Published online Jan 27, 2025. doi: 10.4240/wjgs.v17.i1.100910
Revised: October 3, 2024
Accepted: October 22, 2024
Published online: January 27, 2025
Processing time: 119 Days and 7.1 Hours
Despite improved survival rates in rectal cancer treatment, many patients experience low anterior resection syndrome (LARS). The preoperative LARS score (POLARS) aims to address the limitations of LARS assessment by predicting outcomes preoperatively to enhance surgical planning.
To investigate the predictive accuracy of POLARS in assessing the occurrence of LARS.
This study enrolled a total of 335 patients who underwent laparoscopic or robotic low anal sphincter-preserving surgery for rectal tumors. Patients were categorized into three groups according to their POLARS score: no LARS (score 0-20), minor LARS (score 21-29), and major LARS (score 30-42). The QLQ-C30/CR29 scores were compared among these groups, and the agreement between POLARS predictions and the actual LARS scores was analyzed.
The study population was divided into three groups: major LARS (n = 51, 27.42%), minor LARS (n = 109, 58.6%), and no LARS (n = 26, 13.98%). Significant differences in the QLQ-C30 scales of social function, diarrhea, and financial impact were detected between the no LARS and major LARS groups (P < 0.05) and between the minor LARS and major LARS groups (P < 0.05). Similarly, significant differences were detected in the QLQ-CR29 scales for blood and mucus in the stool, fecal incontinence, and stool frequency between the no LARS and minor LARS groups (P < 0.05), as well as between the minor LARS and major LARS groups (P < 0.05). The predictive precision for major LARS using the POLARS score was 82.35% (42/51), with a recall of 35.89% (42/117). The mean absolute error (MAE) between the POLARS score and the actual LARS score was 8.92 ± 5.47. In contrast, the XGBoost (extreme gradient boosting) model achieved a lower MAE of 6.29 ± 4.77, with a precision of 84.39% and a recall of 74.05% for predicting major LARS.
The POLARS score demonstrated effectiveness and precision in predicting major LARS, thereby providing valuable insights into postoperative symptoms and patient quality of life. However, the XGBoost model exhibited superior performance with a lower MAE and higher recall for predicting major LARS compared to the POLARS model.
Core Tip: The study evaluated the predictive accuracy of the preoperative low anterior resection syndrome score (POLARS) in predicting low anterior resection syndrome (LARS) among 335 patients. Patients were categorized into no LARS, minor LARS, and major LARS groups based on their POLARS scores. Significant differences in the quality of life metrics were identified between the groups. While POLARS effectively predicted major LARS with an 82.35% precision and a mean absolute error (MAE) of 8.92, the XGBoost model outperformed it with an MAE of 6.29 and better recall, highlighting its superior predictive capabilities.
- Citation: Pan YT, Lv YM, Zhou SC, Luo DY, Sun H, Lao WF, Zhou W. Evaluation of surgical strategy for low anterior resection syndrome using preoperative low anterior resection syndrome score in China. World J Gastrointest Surg 2025; 17(1): 100910
- URL: https://www.wjgnet.com/1948-9366/full/v17/i1/100910.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i1.100910
With advancements in medical technology, the cure rate of rectal cancer has significantly improved, leading to a nearly threefold increase in the 5-year survival rate over the past four decades[1]. However, a substantial number of patients (more than 50%) experience postoperative symptoms of impaired rectal function, such as difficulty defecating and increased stool frequency[2]. This constellation of symptoms is collectively referred to as low anterior resection syndrome (LARS)[3,4]. Presently, the evaluation and management of LARS by surgeons, as well as the overall patient care process, face significant challenges, and even skilled surgeons often struggle to address these issues[5].
The LARS score has gained widespread acceptance as a reliable tool for assessing LARS symptoms[6], with its validity confirmed through numerous studies conducted in various regions[7-12]. However, the limitations of the LARS score, which can be evaluated only postoperatively and cannot predict preoperative outcomes, have become apparent. In the era of patient-centered, personalized diagnosis, treatment, and precision medicine, the preoperative LARS (POLARS) scoring model has emerged to address these limitations. The POLARS scoring model helps predict postoperative LARS scores and residual rectal function before surgery, allowing patients to have realistic expectations and aiding in the selection of a suitable surgical plan[13].
A mathematical model for the POLARS score was proposed based on the analysis of multicenter studies conducted in the United Kingdom and Denmark. Preoperative information, including age, sex, surgical method (total/partial mesorectal excision), tumor height, stoma, and preoperative radiotherapy, was collected and analyzed. This model aims to predict postoperative LARS scores before surgery, providing valuable guidance for clinical decision-making[13]. Surgeons can preoperatively predict the recovery of rectal and anal function of patients using this data model.
However, like many previously proposed models, the POLARS scoring model has certain limitations, as recent research has highlighted its suboptimal prediction accuracy[14]. Furthermore, the direct relationship between the POLARS score and postoperative quality of life (QOL) remains unknown. In this study, we sought to analyze the correlation between the POLARS score and postoperative QOL via the EORTC QLQ-C30/CR29 QOL score, which is widely employed for assessing the QOL of patients with colorectal cancer because of its reliability and stability[15-17]. Additionally, a mechanical learning model was trained using data related to the POLARS score, and its accuracy in predicting the POLARS score was evaluated by comparing it with the actual LARS scores obtained from patients after surgery.
A total of 335 patients who underwent anterior rectal resection at Sir Run Shaw Hospital between June 2013 and September 2020 were included in this study. The inclusion criteria were as follows: (1) Pathological confirmation of rectal adenocarcinoma before surgery, with subsequent laparoscopic or robotic low anal sphincter preserving surgery, colon rectal or colon anal anastomosis; (2) Absence of colostomy, enterostomy, intubation, or fistulation during the completion of the questionnaires; and (3) Completion of the LARS score and QLQ-C30/CR29 questionnaires or successful telephone follow-up. The exclusion criteria were as follows: (1) Questionnaire completion rate of less than 90%; and (2) Inability to obtain the preoperative clinical data required for the POLARS score. A total of 335 patients were followed up, and after applying the inclusion and exclusion criteria, 186 patients were deemed eligible. Among those, 172 patients underwent laparoscopic surgery, and 14 underwent robotic surgery.
The preoperative clinical data were analyzed, and based on their POLARS scores, the 186 patients were categorized into three groups: no LARS (POLARS score 0-20, n = 26), minor LARS (POLARS score 21-29, n = 109), and major LARS (POLARS score 30-42, n = 51). The correlation between the POLARS score and QOL score was analyzed, and Cohen’s κ statistic was employed to assess the level of agreement between the POLARS score and the actual LARS score.
The mean absolute error (MAE) was calculated to evaluate the discrepancy range between the POLARS score and the actual LARS score. Additionally, machine learning models were trained using the POLARS scoring data, and their prediction accuracy was evaluated by comparing the predicted results with the actual LARS data. All the statistical analyses and calculations were performed using R software and Python (version 3.8.0; Python Software Foundation). Categorical variables are presented as totals and percentages, and the χ² test was used to compare differences between groups. Continuous variables are presented as the means and standard deviations, with t-tests used to compare the two groups. Multiple imputation procedures were developed for missing values.
The incidence of LARS predicted by the POLARS scoring model following low anterior rectal resection is presented in Table 1. As depicted in Table 1, the study revealed a greater proportion of patients with minor LARS (n = 109, 58.6%) than with major LARS (n = 51, 27.42%) and no LARS (n = 26, 13.98%).
| Parameters | All | No LARS, % | Minor LARS, % | Major LARS, % | P | ||
| No vs minor | No vs major | Minor vs major | |||||
| Patients | 186 | 26 (13.98) | 109 (58.6) | 51 (27.42) | |||
| Age in years | 59.35 ± 10.28 | 69.85 ± 8.03 | 60.51 ± 8.84 | 52.47 ± 9.14 | < 0.001 | < 0.001 | < 0.001 |
| Sex | 0.029 | 0.026 | < 0.001 | ||||
| Male | 129 (69.35) | 23 (12.37) | 73 (39.25) | 33 (17.74) | |||
| Female | 57 (30.65) | 3 (1.61) | 36 (19.35) | 18 (9.68) | |||
| TME/PME | < 0.001 | < 0.001 | < 0.001 | ||||
| TME | 82 (44.09) | 0 (0) | 34 (18.28) | 48 (25.81) | |||
| PME | 104 (55.91) | 26 (13.98) | 75 (40.32) | 3 (1.61) | |||
| Tumor height in cm | 9.08 ± 3.32 | 13.69 ± 2.02 | 9.44 ± 2.60 | 6.08 ± 1.87 | < 0.001 | < 0.001 | < 0.001 |
| Stoma | 86 (46.24) | 3 (1.61) | 37 (19.89) | 46 (24.73) | 0.024 | < 0.001 | < 0.001 |
| Preoperative radiotherapy | 51 (27.42) | 0 (0) | 17 (9.14) | 34 (18.28) | 0.043 | < 0.001 | < 0.001 |
The QOL scores were categorized into three groups based on the POLARS prediction score, namely, no LARS, minor LARS, and major LARS. The QLQ-C30 and CR29 scores for each group are shown in Tables 2 and 3, respectively. Tables 2 and 3 demonstrate significant differences in the QLQ-C30 scales of social function, diarrhea, and financial impact between the no LARS and major LARS groups (P < 0.05), as well as between the minor LARS and major LARS groups (P < 0.05). Furthermore, a significant difference in the QLQ-CR29 scale of embarrassment was observed between the no LARS and minor LARS groups (P < 0.05). Among the QLQ-CR29 scales, blood and mucus in the stool, fecal incontinence, stool frequency, and embarrassment significantly differed between the no LARS and minor LARS groups (P < 0.05). Additionally, the minor LARS and major LARS groups showed significant differences in the QLQ-CR29 scores of blood and mucus in the stool, fecal incontinence, stool frequency, body image, and urinary incontinence (P < 0.05). No statistically significant differences were observed in the remaining scales.
| C30 scales | No LARS | Minor LARS | Major LARS | P | ||
| No vs minor | No vs major | Minor vs major | ||||
| Total | 26 (13.98) | 109 (58.60) | 51 (27.42) | |||
| PF | 87.69 ± 17.96 | 88.38 ± 16.16 | 88.37 ± 12.35 | 0.836 | 0.627 | 0.571 |
| RF | 89.74 ± 17.69 | 85.63 ± 21.33 | 83.99 ± 19.99 | 0.303 | 0.163 | 0.47 |
| EF | 88.78 ± 17.79 | 88.46 ± 15.63 | 87.25 ± 13.16 | 0.773 | 0.284 | 0.243 |
| CF | 91.03 ± 15.08 | 87.31 ± 15.37 | 86.27 ± 15.88 | 0.184 | 0.154 | 0.713 |
| SF | 83.33 ± 25.39 | 81.80 ± 21.58 | 73.53 ± 20.05 | 0.386 | 0.018 | 0.007 |
| Fatigue | 21.37 ± 21.30 | 24.87 ± 22.17 | 28.32 ± 20.53 | 0.473 | 0.118 | 0.215 |
| Nausea and vomiting | 1.92 ± 5.43 | 2.29 ± 7.34 | 2.29 ± 7.47 | 0.963 | 0.863 | 0.851 |
| Pain | 8.33 ± 19.58 | 7.49 ± 13.89 | 11.44 ± 17.79 | 0.503 | 0.157 | 0.166 |
| Dyspnea | 3.85 ± 10.86 | 7.03 ± 14.40 | 8.50 ± 16.12 | 0.304 | 0.204 | 0.613 |
| Sleep disturbance | 10.26 ± 15.69 | 18.96 ± 25.40 | 16.99 ± 23.45 | 0.153 | 0.309 | 0.688 |
| Appetite loss | 8.97 ± 15.08 | 8.56 ± 15.32 | 5.23 ± 13.94 | 0.841 | 0.176 | 0.125 |
| Constipation | 20.51 ± 26.79 | 27.52 ± 26.00 | 20.26 ± 25.01 | 0.157 | 0.938 | 0.069 |
| Diarrhea | 12.82 ± 19.04 | 16.51 ± 19.58 | 24.18 ± 22.19 | 0.354 | 0.027 | 0.036 |
| Financial impact | 11.54 ± 16.17 | 17.74 ± 24.67 | 28.10 ± 30.82 | 0.382 | 0.028 | 0.044 |
| Global quality of life | 66.35 ± 27.54 | 69.50 ± 23.47 | 68.95 ± 18.71 | 0.767 | 0.965 | 0.67 |
| CR29 scales | No LARS | Minor LARS | Major LARS | P | ||
| No vs minor | No vs major | Minor vs major | ||||
| Total | 26 (13.98) | 109 (58.60) | 51 (27.42) | |||
| Urinary frequency | 16.03 ± 19.14 | 16.82 ± 18.36 | 13.07 ± 13.87 | 0.782 | 0.764 | 0.36 |
| Blood and mucus in stool | 3.21 ± 6.70 | 6.57 ± 11.12 | 10.78 ± 13.26 | 0.167 | 0.01 | 0.038 |
| Body image | 14.53 ± 18.53 | 15.09 ± 15.68 | 19.83 ± 14.96 | 0.463 | 0.068 | 0.022 |
| Flatulence | 25.76 ± 30.74 | 28.18 ± 28.60 | 34.67 ± 27.73 | 0.623 | 0.163 | 0.137 |
| Fecal incontinence | 13.04 ± 26.09 | 18.75 ± 23.60 | 30.61 ± 28.74 | 0.145 | 0.006 | 0.013 |
| Sore skin | 1.52 ± 7.11 | 6.25 ± 14.76 | 8.67 ± 17.57 | 0.143 | 0.067 | 0.416 |
| Stool frequency | 23.91 ± 29.23 | 23.20 ± 20.91 | 34.69 ± 20.37 | 0.703 | 0.039 | 0.002 |
| Embarrassment | 21.21 ± 37.86 | 32.64 ± 31.71 | 40.28 ± 33.66 | 0.046 | 0.012 | 0.215 |
| Sexual interest (men) | 21.05 ± 25.36 | 19.44 ± 23.57 | 23.53 ± 25.33 | 0.796 | 0.707 | 0.384 |
| Impotence | 11.76 ± 20.21 | 23.23 ± 29.22 | 28.13 ± 35.02 | 0.137 | 0.128 | 0.667 |
| Sexual interest (women) | 1 | 1 | 1 | |||
| Dyspareunia | 1 | 1 | 1 | |||
| Urinary incontinence | 2.56 ± 9.06 | 5.50 ± 13.24 | 1.31 ± 6.53 | 0.295 | 0.484 | 0.033 |
| Dysuria | 5.13 ± 15.47 | 2.14 ± 8.21 | 1.96 ± 7.92 | 0.35 | 0.366 | 0.896 |
| Abdominal pain | 7.69 ± 14.32 | 6.73 ± 14.89 | 9.15 ± 16.44 | 0.619 | 0.784 | 0.308 |
| Buttock pain | 6.41 ± 18.90 | 6.73 ± 15.57 | 11.76 ± 20.90 | 0.548 | 0.116 | 0.088 |
| Bloating | 16.67 ± 21.60 | 10.09 ± 16.67 | 11.11 ± 18.46 | 0.134 | 0.244 | 0.842 |
| Dry mouth | 12.82 ± 19.04 | 19.57 ± 20.39 | 20.26 ± 23.17 | 0.111 | 0.18 | 0.976 |
| Hair loss | 6.41 ± 13.40 | 12.23 ± 22.53 | 12.42 ± 18.81 | 0.316 | 0.177 | 0.577 |
| Taste | 8.97 ± 17.78 | 3.67 ± 10.48 | 5.88 ± 14.46 | 0.094 | 0.421 | 0.385 |
| Anxiety | 23.08 ± 26.28 | 27.52 ± 25.19 | 32.03 ± 26.63 | 0.383 | 0.157 | 0.304 |
| Weight | 11.54 ± 18.72 | 16.51 ± 22.96 | 15.69 ± 21.45 | 0.355 | 0.43 | 0.924 |
The agreement between the POLARS score and the actual LARS score, as shown in Table 4, was found to be only 38.71% (72/186). Among patients predicted to have no LARS, only 40% (10/25) were accurately identified. Similarly, a mere 18.28% (20/110) of patients who were predicted to have minor LARS were accurately classified postoperatively. The POLARs score was most accurate for patients predicted to have major LARS, with 82.35% (42/51) precision, but a low recall rate of 35.89% (42/117). The imprecision of POLARS predictions primarily stemmed from underestimating the LARS score. Specifically, 60% (15/25) of patients predicted to have no LARS had minor or major LARS, whereas 59.09% (65/110) of patients predicted to have minor LARS were actually classified as major LARS. Conversely, the overestimation of LARS occurred in only 17.65% (9/51) of the cases where major LARS was predicted, but the actual outcome was no LARS or minor LARS. Cohen’s κ statistic for the level of agreement was 0.126, indicating a low level of agreement (P = 0.002).
| Parameter | POLARS score | Total | |||
| No LARS | Minor LARS | Major LARS | |||
| Actual LARS score | No LARS | 10 | 25 | 3 | 38 |
| Minor LARS | 6 | 19 | 6 | 31 | |
| Major LARS | 10 | 65 | 42 | 117 | |
| Total | 26 | 109 | 51 | 186 | |
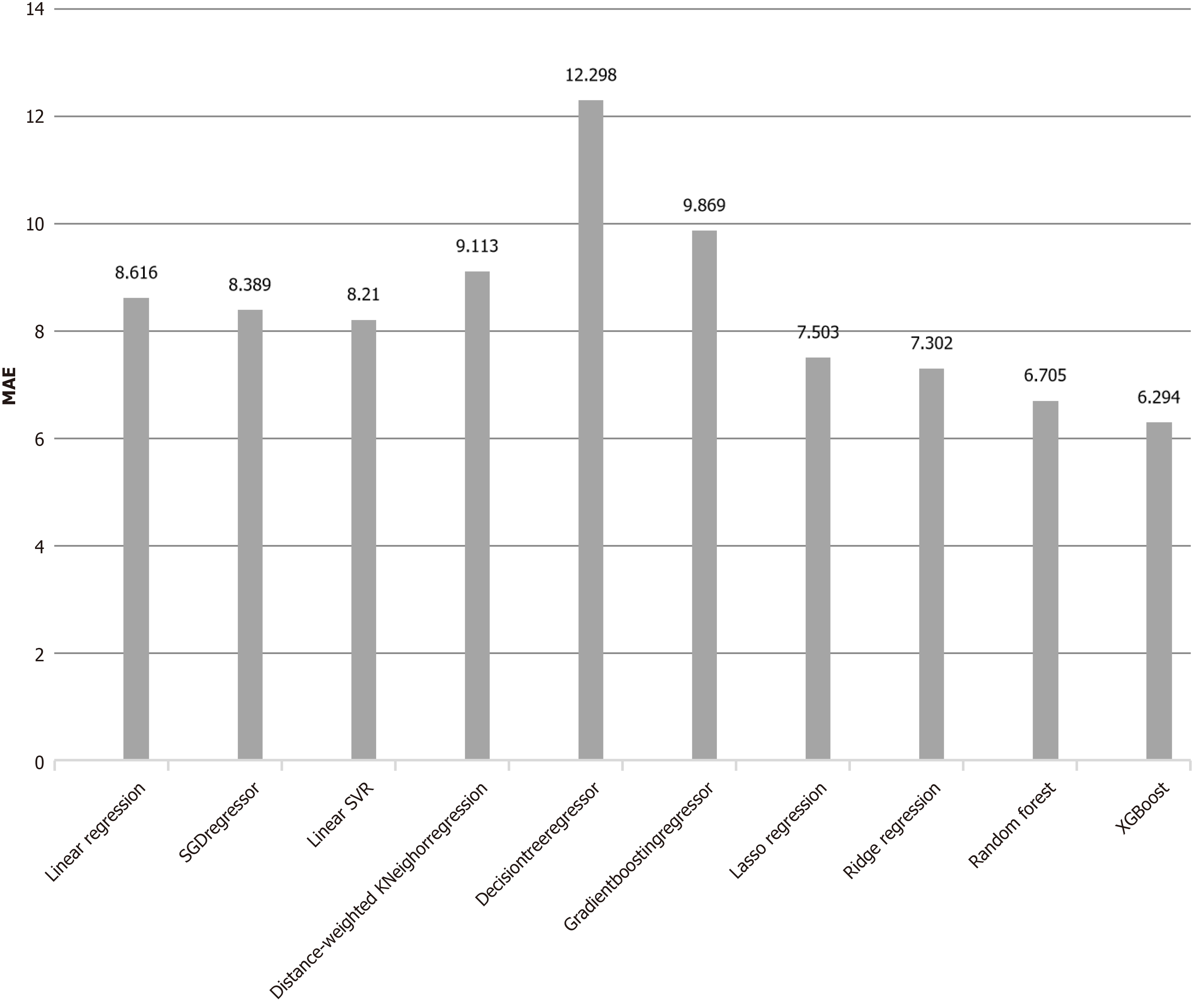
The MAE between the predicted POLARS score and the actual LARS score was 8.92 ± 5.47. To address the issue of high MAEs in POLARS, several classical machine learning models (XGBoost, random forest regression, logistic regression, ridge regression, LASSO regression, etc.) have been employed to learn the clinical data used by POLARS. The data were randomly divided into training (80%) and validation (20%) sets. Among these models, XGBoost demonstrated the best performance, with a mean MAE of 6.29 ± 4.77 after 500 random runs, as shown in Figure 1. Furthermore, XGBoost exhibited higher precision and recall rates than the POLARS scoring model did, particularly in predicting major LARS. The precision and recall rates for the major LARS were 84.39% and 74.05%, respectively, for XGBoost, whereas the corresponding rates for the POLARS scoring model were 82.35% and 35.89%, respectively.
As presented in Table 1, the prevalence of minor LARS predicted by the POLARS scoring model was notably high, accounting for 58.6% of patients. This differs from the findings of foreign epidemiological investigations[18,19], which reported a minor LARS prevalence of 27% and a major LARS prevalence of 46%. The conservative nature of the POLARS score may contribute to its tendency to underestimate the actual LARS score. However, it is important to acknowledge the bias in our dataset, where most of the patients had major LARS (117/186).
The relationships between the POLARS-predicted LARS score and the QLQ-C30/CR29 scores are depicted in Tables 2 and 3. Notably, LARS-related symptoms such as diarrhea, defecation incontinence, frequent stools, presence of blood in the stool, body image, and social function were found to have statistically significant associations with the corresponding QOL scales, particularly on the QLQ-CR29 scale. These findings align with existing research demonstrating the close connection between the LARS score and the QLQ-C30/CR29 score[20,21]. Although the number of scales with statistically significant differences was not as extensive as anticipated, these findings indicate the potential of POLARS for predicting LARS symptoms and postoperative QOL.
Cohen’s κ coefficient analysis revealed a disparity between the POLARS score and the actual LARS score. Presently, the POLARS scoring model tends to underestimate the actual LARS score because of various factors, necessitating further optimization and improvement in the future. Notably, the POLARS scoring model exhibited a high positive predictive rate of 82.35% (42/51) in predicting major LARS. This underscores the practicality of the POLARS score in clinical settings. Clinicians and patients should exercise caution when considering low anal sphincter-preserving surgery and anticipate a decline in postoperative anal function when the POLARS scoring model is used to predict major LARS. Patients can also engage in preventive Kegel exercises targeting the anal sphincter to mitigate LARS symptoms following surgery[22,23]. These insights significantly impact clinical decision-making.
The MAE between the POLARS score and the actual LARS score was also unsatisfactory. Given that the scale for the minor LARS score ranges only from 21 to 29, an MAE of 8.92 is too high to accurately predict the actual LARS score. Reducing the MAE is essential to achieve a high predictive concordance. With the rapid advancement of data science, the integration of machine learning and artificial intelligence in clinical medicine is becoming increasingly feasible. In this study, we explored the application of several classical machine learning models to clinical data, aiming to address the high MAE of the POLARS model in predicting LARS scores. After randomly dividing the data, with 80% used for training and 20% for validation, we found that the XGBoost model performed the best, achieving an MAE of 6.29 ± 4.77 across 500 runs, outperforming the other models. Qin Q and Huang B mentioned the PORTLARS prediction model in their article, which employs logistic regression algorithms to predict postoperative LARS scores for patients who have undergone radiotherapy[24]. However, their model is limited to patients after radiotherapy and uses anastomotic leakage as a predictive indicator, which cannot be obtained preoperatively. This limitation significantly reduces its applicability for preoperative clinical guidance.
Notably, XGBoost exhibited higher precision and recall rates in predicting major LARS, reaching 84.39% and 74.05%, respectively. In contrast, the POLARS model achieves precision and recall rates of only 82.35% and 35.89%, respectively. These results suggest that, compared with the POLARS model, XGBoost possesses stronger predictive capabilities and can better identify high-risk patients.
The superior performance of XGBoost may be attributed to its robust features, which combine gradient-boosting algorithms along with its ability to handle missing values and outliers, making it suitable for complex clinical data. This suggests that XGBoost could significantly enhance the accuracy of major LARS predictions. By providing surgeons and oncologists with precise preoperative predictions, XGBoost has the potential to support more personalized treatment strategies and improve patient outcomes.
While discussing the predictive accuracy and clinical utility of the POLARS tool, it is essential to acknowledge the limitations of this study. With a relatively small sample size, this preliminary evaluation of predictions made using the POLARS model highlights the need for further data and research to validate its accuracy and reliability. Conducted as a retrospective study utilizing questionnaires and telephone follow-up, this research faced challenges, including limited patient cooperation, selection bias, and missing data. Specifically, there was a notable lack of retrospective data on sexual interest (particularly in women) and dyspareunia in the CR29 scale. As more data become available, future prospective studies that are both safe and controllable will provide more reliable conclusions. With the rapid advancement of technology, the widespread application of big data models, machine learning, and artificial intelligence in clinical medicine is expected. We look forward to further research exploring the potential of these technologies in predicting postoperative complications and guiding clinical decision-making to achieve better clinical treatment outcomes.
The predictive accuracy of the POLARS model fell short of our expectations, showing a tendency to underestimate the actual LARS score. However, it demonstrated precision in predicting major LARS cases. Furthermore, a notable association was observed between POLARS scores and QLQ-C30/CR29 scores, particularly on the QLQ-CR29 scales pertaining to LARS symptoms. Compared to the POLARS model, the XGBoost model exhibited superior performance in terms of MAE, precision, and recall in predicting major LARS.
We would like to thank all the physicians in the Department of Colorectal Surgery at the Sir Run Shaw Hospital, Zhejiang University School of Medicine, particularly Professor Chao He, our mentor, and Director Xue-Feng Huang.
| 1. | Quaresma M, Coleman MP, Rachet B. 40-year trends in an index of survival for all cancers combined and survival adjusted for age and sex for each cancer in England and Wales, 1971-2011: a population-based study. Lancet. 2015;385:1206-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 343] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 2. | Bryant CL, Lunniss PJ, Knowles CH, Thaha MA, Chan CL. Anterior resection syndrome. Lancet Oncol. 2012;13:e403-e408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 435] [Article Influence: 33.5] [Reference Citation Analysis (1)] |
| 3. | Battersby NJ, Juul T, Christensen P, Janjua AZ, Branagan G, Emmertsen KJ, Norton C, Hughes R, Laurberg S, Moran BJ; United Kingdom Low Anterior Resection Syndrome Study Group. Predicting the Risk of Bowel-Related Quality-of-Life Impairment After Restorative Resection for Rectal Cancer: A Multicenter Cross-Sectional Study. Dis Colon Rectum. 2016;59:270-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 140] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 4. | Keane C, Wells C, O'Grady G, Bissett IP. Defining low anterior resection syndrome: a systematic review of the literature. Colorectal Dis. 2017;19:713-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 5. | Andreyev HJ, Davidson SE, Gillespie C, Allum WH, Swarbrick E; British Society of Gastroenterology; Association of Colo-Proctology of Great Britain and Ireland; Association of Upper Gastrointestinal Surgeons; Faculty of Clinical Oncology Section of the Royal College of Radiologists. Practice guidance on the management of acute and chronic gastrointestinal problems arising as a result of treatment for cancer. Gut. 2012;61:179-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 214] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 6. | Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 1586] [Article Influence: 264.3] [Reference Citation Analysis (2)] |
| 7. | Eid Y, Bouvier V, Dejardin O, Menahem B, Chaillot F, Chene Y, Dutheil JJ, Juul T, Morello R, Alves A. 'French LARS score': validation of the French version of the low anterior resection syndrome (LARS) score for measuring bowel dysfunction after sphincter-preserving surgery among rectal cancer patients: a study protocol. BMJ Open. 2020;10:e034251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Akizuki E, Matsuno H, Satoyoshi T, Ishii M, Usui A, Ueki T, Nishidate T, Okita K, Mizushima T, Mori M, Takemasa I. Validation of the Japanese Version of the Low Anterior Resection Syndrome Score. World J Surg. 2018;42:2660-2667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Samalavicius NE, Dulskas A, Lasinskas M, Smailyte G. Validity and reliability of a Lithuanian version of low anterior resection syndrome score. Tech Coloproctol. 2016;20:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Hupkens BJP, Breukink SO, Olde Reuver Of Briel C, Tanis PJ, de Noo ME, van Duijvendijk P, van Westreenen HL, Dekker JWT, Chen TYT, Juul T. Dutch validation of the low anterior resection syndrome score. Colorectal Dis. 2018;20:881-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Hou XT, Pang D, Lu Q, Yang P, Jin SL, Zhou YJ, Tian SH. Validation of the Chinese version of the low anterior resection syndrome score for measuring bowel dysfunction after sphincter-preserving surgery among rectal cancer patients. Eur J Oncol Nurs. 2015;19:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Juul T, Ahlberg M, Biondo S, Emmertsen KJ, Espin E, Jimenez LM, Matzel KE, Palmer G, Sauermann A, Trenti L, Zhang W, Laurberg S, Christensen P. International validation of the low anterior resection syndrome score. Ann Surg. 2014;259:728-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 219] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 13. | Battersby NJ, Bouliotis G, Emmertsen KJ, Juul T, Glynne-Jones R, Branagan G, Christensen P, Laurberg S, Moran BJ; UK and Danish LARS Study Groups. Development and external validation of a nomogram and online tool to predict bowel dysfunction following restorative rectal cancer resection: the POLARS score. Gut. 2018;67:688-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 14. | Bogacki P, Krzak J, Gach T, Szwed W, Szura M. Can the POLARS tool accurately predict low anterior resection syndrome in rectal cancer patients undergoing laparoscopic resection? Arch Med Sci. 2023;19:365-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9802] [Cited by in RCA: 11469] [Article Influence: 358.4] [Reference Citation Analysis (0)] |
| 16. | Brown LF, Kroenke K, Theobald DE, Wu J, Tu W. The association of depression and anxiety with health-related quality of life in cancer patients with depression and/or pain. Psychooncology. 2010;19:734-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 17. | Engel J, Kerr J, Schlesinger-Raab A, Eckel R, Sauer H, Hölzel D. Quality of life in rectal cancer patients: a four-year prospective study. Ann Surg. 2003;238:203-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 272] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 18. | Sturiale A, Martellucci J, Zurli L, Vaccaro C, Brusciano L, Limongelli P, Docimo L, Valeri A. Long-term functional follow-up after anterior rectal resection for cancer. Int J Colorectal Dis. 2017;32:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 19. | Chen TY, Wiltink LM, Nout RA, Meershoek-Klein Kranenbarg E, Laurberg S, Marijnen CA, van de Velde CJ. Bowel function 14 years after preoperative short-course radiotherapy and total mesorectal excision for rectal cancer: report of a multicenter randomized trial. Clin Colorectal Cancer. 2015;14:106-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 222] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 20. | Kupsch J, Kuhn M, Matzel KE, Zimmer J, Radulova-Mauersberger O, Sims A, Witzigmann H, Stelzner S. To what extent is the low anterior resection syndrome (LARS) associated with quality of life as measured using the EORTC C30 and CR38 quality of life questionnaires? Int J Colorectal Dis. 2019;34:747-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 21. | Lao W, Prasoon P, Pan Y, Lv Y, Tan LT. The EORTC QLQ-C30 and QLQ-CR29 may play a complementary role to LARS score in evaluating the quality of life for patients following laparoscopic and robotic rectal cancer surgery. Laparosc Endosc Robotic Surg. 2021;4:79-84. [RCA] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Sakr A, Sauri F, Alessa M, Zakarnah E, Alawfi H, Torky R, Kim HS, Yang SY, Kim NK. Assessment and management of low anterior resection syndrome after sphincter preserving surgery for rectal cancer. Chin Med J (Engl). 2020;133:1824-1833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Liu CH, Chen CH, Lee JC. Rehabilitation exercise on the quality of life in anal sphincter-preserving surgery. Hepatogastroenterology. 2011;58:1461-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Qin Q, Huang B, Wu A, Gao J, Liu X, Cao W, Ma T, Kuang Y, Guo J, Wu Q, Shao B, Guan Q, Yao H, Zhang X, Wang H; Chinese Radiation Intestinal Injury Research Group. Development and Validation of a Post-Radiotherapy Prediction Model for Bowel Dysfunction After Rectal Cancer Resection. Gastroenterology. 2023;165:1430-1442.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |