INTRODUCTION
Rectal cancer remains a significant health concern worldwide, with neoadjuvant chemoradiotherapy (nCRT) followed by total mesorectal excision (TME) being the standard treatment for locally advanced cases. However, TME is burdened by several relevant postoperative complications. Anastomotic leak (AL) affects up to 20% of patients undergoing rectal resection, impacting quality of life and bowel function, and often necessitating temporary or permanent stoma[1]. Moreover, AL can adversely affect oncological outcomes by increasing the risk of local recurrence and delaying the initiation of adjuvant therapy[2-4]. Identified risk factors for AL include sex, obesity, the distance of the anastomosis from the anal verge, and preoperative nCRT[5-7].
Given the substantial impact of AL on both quality of life and oncological outcomes, it is crucial to develop reliable predictive and preventive measures. In this endeavor, advances in artificial intelligence (AI) show great potential.
Machine learning (ML), a subset of AI, enables systems to learn from data and improve their performance without explicit programming. Through algorithms that detect patterns and make data-driven decisions, ML is transforming predictive models. Within ML, deep learning (DL) is a specialized area that uses multilayered neural networks (or “deep networks”) to model complex patterns in extensive datasets. This approach is particularly effective for tasks such as image recognition, natural language processing, and speech recognition. Although both ML and DL involve data-driven learning, DL typically requires larger datasets and greater computational resources to achieve high accuracy. Together, ML and DL excel at handling vast amounts of complex and clinical data, supporting the development of models that enhance patient care[8]. In healthcare and clinical settings, these models play a crucial role in applications such as diagnosis, treatment planning, intraoperative guidance, and predicting prognosis and outcomes[9,10].
In the management of patients with rectal cancer, various applications of DL have been investigated. DL techniques are applied in radiological imaging (e.g., computed tomography and magnetic resonance imaging [MRI]) and histopathological analysis to support clinical decision-making[11].
DL techniques have also been used to predict tumor response to neoadjuvant treatment. Moreover, ML and DL have been applied to anticipate postoperative complications following TME by analyzing preoperative factors such as patient characteristics, radiological findings, surgical details, and preoperative treatments. Given the incidence and clinical impact of AL, ML, and DL can be valuable tools for predicting and potentially preventing anastomosis-related complications.
RISK FACTORS FOR ALS
ML systems, trained on diverse and extensive data, can generalize from past experiences to make accurate predictions about future events or outcomes. A recent study showed that neural networks have higher predictive power than regression models for major surgical complications and for AL after colorectal surgery[12]. In an attempt to identify possible preoperative, clinical, or radiological risk factors for AL, several ML models have been developed.
Preoperative risk factors
Malnutrition is a well-documented preoperative risk factor for AL, significantly affecting patient outcomes by increasing morbidity, mortality, length of hospital stay, and healthcare costs[13,14]. It negatively impacts wound healing and tissue integrity, leading to decreased collagen synthesis and reduced vascularization at the anastomotic site[15-17]. ML models have been developed to predict the impact of malnutrition on surgical outcomes and have identified forearm circumference and forearm muscle area as the most relevant criteria for predicting the occurrence of surgical complications[18].
In addition to malnutrition, body composition, particularly visceral fat, has been identified as a significant risk factor for surgical complications[19]. A high visceral fat area (VFA) is a notable predictor of postoperative complications in colorectal cancer surgeries, with patients exhibiting higher VFA facing increased risks of major complications compared to those with lower VFA. Body fat ratio as a novel predictor of complications and survival after rectal cancer surgery[20]. Based on these premises, Shao et al[21] used random forest analysis to test the hypothesis that abdominal composition factors contribute to AL. Using feature importance analysis, an AI method for assessing the importance of each included feature, the authors quantified the contribution of various abdominal composition indicators to the development of AL. In their study of 156 patients who underwent rectal surgery, they identified the transverse diameter of the abdominal cavity, the anterior-to-posterior diameter of the abdominal cavity, and the VFA as the three most critical features.
The use of AI-based models to determine malnutrition and body composition preoperatively could thus be used in clinical practice to identify patients at risk for AL and guide surgical decisions.
Perioperative risk factors
While several predictive models are available to assess the risk of postoperative complications in rectal cancer patients, artificial neural network (ANN) and AI-based predictive models outperform traditional statistical models due to their ability to handle complex, non-linear data and continuously learn from new information. These models increase predictive accuracy and robustness, leading to improved surgical outcomes and personalized patient care[22].
These tools have been used to predict postoperative outcomes in patients with colorectal cancer. Adams and Papagrigoriadis[23] developed an ANN-based prediction model for AL based on preoperative and postoperative clinical data. The main predictors were C-reactive protein on postoperative day (POD) IV-V, platelet count on POD I-V, and preoperative hemoglobin. Baker et al[24] used backward propagation neural net enhanced multivariable regression and propensity score modified analysis applied to the American College of Surgeons National Surgical Quality Improvement Program database. Clostridium difficile infection significantly increased AL in a cohort of 46735 colectomies.
Radiomics features
To date, the role of radiomics in predicting AL has been limited. However, the potential to predict such a significant complication based on preoperative imaging is highly valuable. Cai et al[25] and Fu et al[26] developed two DL models to predict the use of multiple linear suture loads, an intraoperative factor associated with increased AL after laparoscopic low anterior resection. These models used clinical and radiomics data to identify patients at risk of ≥ 3 stapler firings on staging MRI. Tumor size ≥ 5 cm and preoperative carcinoembryonic antigen level > 5 ng/mL were identified as independent risk factors for ≥ 3 linear stapler firings. Notably, the integrated model demonstrated superior diagnostic performance compared to both the clinical and image-based models, underscoring the potential of integrated models to improve the identification of patients at risk for surgical complications.
STOMA PREDICTION
Some authors emphasize the need to determine whether a temporary or permanent stoma is required after TME, primarily because of the risk of AL. The key consideration is to avoid unnecessary temporary ileostomies in patients who do not require them, while ensuring that patients at high risk of AL following low anterior resection receive an ileostomy to mitigate the clinical impact of this complication[27]. Shao et al[28] analyzed medical data from 2240 patients who underwent rectal resection to predict the risk of AL and the need for a temporary ileostomy. They used a support vector machine (SVM) model, a supervised ML algorithm commonly applied to classification and regression tasks. SVM works by maximizing the margin between classes, enhancing generalization. Their findings suggest that the model would have recommended a protective ileostomy for 40% of patients who developed AL but did not receive an ileostomy during surgery. Likewise, it would have avoided the placement of a temporary ileostomy in 17% of patients who did not develop AL.
Liu et al[29] developed an XGBoost-ML model, while Kuo et al[30] identified two models, decision tree and light gradient boosting machine, to predict the risk of non-sphincter-saving surgery and permanent stoma in rectal cancer patients. Their studies identified several risk factors, including tumor distance from the dentate line, advanced age, clinical N stage, and previous chemoradiotherapy. Both groups concluded that AI-based models outperformed traditional methods and could be more amenable for extensive clinical application.
AL SCORES AND CALCULATORS
Beyond identifying risk factors for AL, AI models have been utilized to validate or develop predictive scoring systems. Sammour et al[31] evaluated and compared three AL risk calculators: The anastomoticleak.com calculator, the American College of Surgeons National Surgical Quality Improvement Program calculator, and the colon leakage score calculator. Using a cohort of 402 patients from a single institution who underwent colon cancer surgery, the authors conducted AI analysis with IBM Watson analytics (IBM Co., Armonk, NY, United States). In their dataset, only the anastomoticleak.com calculator score and patient age emerged as independent predictors of AL.
Arezzo et al[32] applied a ML technique, multiple imputation, to identify and quantify risk factors for AL. Through a meta-analysis of AL studies, they extracted pre-, intra-, and postoperative data to compare AL rates. Using a multivariate logistic regression model, they developed a predictive score that incorporates several preoperative variables. Significant risk factors identified included sex, short-course radiotherapy, T and N staging, perioperative blood transfusion requirements, and the anastomotic distance from the anal verge. This predictive score is available online at real-score.org.
INTRAOPERATIVE GUIDANCE: FROM INTRAOPERATIVE INDOCYANINE GREEN TO COMPUTED-ASSISTED SURGERY
Intraoperative indocyanine green
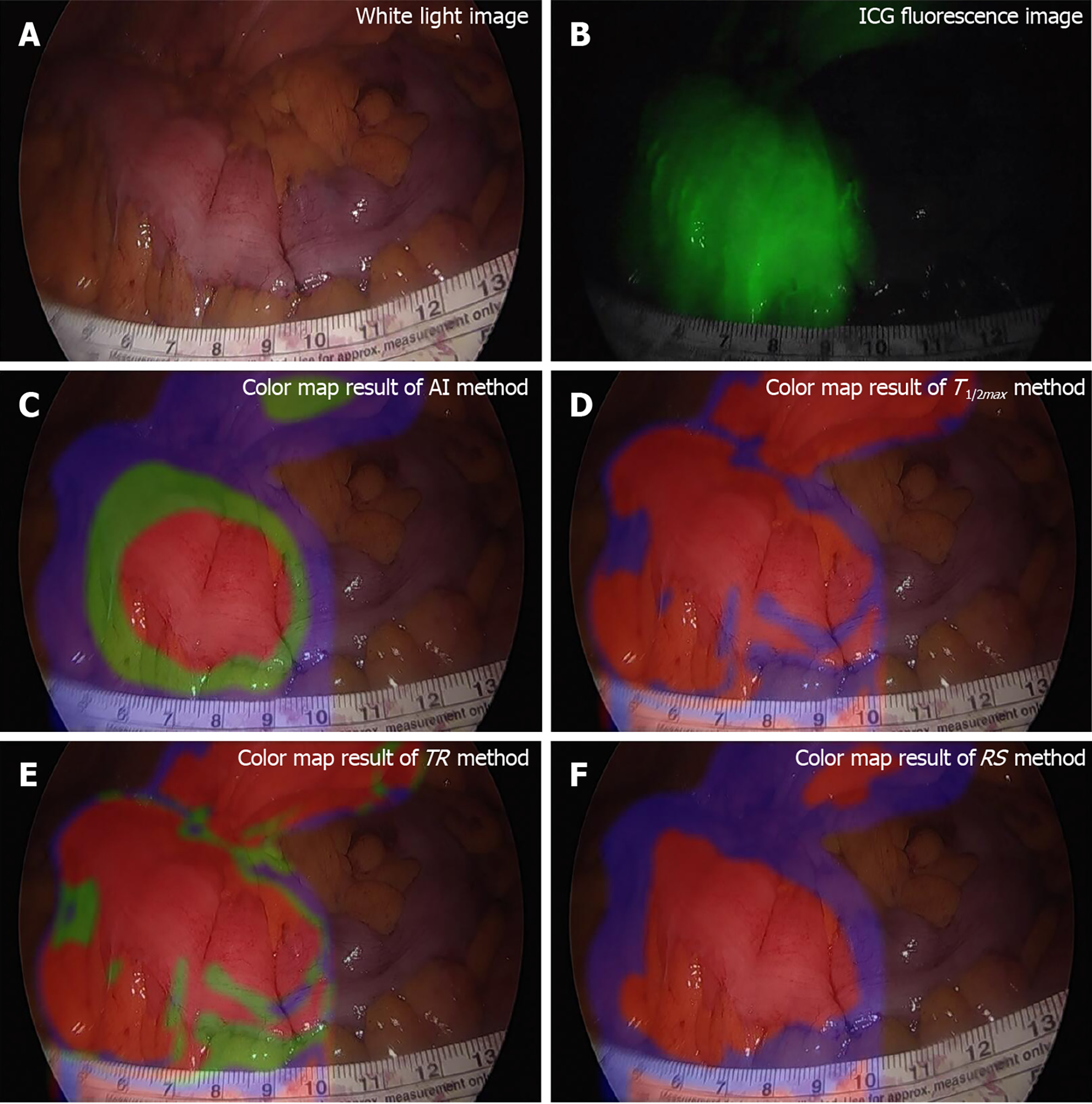
Intraoperative factors play a critical role in the success of anastomosis, directly impacting the risk of postoperative anastomotic complications. One crucial factor in preventing AL is ensuring proper bowel blood perfusion. A fluorescence laparoscopic system can be used to assess tissue microcirculation, and intraoperative indocyanine green (ICG) angiography helps predict hypoperfusion-related complications[33]. Randomized clinical trials have reported a reduction in AL rates with the use of ICG fluorescence imaging[34]. In this context, AI techniques can be valuable for real-time monitoring of adequate blood perfusion at the bowel and colorectal anastomosis sites[35]. Park et al[36] developed a ML model for real-time AI-based analysis of the microcirculation system. Their ANN effectively analyzed fluorescence curves, accurately distinguishing between well-vascularized and poorly vascularized intestinal tissue (Figure 1).
Figure 1 Colonic tissue perfusion per indocyanine green injection.
A: White light image; B: Indocyanine green fluorescence image, very well observed; C-F: Images resulting from elaboration of fluorescence data. AI: Artificial intelligence; ICG: Indocyanine green; RS: Rising slope; TR: Time ratio. Citation: Park SH, Park HM, Baek KR, Ahn HM, Lee IY, Son GM. Artificial intelligence based real-time microcirculation analysis system for laparoscopic colorectal surgery. World J Gastroenterol 2020; 26: 6945-6962. ©The Author(s) 2020. Published by Baishideng Publishing Group Inc[36].
Image segmentation
Accurate anatomical dissection during surgery is essential for preventing major intraoperative complications, such as bleeding and ischemia, which can subsequently lead to postoperative complications and AL. Image segmentation can support surgeons during procedures by providing guidance and facilitating their work. Semantic segmentation, a technique in image recognition, has emerged as a transformative tool in surgical applications. It can assist in computer-assisted surgery by enabling real-time identification and delineation of organs and anatomical structures, thereby enhancing surgical accuracy, safety, and effectiveness. Numerous studies have investigated the use of image segmentation for various purposes, including the recognition of blood vessels and dissection planes[37-39]. Igaki et al[38] developed a DL model to recognize the plane of mesorectal dissection for accurate surgical laparoscopic TME. Kitaguchi et al[39] developed a DL model for identifying the inferior mesenteric artery during sigmoidectomy, demonstrating its potential for real-time use.
Hyperspectral imaging
Hyperspectral imaging (HSI) is a contrast-free optical imaging technology that facilitates the quantitative imaging of physiological tissue parameters and the visualization of anatomical structures. In surgery, HSI is a powerful tool that offers numerous advantages. It enables the quantification of essential physiological tissue parameters, providing deeper insights into tissue composition, oxygenation levels, and metabolic activity. This quantitative information empowers surgeons to make informed decisions, improving the precision of interventions and reducing the risk of complications. Unlike ICG, HSI can assess and quantify tissue perfusion more comprehensively. The potential of HSI in colorectal surgery has been demonstrated in both animal models and surgical specimens[11,34,35]. In vivo HSI has been applied to determine tissue perfusion in intestinal ischemia and direct intraoperative decision-making[40]. Although still in its infancy, HSI shows potential in assessing tissue perfusion and could be useful in the intraoperative assessment of the viability of stumps to be anastomosed during colorectal resection[41].
CHALLENGE AND LIMITATIONS
The clinical application of AI to predict AL faces several challenges and limitations, despite its potential to improve patient outcomes. One major barrier is the integration of AI models into existing clinical workflows. While ML models can analyze complex, non-linear data and generate personalized predictions, they often require extensive, high-quality datasets to function accurately. In many clinical settings, access to such comprehensive data can be limited. If models are trained on insufficient or biased data, their performance may degrade, leading to inaccurate predictions. Validation in large, external patient cohorts is essential for ensuring the universal applicability of these models.
Additionally, using models for intraoperative guidance presents significant challenges. For instance, there is currently no standardized protocol for administering ICG or quantifying fluorescence. Similarly, the effectiveness of HSI can vary significantly depending on the camera and technology used for data acquisition. Given the potential of these models, it is crucial to establish expert consensus and guidelines for their implementation in daily practice.
Lastly, rigorous validation through multicenter randomized clinical trials is necessary to ensure that AI-based predictions are reliable and generalizable within healthcare settings. While the clinical use of AI models for predicting and preventing AL is challenging, ongoing research and innovation offer hope for overcoming these limitations in the future.
CONCLUSION
ML and DL techniques hold significant promise in preventing and predicting AL following TME for rectal cancer. The integration of AI can enhance patient stratification, refine surgical techniques, and improve overall accuracy. Furthermore, ML and DL have been employed to develop predictive models and probability scores that identify high-risk patients, facilitating better planning and management of rectal cancer treatment. In addition, AI enables real-time intraoperative evaluation of bowel and anastomotic perfusion. These technological advancements provide substantial benefits for both clinicians and patients, improving treatment planning and delivery. With the potential for widespread adoption in clinical practice, AI could significantly enhance the ability to offer tailored treatments and achieve better outcomes.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report’s classification
Scientific Quality: Grade B, Grade B, Grade C, Grade D, Grade E
Novelty: Grade B, Grade B, Grade B, Grade C, Grade C
Creativity or Innovation: Grade B, Grade B, Grade C, Grade C, Grade C
Scientific Significance: Grade B, Grade B, Grade B, Grade C, Grade D
P-Reviewer: Augustin G; Chen KJ; Kolokotronis T S-Editor: Fan M L-Editor: Filipodia P-Editor: Wang WB