (77.6) | 0.046 |
| D1 LMR | 1.6 (0.8) | 1.5 (0.8) | 1.6 (0.7) | 0.619 |
| D1 SII | 1306.9 (813.9) | 1197.3 (644.4) | 1416.5 (948.4) | 0.193 |
| D3 NLR | 5.4 (4.1) | 4.5 (2.9) | 6.3 (4.9) | 0.033 |
| D3 PLR | 107.7 (61.0) | 102.0 (40.0) | 113.3 (76.6) | 0.373 |
| D3 LMR | 2.4 (3.9) | 2.0 (0.8) | 2.9 (5.5) | 0.278 |
| D3 SII | 708.7 (569.3) | 601.3 (451.5) | 816.1 (654.0) | 0.067 |
Compared with traditional open hepatectomy, laparoscopic hepatectomy has advantages such as the use of smaller incisions, lower blood loss, less postoperative pain, and significantly shorter hospital stays[3,4]. Consequently, it has gradually become the primary surgical option for treating liver tumors. Nonetheless, during laparoscopic operation, surgeons cannot palpate the liver to detect tumors using tactile sensation, making the detection of microscopic tumors and satellite nodules dependent on the surgeons’ intraoperative ultrasonography skills.
The application of ICG fluorescence fusion image-guided technology in laparoscopic hepatectomy not only enables surgeons detect tumors more accurately, rapidly, and intuitively, but also has an essential role in safeguarding surgical margins[9,11]. ICG emits near-infrared light when stimulated by external light, and after being absorbed by hepatic parenchymal cells, it is excreted into the biliary system without metabolism, bypassing the enterohepatic circulation[8,10,12]. This characteristic forms the foundation for its application in liver surgery.
Inflammation-related markers have been confirmed to be associated with postoperative recovery[15,16], and post
After analyzing the general clinicopathological characteristics and applying 1:1 PSM, we found no significant difference between the two groups (Table 1), which helped mitigate the bias associated with an insufficiency sample size. One notable observation was that using ICG fluorescence resulted in a shorter surgical time, lower blood loss, and milder HIO (Table 2). The fluorescence effect of the tumors allowed for an accurate and rapid localization and marking of surgical margins facilitating the dissection of the liver parenchyma. The use of special imaging techniques also made the assessment of the location of the tumors and surrounding blood vessels easy, thus reducing the amount of intraoperative bleeding. Additionally, reduced bleeding, improved the visibility of the liver sections, which in turn minimized the amount and time of HIO, and further shortening the surgical time. In this study, we found no significant difference in the surgical margins or postoperative elevation of transaminase and bilirubin levels (Tables 1 and 3), suggesting that the two surgical methods had little effect on postoperative hepatic functions. A possible reason for this is that we routinely use intraoperative ultrasonography and constantly adjust the resection line according to the boundaries between the tumor and liver parenchyma, and the essential vessels. Therefore, even without fluorescence-based navigation, we can ensure sufficient safe margins, avoid damage to normal hepatic tissues, and reduce the impact on postoperative hepatic function. However, the surgical time was prolonged to a certain extent due to the constant use of ultrasonography for intraoperative observation, which aligns with the findings of this study.
We also reimaged the surgical sections using intraoperative ICG fluorescence and detected two tiny lesions (Figure 2). Both of them were located superficially but could not be explored and distinguished from fibrotic nodules visually or through intraoperative ultrasonography and preoperative medical imaging. This highlights the crucial role of ICG fluorescence in detecting microscopic lesions. Kose et al[6] reported that the identification rates of superficial lesions were 95% with ICG fluorescence imaging and 89% with intraoperative ultrasonography, whereas those of deep lesions were only 4% with ICG fluorescence imaging and 94% with intraoperative ultrasonography. Thus, intraoperative ultrasonography is still required for deep lesions. In consequence, switching modes (ICG or white light) properly, employing ultrasonography for deep lesions, and reviewing preoperative imaging findings if necessary can help identify the characteristics of the lesions accurately.
A study[27] suggested that laparoscopic hepatectomy using ICG fluorescence improves the prognosis of patients because of better margins and R0 resection rates. However, our study presents a different view (Figure 3), as we did not observed any statistical difference in the margins and R0 resection rates (Table 2). However, as mentioned above, we did identify two tiny additional small lesions via ICG fluorescence, which could be helpful in improving the prognosis if a larger sample size and longer follow-up period were applied.
In postoperative blood tests, we identified differences in the values for inflammation-related markers (Table 4). On the first day after surgery, patients in the ICG group exhibited a lower PLR (134.3 ± 53.6 vs 162.1 ± 77.6, P = 0.046), and on the third day, they exhibited a lower NLR (4.5 ± 2.9 vs 6.3 ± 4.9, P = 0.033) compared with patients in the non-ICG group. Although no significant difference was detected, the values for almost all markers were lower in the ICG group.
Several studies have exhibited lower levels of serum inflammatory cytokines with laparoscopic surgery than with open surgery, suggesting that laparoscopic surgeries are associated with a lower surgical stress response[28-30]. And such findings are consistent with those of our research. We attributed the lower postoperative inflammatory response in the ICG group to less intraoperative trauma (Table 2) including shorter surgical time, less blood loss, and milder HIO. Inflammation-related markers are considered to be associated with perioperative outcomes[31], and better attenuation of the postoperative inflammatory response anticipated to enable faster recovery after surgery. Patients in the ICG group had a shorter duration of hospitalization (Table 3). Although significant differences were not observed in some of the inflammatory markers, we believe that ICG fluorescence offers postoperative inflammatory response attenuation, which provides several perioperative benefits.
The introduction of ICG fluorescence improves surgical outcomes and patients’ recovery after surgery and may be favorable for long-term survival. Together with other emerging technologies, such as photothermal therapy[32-34], ICG fluorescence could provide a synergistic effect. Furthermore, precise, varied, and personalized treatments can lead to more benefits.
This study has several limitations. First, it has an inherent bias and the risk of data missing due to the retrospective study and single center study design. Second, the follow-up period was not sufficiently long which leads to an insufficient power for a long-term survival analysis. In addition, the sample size was relatively small. The follow-up period will be extended to observe long-term survival effects, and the inclusion of study participants will continually be conducted.
In this study, we compared the perioperative outcomes between laparoscopic hepatectomy with and without ICG fluorescence and found that ICG reduced intraoperative trauma and attenuated the postoperative inflammatory response. Laparoscopic hepatectomy using ICG fluorescence offers benefits in the perioperative period. Although some benefits have been observed, additional in-depth studies would help to further validate our conclusions, and assist in enabling the analysis of long-term prognosis. Prospective studies conducted over long follow-up periods and at multiple centers are essential to strengthen the effectiveness of our research.
| 1. | Feng R, Su Q, Huang X, Basnet T, Xu X, Ye W. Cancer situation in China: what does the China cancer map indicate from the first national death survey to the latest cancer registration? Cancer Commun (Lond). 2023;43:75-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 69] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 2. | Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400:1345-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1284] [Cited by in RCA: 1214] [Article Influence: 404.7] [Reference Citation Analysis (41)] |
| 3. | Franken C, Lau B, Putchakayala K, DiFronzo LA. Comparison of short-term outcomes in laparoscopic vs open hepatectomy. JAMA Surg. 2014;149:941-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Biehl TR. A comparison of laparoscopic vs open hepatectomy: good try, but we still have selection bias. JAMA Surg. 2014;149:947. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Kokudo N, Takemura N, Ito K, Mihara F. The history of liver surgery: Achievements over the past 50 years. Ann Gastroenterol Surg. 2020;4:109-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 6. | Kose E, Kahramangil B, Aydin H, Donmez M, Takahashi H, Acevedo-Moreno LA, Sasaki K, Aucejo F, Berber E. A comparison of indocyanine green fluorescence and laparoscopic ultrasound for detection of liver tumors. HPB (Oxford). 2020;22:764-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Chauhan N, Cabrera M, Chowdhury P, Nagesh PKB, Dhasmana A, Pranav, Jaggi M, Chauhan SC, Yallapu MM. Indocyanine Green-based Glow Nanoparticles Probe for Cancer Imaging. Nanotheranostics. 2023;7:353-367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Nishino H, Hatano E, Seo S, Nitta T, Saito T, Nakamura M, Hattori K, Takatani M, Fuji H, Taura K, Uemoto S. Real-time Navigation for Liver Surgery Using Projection Mapping With Indocyanine Green Fluorescence: Development of the Novel Medical Imaging Projection System. Ann Surg. 2018;267:1134-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 9. | Zhang Y, Zhang Y, Zhu J, Tao H, Liang H, Chen Y, Zhang Z, Zhao J, Zhang W. Clinical application of indocyanine green fluorescence imaging in laparoscopic lymph node dissection for intrahepatic cholangiocarcinoma: A pilot study (with video). Surgery. 2022;171:1589-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Terasawa M, Ishizawa T, Mise Y, Inoue Y, Ito H, Takahashi Y, Saiura A. Applications of fusion-fluorescence imaging using indocyanine green in laparoscopic hepatectomy. Surg Endosc. 2017;31:5111-5118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 11. | Ishizawa T, Saiura A, Kokudo N. Clinical application of indocyanine green-fluorescence imaging during hepatectomy. Hepatobiliary Surg Nutr. 2016;5:322-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 12. | Tao H, Wang Z, Zeng X, Hu H, Li J, Lin J, Lin W, Fang C, Yang J. Augmented Reality Navigation Plus Indocyanine Green Fluorescence Imaging Can Accurately Guide Laparoscopic Anatomical Segment 8 Resection. Ann Surg Oncol. 2023;30:7373-7383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Agarwal V, Divatia JV. Enhanced recovery after surgery in liver resection: current concepts and controversies. Korean J Anesthesiol. 2019;72:119-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 14. | Jia W, Liu W, Qiao X. Chinese Expert Consensus on Enhanced Recovery After Hepatectomy (Version 2017). Asian J Surg. 2019;42:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Fu XT, Tang Z, Chen JF, Shi YH, Liu WR, Gao Q, Ding GY, Song K, Wang XY, Zhou J, Fan J, Ding ZB. Laparoscopic hepatectomy enhances recovery for small hepatocellular carcinoma with liver cirrhosis by postoperative inflammatory response attenuation: a propensity score matching analysis with a conventional open approach. Surg Endosc. 2021;35:910-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Song DJ, Zhu K, Tan JP, Cai JB, Lv MZ, Hu J, Ding ZB, Shi GM, Ren N, Huang XW, Shi YH, Qiu SJ, Ye QH, Sun HC, Gao Q, Zhou J, Fan J, Wang XY. Perioperative and oncologic outcomes of laparoscopic versus open liver resection for combined hepatocellular-cholangiocarcinoma: a propensity score matching analysis. Surg Endosc. 2023;37:967-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 17. | Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2279] [Cited by in RCA: 4652] [Article Influence: 4652.0] [Reference Citation Analysis (3)] |
| 18. | Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, Zhou W, Bie P, Liu L, Wen T, Han G, Wang M, Liu R, Lu L, Ren Z, Chen M, Zeng Z, Liang P, Liang C, Chen M, Yan F, Wang W, Ji Y, Yun J, Cai D, Chen Y, Cheng W, Cheng S, Dai C, Guo W, Hua B, Huang X, Jia W, Li Y, Li Y, Liang J, Liu T, Lv G, Mao Y, Peng T, Ren W, Shi H, Shi G, Tao K, Wang W, Wang X, Wang Z, Xiang B, Xing B, Xu J, Yang J, Yang J, Yang Y, Yang Y, Ye S, Yin Z, Zhang B, Zhang B, Zhang L, Zhang S, Zhang T, Zhao Y, Zheng H, Zhu J, Zhu K, Liu R, Shi Y, Xiao Y, Dai Z, Teng G, Cai J, Wang W, Cai X, Li Q, Shen F, Qin S, Dong J, Fan J. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer. 2020;9:682-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 571] [Article Influence: 114.2] [Reference Citation Analysis (1)] |
| 19. | Demirtas CO, D'Alessio A, Rimassa L, Sharma R, Pinato DJ. ALBI grade: Evidence for an improved model for liver functional estimation in patients with hepatocellular carcinoma. JHEP Rep. 2021;3:100347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 121] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 20. | Vlahcevic ZR, Buhac I, Bell CC Jr, Swell L. Abnormal metabolism of secondary bile acids in patients with cirrhosis. Gut. 1970;11:420-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 45] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24779] [Article Influence: 1180.0] [Reference Citation Analysis (0)] |
| 22. | Wakabayashi T, Cacciaguerra AB, Abe Y, Bona ED, Nicolini D, Mocchegiani F, Kabeshima Y, Vivarelli M, Wakabayashi G, Kitagawa Y. Indocyanine Green Fluorescence Navigation in Liver Surgery: A Systematic Review on Dose and Timing of Administration. Ann Surg. 2022;275:1025-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 97] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 23. | Liang J, Hu Z, Zhan C, Wang Q. Using Propensity Score Matching to Balance the Baseline Characteristics. J Thorac Oncol. 2021;16:e45-e46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 24. | Bi G, Liang J, Shan G, Zhan C. Propensity Score Matching for Bias Reduction in Genomic Profiling. J Clin Oncol. 2022;40:1259-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Chen JW, Maldonado DR, Kowalski BL, Miecznikowski KB, Kyin C, Gornbein JA, Domb BG. Best Practice Guidelines for Propensity Score Methods in Medical Research: Consideration on Theory, Implementation, and Reporting. A Review. Arthroscopy. 2022;38:632-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 76] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 26. | Wang Z, Ren Z, Chen Y, Hu J, Yang G, Yu L, Yang X, Huang A, Zhang X, Zhou S, Sun H, Wang Y, Ge N, Xu X, Tang Z, Lau W, Fan J, Wang J, Zhou J. Adjuvant Transarterial Chemoembolization for HBV-Related Hepatocellular Carcinoma After Resection: A Randomized Controlled Study. Clin Cancer Res. 2018;24:2074-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 204] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 27. | Liu F, Wang H, Ma W, Li J, Liu Y, Tang S, Li K, Jiang P, Yang Z, He Y, Liu Z, Zhang Z, Yuan Y. Short- and Long-Term Outcomes of Indocyanine Green Fluorescence Navigation- Versus Conventional-Laparoscopic Hepatectomy for Hepatocellular Carcinoma: A Propensity Score-Matched, Retrospective, Cohort Study. Ann Surg Oncol. 2023;30:1991-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 28. | Zhu P, Miao W, Gu F, Xing C. Changes of serum and peritoneal inflammatory mediators in laparoscopic radical resection for right colon carcinoma. J Minim Access Surg. 2019;15:115-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Okholm C, Goetze JP, Svendsen LB, Achiam MP. Inflammatory response in laparoscopic vs. open surgery for gastric cancer. Scand J Gastroenterol. 2014;49:1027-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Naqvi SEH, Zaka-Ur-Rab A, Islam N, Ali E. A Prospective Study of Altered Inflammatory Response and Its Clinical Outcome following Laparoscopic and Open Cholecystectomy. Iran J Med Sci. 2017;42:347-353. [PubMed] |
| 31. | Ishikawa Y, Kojima F, Ishii T, Yoshiyasu N, Ohde S, Bando T. Early postoperative inflammatory response by procedure types: stapler-based segmentectomy versus lobectomy. Gen Thorac Cardiovasc Surg. 2020;68:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Wei Z, Xin F, Zhang J, Wu M, Qiu T, Lan Y, Qiao S, Liu X, Liu J. Donor-acceptor conjugated polymer-based nanoparticles for highly effective photoacoustic imaging and photothermal therapy in the NIR-II window. Chem Commun (Camb). 2020;56:1093-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 33. | Huang M, Xu C, Yang S, Zhang Z, Wei Z, Wu M, Xue F. Vehicle-Free Nanotheranostic Self-Assembled from Clinically Approved Dyes for Cancer Fluorescence Imaging and Photothermal/Photodynamic Combinational Therapy. Pharmaceutics. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Cai H, Dai X, Guo X, Zhang L, Cao K, Yan F, Ji B, Liu Y. Ataxia telangiectasia mutated inhibitor-loaded copper sulfide nanoparticles for low-temperature photothermal therapy of hepatocellular carcinoma. Acta Biomater. 2021;127:276-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |