Published online Jan 27, 2025. doi: 10.4240/wjgs.v17.i1.100119
Revised: September 16, 2024
Accepted: October 18, 2024
Published online: January 27, 2025
Processing time: 142 Days and 2.5 Hours
Postpancreatectomy hemorrhage is one of the most severe and life-threatening complications after pancreaticoduodenectomy. We present four cases of gas
The main symptoms included black stool, hematochezia, haematemesis, blood in the nasogastric tube, and hemorrhagic shock. The mean age was 66.25 years old and the median onset time was 340 d after the surgery. The bleeding location comprised gastrointestinal anastomosis, bile duct-jejunum anastomosis, and ex
Early and effective endoscopic intervention is the key to successful hemostasis in patients with gastrointestinal bleeding after pancreatoduodenectomy.
Core Tip: We present four cases of gastrointestinal bleeding patients to clarify its appropriate treatment and prevention. The bleeding location comprised gastrointestinal anastomosis, bile duct-jejunum anastomosis, and extraluminal bleeding. The possible etiology included marginal ulcer, jejunal varix, and abdominal infection. Endoscopic hemostatic clips, as well as a covered stent using angiography, were done to stop the bleeding and three patients survived. Only one patient died of gastrointestinal bleeding, abdominal bleeding, abdominal infection, hypovolemic shock, and disseminated intravascular coagulation. In conclusion, early and effective endoscopic intervention is the key to successful hemostasis in patients with gastrointestinal bleeding after pancreatoduodenectomy.
- Citation: Liu ZJ, Hong JY, Zhang C, She J, Zhai HH. Gastrointestinal bleeding after pancreatoduodenectomy: Report of four cases. World J Gastrointest Surg 2025; 17(1): 100119
- URL: https://www.wjgnet.com/1948-9366/full/v17/i1/100119.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i1.100119
Pancreaticoduodenectomy (PD) is commonly performed for malignant tumors of the pancreatic head, ampulla, and distal bile duct, benign tumors, trauma of the pancreatic head and duodenum, and chronic pancreatitis[1]. The procedure involves resection of the head of the pancreas, the duodenum, the proximal jejunum, the distal third of the stomach, and the lower half of the common bile duct followed by biliary, pancreatic, and gastric anastomoses to the jejunum. Postpancreatectomy hemorrhage (PPH) is one of the most severe and life-threatening complications, with an incidence of nearly 30%. Almost 5% of PPH patients require interventional (endoscopy or angiography) and/or relaparotomy. PPH can be categorized into early (< 24 h) and late PPH based on onset time. Early bleeding tends to be related to technical failure during the index operation, while late bleeding tends to result from intestinal ulcerations, which is relatively uncommon (< 1.5%) because most PD patients are elderly with hypochlorhydria or achlorhydria and seldom develop gastrointestinal ulcers[2-5]. Here, we report four cases of gastrointestinal bleeding in patients after PD surgery and review the literature to figure out the proper management for these patients. Written informed consent was obtained from all patients. The case report follows the CARE guidelines.
Case 1: A 70-year-old man was admitted because of intermittent black stool for 1 mo.
Case 2: A 65-year-old woman was admitted due to haematemesis for 1 d.
Case 3: A 69-year-old male patient was admitted because of recurrent abdominal pain for 6 years.
Case 4: A 71-year-old man was admitted because of jaundice for 1 wk.
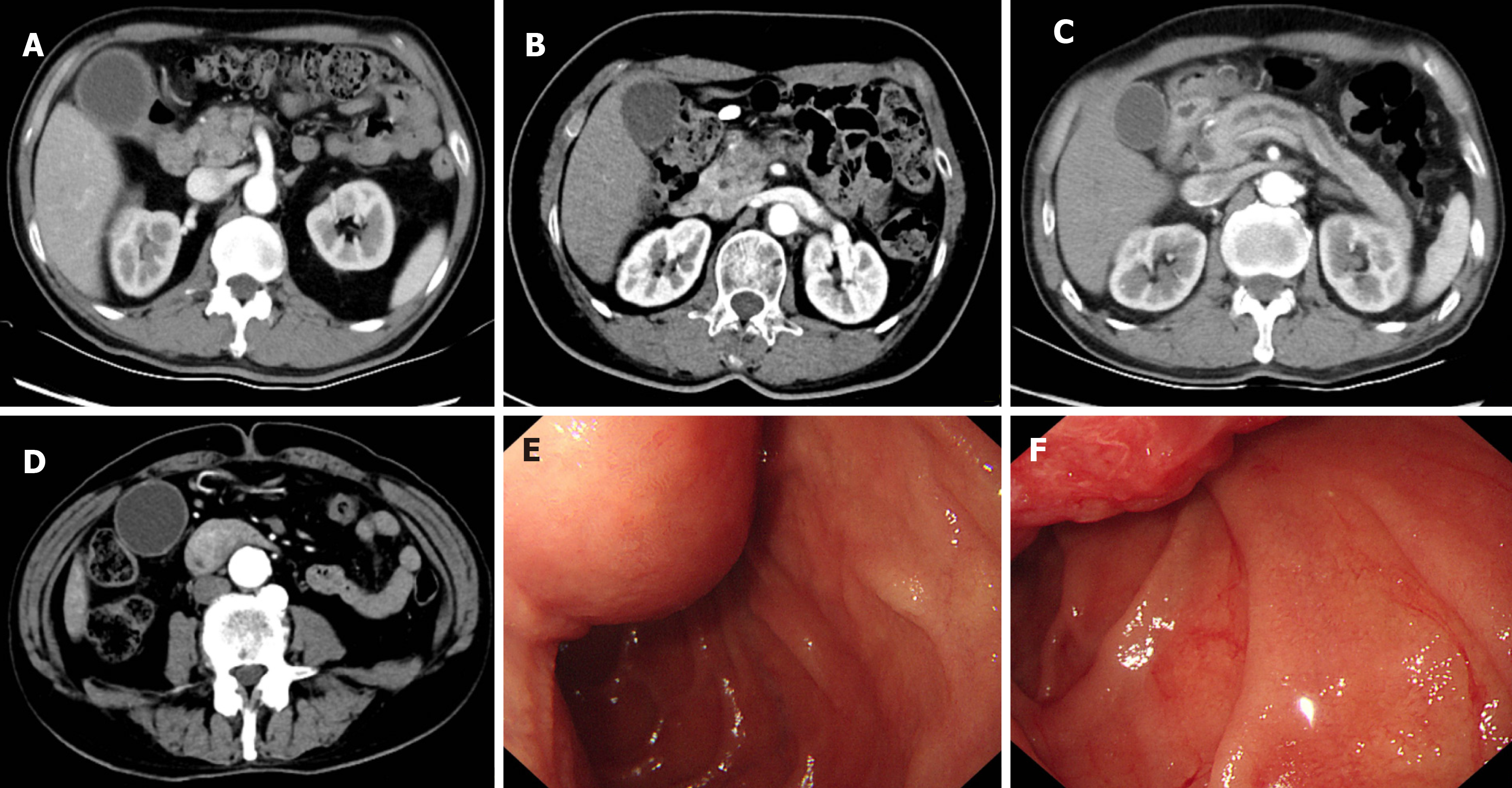
Case 1: The patient had pancreatic ductal adenocarcinoma (PDAC) and received an open radical PD with Roux-en-Y reconstruction procedure over 2 years ago (Figure 1A). Since then, he has received regular chemotherapy. He had an intermittent black stool 1 mo ago.
Case 2: The patient received an open radical PD with Roux-en-Y because of PDAC about a year ago and had regular chemotherapy after that (Figure 1B). She had hematemesis 1 d ago.
Case 3: The patient was diagnosed with chronic pancreatitis and given an open radical PD with Roux-en-Y since the pancreatic head enlargement compressed the common bile duct and pancreatic duct (Figure 1C). On the same day of the surgery, there was blood coming from the nasogastric tube, suggesting the possibility of gastrointestinal bleeding.
Case 4: Esophagogastroduodenoscopy (EGD) and computed tomography confirmed periampullary adenocarcinoma (Figure 1D-F), and the patient received an open radical PD with Roux-en-Y procedure. On the first 2 d after the surgery, there was light red fluid coming from the abdominal drainage tube and nasogastric tube.
The four patients had no previous abnormalities.
The four patients reported no family history of relative diseases.
Physical examination showed hyperactive bowel sounds for all patients.
The hemoglobin changes are shown in Table 1.
| Case number | Underlying disease | Age (years) | Gender | Postoperative days | Blood loss | Main symptoms | Bleeding location | Interventions | Outcome |
| 1 | PDAC | 70 | Male | 874 | Decrease in hemoglobin concentration less than 3 g/dL | Black stool and hematochezia | Gastrointestinal anastomosis and bile duct-jejunum anastomosis | Endoscopy | Successful hemostasis |
| 2 | PDAC | 65 | Female | 477 | Decrease in hemoglobin concentration over 3 g/dL | Haematemesis | Bile duct-jejunum anastomosis vascular malformation | Endoscopy | Successful hemostasis |
| 3 | Chronic pancreatitis | 69 | Male | 1 | Decrease in hemoglobin concentration over 3 g/dL | Blood in nasogastric tube | Gastrointestinal anastomosis | Endoscopy | Successful hemostasis |
| 4 | Periampullary adenocarcinoma | 71 | Male | 10 | Decrease in hemoglobin concentration over 3 g/dL | Blood in nasogastric tube, hematochezia, and hemorrhagic shock | Gastrointestinal anastomosis and extraluminal bleeding | Endoscopy and angiography | Dead |
Gastrointestinal bleeding after pancreatoduodenectomy.
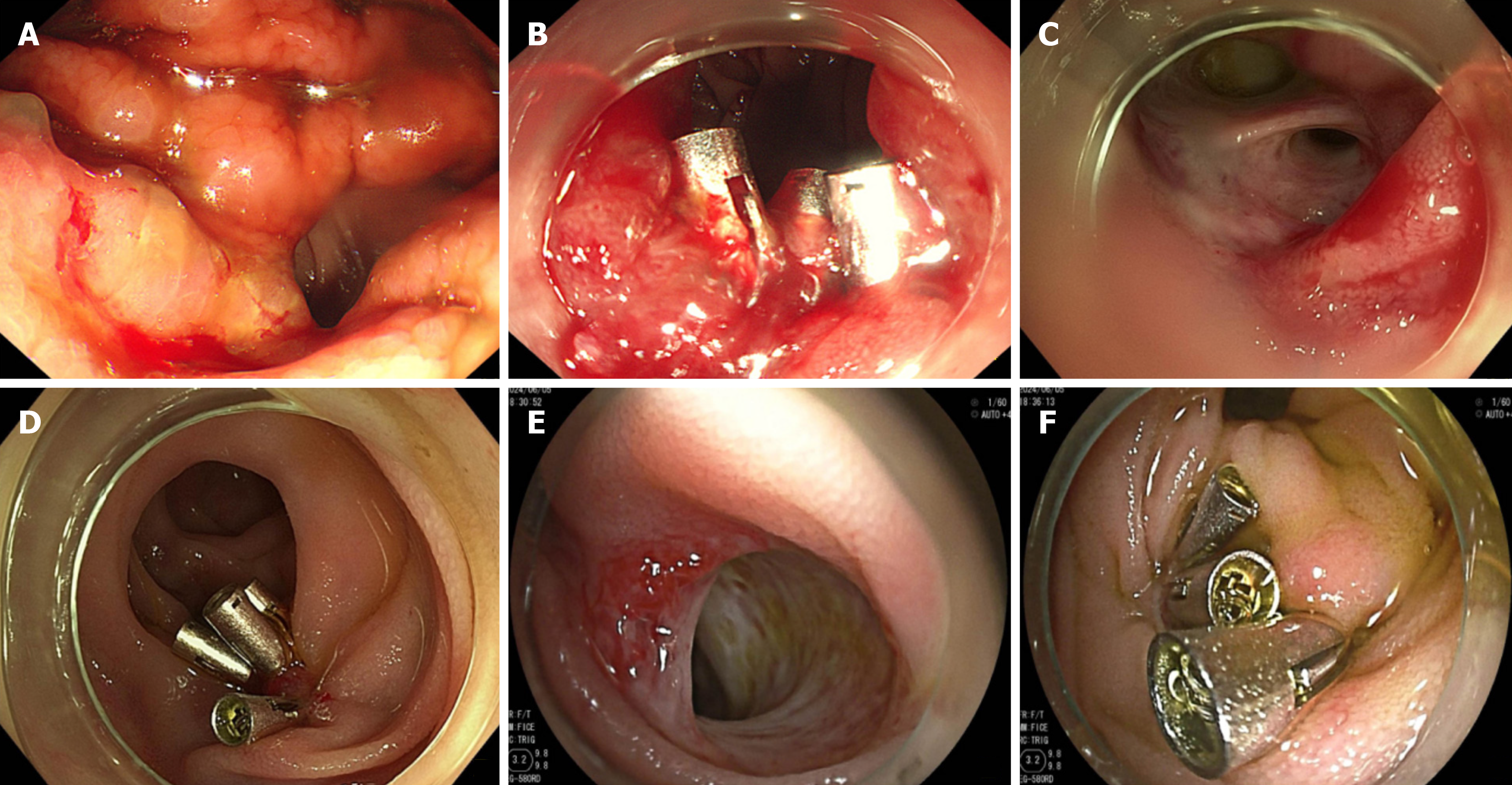
The patient received an emergency EGD in the emergency department but no bleeding spot was found in his upper gastrointestinal tract. Then, he was fasted and immediately received proton pump inhibitor (PPI) and intravenous nutrition supplement treatment. After his vital signs became stable, he received another EGD, and the result showed an oozing bleeding spot at his gastrointestinal anastomosis. He received three endoscopic hemostatic clips, and the bleeding was successfully stopped (Figure 2A and B). Ten days after discharge, he had hematochezia and loss of consciousness. He soon had EGD again, and the result showed an oozing bleeding spot at his bile duct-jejunum anastomosis. He received five endoscopic hemostatic clips, and hemostasis was successful (Figure 2C and D). Before discharge, he received another EGD, which showed no active bleeding (Table 1).
The patient immediately received an EGD, which showed only mucosal swelling at the bile duct-jejunum and pancreatic duct-jejunum anastomosis. A colonoscopy was conducted to rule out other possible etiologies, and no bleeding spot was found either. She was given the mucosa protective agent and a PPI and took a fluid diet. After that, the patient had an intermediate black stool; however, as the vital signs and hemoglobin were stable, the left blood was assumed to be discharged. To further find out the cause, a thoroughgoing EGD and colonoscopy with sedation were performed. At this moment, the EGD showed vascular malformation exposed at the bile duct-jejunum anastomosis, which was considered as the possible source of bleeding; thus, five endoscopic hemostatic clips were applied to prevent future bleeding (Figure 2E and F). An enteroscopy was also conducted, and the result was negative, which supported the possibility of vascular malformation bleeding at the duct-jejunum anastomosis. Her clinical status continued to improve, and she was discharged home in a stable condition (Table 1).
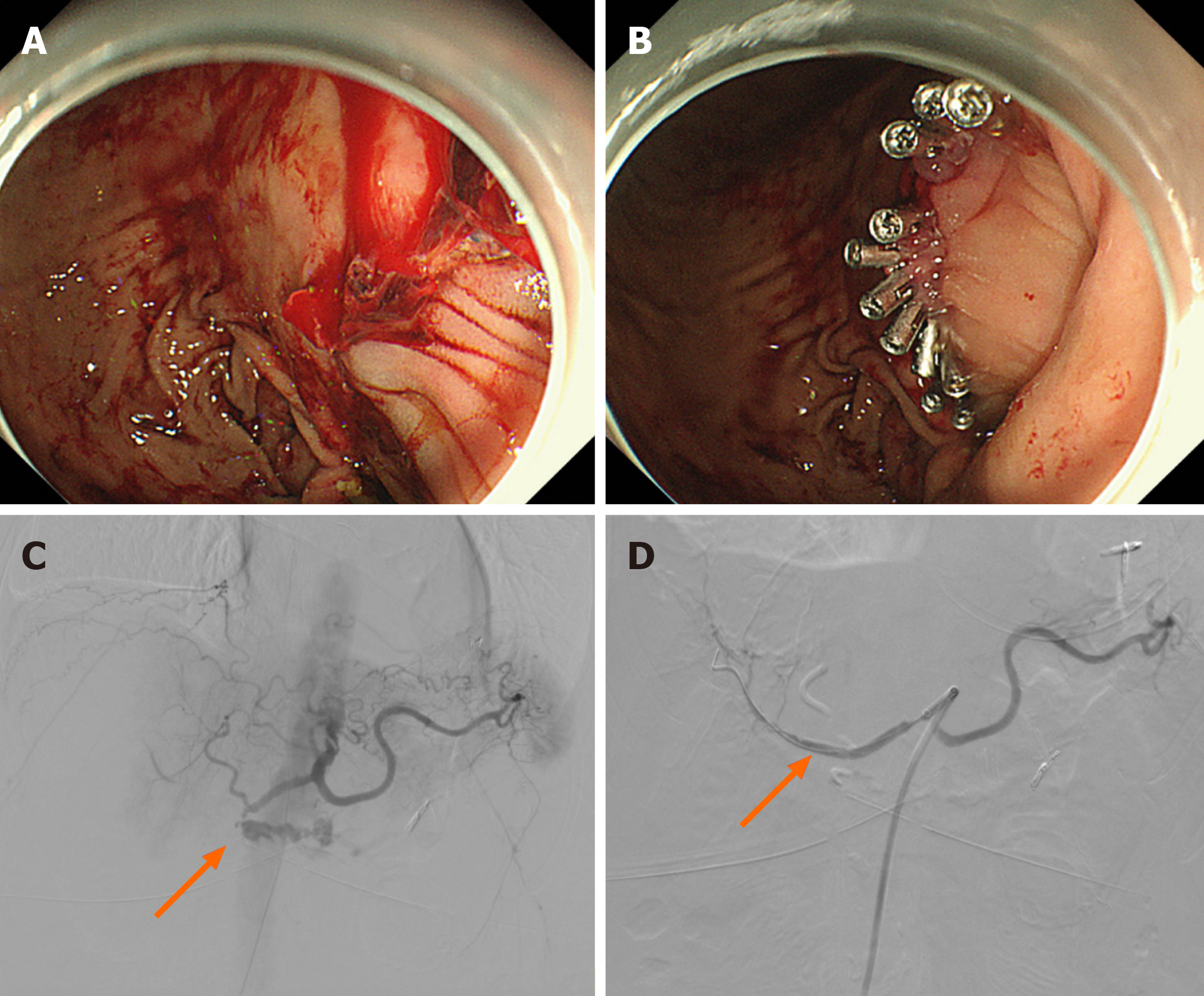
Cold normal saline was given through the nasogastric tube to constrict blood vessels. A PPI and intravenous hemostatic drugs were also applied. The patient received EGD, and the result showed a bleeding spot at the gastrointestinal anastomosis. Eleven endoscopic hemostatic clips were applied, and the bleeding was stopped successfully (Figure 3A and B). He was discharged in a stable condition a few days later (Table 1).
The patient was given blood transfusion therapy. Then, purulence mixed with blood in his bile duct-jejunum drainage tube and high body temperature indicated an abdominal infection, so he had to receive an abdominal abscess puncture. About 10 d after the procedure, the patient had a bloody stool of 300 mL and fresh blood draining from the nasogastric tube. An emergency EGD showed many gastrointestinal anastomosis ulcers, one of which had active bleeding, so 1:10000 adrenaline was injected into the bleeding mucosa, and thrombin was sprayed. After that, the patient’s abdominal and drainage tube and nasogastric tube continued to drain fresh blood, so he had to receive three EGDs and two abdominal aortographies while the hemoglobin was still unstable. About 20 d after the surgery, the patient’s blood pressure decreased to 73/48 mmHg, with 1000 mL of fresh blood draining from the abdominal tube. An emergency abdominal aortography had to be carried out, which revealed a bleeding spot of the gastroduodenal artery, and then a covered stent was placed at the bleeding spot (Figure 3C and D). Despite this, the patient had disseminated intravascular coagulation (DIC) and more fresh blood from his abdominal drainage tube and nasogastric tube. He died on the 23rd day after PD (Table 1).
The patient was continuously followed in the out-patient department and the last visit occurred three years after PD. He had local PDAC recurrence and received percutaneous transhepatic cholangial drainage. He did not experience gastrointestinal bleeding again.
The last visit occurred one and half years after PD and the patient had abdominal metastasis. She experienced two more times of gastrointestinal bleeding and the bleeding location became gastrointestinal anastomosis.
The patient was continuously followed and the last visit occurred 9 mo after surgery. He only experienced mild dyspepsia symptoms.
Dead.
PPH is one of the most serious and life-threatening complications of pancreaticoduodenectomy and is associated with high mortality. According to the International Study Group of Pancreatic Surgery definition, PPH is graded as A, B, or C according to three parameters: Onset (early PPH < 24 h post-surgery; late PPH ≥ 24 h after the end of the index operation), location (intra- or extraluminal), and severity. General management of PPH includes EGD, surgical re-exploration, and angiography[2]. Here we presented the diagnosis and treatment process of four cases of endoscopy-confirmed gastrointestinal bleeding after PD procedure, which was quite rare.
For the first two cases, they were both delayed gastrointestinal bleeding. Marginal ulcers were the main cause in Case 1, yet Case 2 was caused by vascular malformation. A recent study reported that marginal ulcer incidence after PD was 2.4%, occurring at a median time of 15.5 mo after surgery[6,7]. The risk factors include delayed gastric emptying, Helicobacter pylori infection, younger age, underlying disease of chronic pancreatitis, smoking, alcohol consumption, and elevated levels of carcinoembryonic antigen[8,9]. Previous research proved that somatostatin usage after PD could prevent bleeding and other complications[10]. However, postoperative PPI use was related to a higher rate of marginal ulcers and bleeding[11]. That can be explained that ulcerations on the gastric side are usually due to alkaline reflux and antiacid therapy is often of no use. Concerning that, some research proved that Braun enteroenterostomy reconstruction following PD, which reduced alkaline reflux, could largely decrease the marginal ulcer rate[12,13], yet the gastrointestinal bleeding rate might remain unchanged[14]. In addition, drugs like aluminum hydroxide and ursodeoxycholic acid could neutralize bile acid, but little evidence supported the prescription after PD. Future study is supposed to explore surgical and non-surgical methods in gastrointestinal bleeding prevention.
For the patient in Case 2, jejunal varix might explain the vascular malformation. Previous research reported that 11.7% of patients developed jejunal varix at a median of 12 mo after PD. Portal vein stenosis and superior mesenteric vein resection were independent risk factors for jejunal varix. Early portal vein stent placement and maintaining stent patency could reduce the risk of variceal bleeding in patients with portal vein stenosis[15]. Notably, the bleeding spots of these two cases were difficult to identify. This might be explained by the fact that the PD procedure largely changes the normal anatomy, so the bleeding spots were usually in untypical areas compared with normal individuals. Repeated EGD, colonoscopy, or even enteroscopy might be necessary to confirm the bleeding spot.
Early PPH can be induced by enzymatic digestion of the blood vessel wall by trypsin, elastase, and other pancreatic exocrine enzymes secondary to a pancreatic leak, intrabdominal infection involving peripancreatic vessels, or vascular injury during resection that leads to a pseudoaneurysm. What’s more, technical failure of appropriate hemostasis during the index operation or an underlying perioperative coagulopathy could be fatal for such patients[16]. For the patient in Case 3, in the absence of extraluminal bleeding, early and effective endoscopic hemostasis is of great importance. The European Society of Gastrointestinal Endoscopy recommends using epinephrine injection plus a second hemostasis modality (contact thermal or mechanical therapy) for non-variceal upper gastrointestinal bleeding, and the American Gastroenterology Association recommends that an over-the-scope clip should be considered if conventional elec
The only dead patient suffered from gastrointestinal bleeding, abdominal bleeding, abdominal infection, hypovolemic shock, and DIC. Presumably, the intra-abdominal infection involving peripancreatic vessels might be the main reason for the progression of his abdominal bleeding. Previous research found that abdominal infection was a risk factor for abdominal bleeding with an odds ratio of 3.6-4.5. The most common source of bleeding was the gastroduodenal artery (24.3%), which was consistent with our Case 4. Although almost 58%-78% of patients with arterial bleeding could be cured by angiography and embolization, the mortality remained at 22%. For our patient, emergency laparotomy might be an option (78%-90% of successful rate) since repeated EGD and intervention therapy failed to stop the bleeding[19,20].
In conclusion, four patients who experienced gastrointestinal bleeding after PD are presented in this paper. Marginal peptic ulcer and jejunal varix are the reasons for delayed bleeding after PD, and it may be prevented by Braun enteroenterostomy reconstruction, bind bile neutralizer, and portal vein stents. Early and effective endoscopic intervention, such as contact thermal, mechanical therapy, or over-the-scope clip, is the key to successful hemostasis. For the patients accompanied by abdominal bleeding, the mortality is extremely high. Laparotomy can be the rescue plan after the failure of repeated EGDs and angiographies.
| 1. | Karim SAM, Abdulla KS, Abdulkarim QH, Rahim FH. The outcomes and complications of pancreaticoduodenectomy (Whipple procedure): Cross sectional study. Int J Surg. 2018;52:383-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 143] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 2. | Welsch T, Eisele H, Zschäbitz S, Hinz U, Büchler MW, Wente MN. Critical appraisal of the International Study Group of Pancreatic Surgery (ISGPS) consensus definition of postoperative hemorrhage after pancreatoduodenectomy. Langenbecks Arch Surg. 2011;396:783-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Simon R. Complications After Pancreaticoduodenectomy. Surg Clin North Am. 2021;101:865-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 4. | Lwin TM, Leigh N, Iskandar ME, Steele JG, Wayne MG, Cooperman AM. Rare, Uncommon, and Unusual Complications After Pancreaticoduodenal Resection. Surg Clin North Am. 2018;98:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Malgras B, Dokmak S, Aussilhou B, Pocard M, Sauvanet A. Management of postoperative pancreatic fistula after pancreaticoduodenectomy. J Visc Surg. 2023;160:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (1)] |
| 6. | Gagnier JJ, Riley D, Altman DG, Moher D, Sox H, Kienle G; CARE Group. The CARE guidelines: consensus-based clinical case reporting guideline development. Dtsch Arztebl Int. 2013;110:603-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Butler JR, Rogers T, Eckart G, Martens GR, Ceppa EP, House MG, Nakeeb A, Schmidt CM, Zyromski NJ. Is antisecretory therapy after pancreatoduodenectomy necessary? Meta-analysis and contemporary practices of pancreatic surgeons. J Gastrointest Surg. 2015;19:604-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Luu AM, Vogel SR, Braumann C, Praktiknjo M, Höhn P, Förster S, Janot M, Uhl W, Belyaev O. Risk factors for perforated marginal ulcers following pancreaticoduodenectomy and prospective analysis of marginal ulcer development. Gland Surg. 2021;10:739-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 9. | Oida T, Kano H, Mimatsu K, Kawasaki A, Kuboi Y, Fukino N, Kida K, Amano S. Gastric marginal ulcer after pancreaticoduodenectomy with pancreaticogastrostomy due to delayed gastric emptying and Helicobacter pylori infection. Hepatogastroenterology. 2012;59:899-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Jin K, Zhou H, Zhang J, Wang W, Sun Y, Ruan C, Hu Z, Wang Y. Systematic review and meta-analysis of somatostatin analogues in the prevention of postoperative complication after pancreaticoduodenectomy. Dig Surg. 2015;32:196-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Panni U, Srivastava R, Bewley A, Williams GA, Fields RC, Sanford DE, Hawkins WG, Leigh N, Hammill CW. Postoperative Proton Pump Inhibitors are associated with a significantly higher rate of delayed gastric emptying after pancreatoduodenectomy. HPB (Oxford). 2023;25:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Su AP, Ke NW, Zhang Y, Wang WG, Zhang ZD, Liu XB, Hu WM, Tian BL. Does modified Braun enteroenterostomy improve alkaline reflux gastritis and marginal ulcer after pancreaticoduodenectomy? Dig Dis Sci. 2013;58:3224-3231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Wang L, Su Ap, Zhang Y, Yang M, Yue Pj, Tian Bl. Reduction of alkaline reflux gastritis and marginal ulcer by modified Braun enteroenterostomy in gastroenterologic reconstruction after pancreaticoduodenectomy. J Surg Res. 2014;189:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Xu B, Zhu YH, Qian MP, Shen RR, Zheng WY, Zhang YW. Braun Enteroenterostomy Following Pancreaticoduodenectomy: A Systematic Review and Meta-Analysis. Medicine (Baltimore). 2015;94:e1254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | D'Silva M, Yoon YS, Lee JS, Cho JY, Lee HW, Lee B, Kim M, Han HS. Incidence, risk factors, and outcomes of jejunal varix of the afferent loop after pancreatoduodenectomy. HPB (Oxford). 2022;24:2193-2201. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Yeo CJ, Büchler MW. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1411] [Cited by in RCA: 1944] [Article Influence: 108.0] [Reference Citation Analysis (0)] |
| 17. | Gralnek IM, Stanley AJ, Morris AJ, Camus M, Lau J, Lanas A, Laursen SB, Radaelli F, Papanikolaou IS, Cúrdia Gonçalves T, Dinis-Ribeiro M, Awadie H, Braun G, de Groot N, Udd M, Sanchez-Yague A, Neeman Z, van Hooft JE. Endoscopic diagnosis and management of nonvariceal upper gastrointestinal hemorrhage (NVUGIH): European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2021. Endoscopy. 2021;53:300-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 264] [Article Influence: 66.0] [Reference Citation Analysis (1)] |
| 18. | Mullady DK, Wang AY, Waschke KA. AGA Clinical Practice Update on Endoscopic Therapies for Non-Variceal Upper Gastrointestinal Bleeding: Expert Review. Gastroenterology. 2020;159:1120-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (39)] |
| 19. | Lu JW, Ding HF, Wu XN, Liu XM, Wang B, Wu Z, Lv Y, Zhang XF. Intra-abdominal hemorrhage following 739 consecutive pancreaticoduodenectomy: Risk factors and treatments. J Gastroenterol Hepatol. 2019;34:1100-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Wei HK, Wang SE, Shyr YM, Tseng HS, Tsai WC, Chen TH, Su CH, Wu CW, Lui WY. Risk factors for post-pancreaticoduodenectomy bleeding and finding an innovative approach to treatment. Dig Surg. 2009;26:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |