Published online Sep 27, 2024. doi: 10.4240/wjgs.v16.i9.3065
Revised: August 14, 2024
Accepted: August 22, 2024
Published online: September 27, 2024
Processing time: 89 Days and 22.5 Hours
Primary lung cancer is the leading cause of cancer-related death worldwide. Common metastatic sites include the brain, liver, bones, and adrenal glands. However, gastric metastases from lung cancer are rare. This case may be the first report of a combined gastroscopic and laparoscopic resection for gastric metasta
We report a case of gastric metastasis from lung cancer. The patient was a 61-year-old Han Chinese female who first attended our hospital complaining of a per
Gastric metastases from lung cancer are rare. Endoscopy, histological and immunohistochemical staining are useful for diagnosing metastatic lesions. Surgical management may provide extended survival in appropriately selected patients.
Core Tip: We report a case of primary lung cancer that metastasized to the stomach after more than four years of chemotherapy and was successfully resected by a cooperative operation involving gastroscopy and laparoscopy. Histopathological examination of the gastric postoperative specimen revealed adenosquamous carcinoma (ASC). This may be the first report of gastric metastatic ASC from lung resected using a combinaton of gastroscopy and laparoscopy.
- Citation: Lin Y, Wu YL, Zou DD, Luo XL, Zhang SY. Combined gastroscopic and laparoscopic resection of gastric metastatic adenosquamous carcinoma from lung: A case report. World J Gastrointest Surg 2024; 16(9): 3065-3073
- URL: https://www.wjgnet.com/1948-9366/full/v16/i9/3065.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i9.3065
Lung cancer is one of the most common cancers worldwide. The incidence and mortality rates of lung cancer rank first or second among malignant tumors in both China and the United States[1,2]. The most common metastatic sites are the liver, bones, brain, and adrenal glands[3]. However, gastrointestinal metastases, particularly gastric metastases from lung cancer, are rare. Here we report a case of primary lung cancer that metastasized to the stomach after more than four years of chemotherapy and was successfully resected by combined gastroscopy and laparoscopy. We discuss the diagnostic and histologic characteristics, as well as treatment strategies, in order to provide additional information and references for diagnosing and treating such uncommon cases.
A 61-year-old Han Chinese female was referred to our hospital in April 2024 due to epigastric pain.
The patient had previously been diagnosed with advanced lung adenocarcinoma (AC) after experiencing a persistent cough. She was referred to our hospital in April 2024 due to new symptoms of epigastric pain.
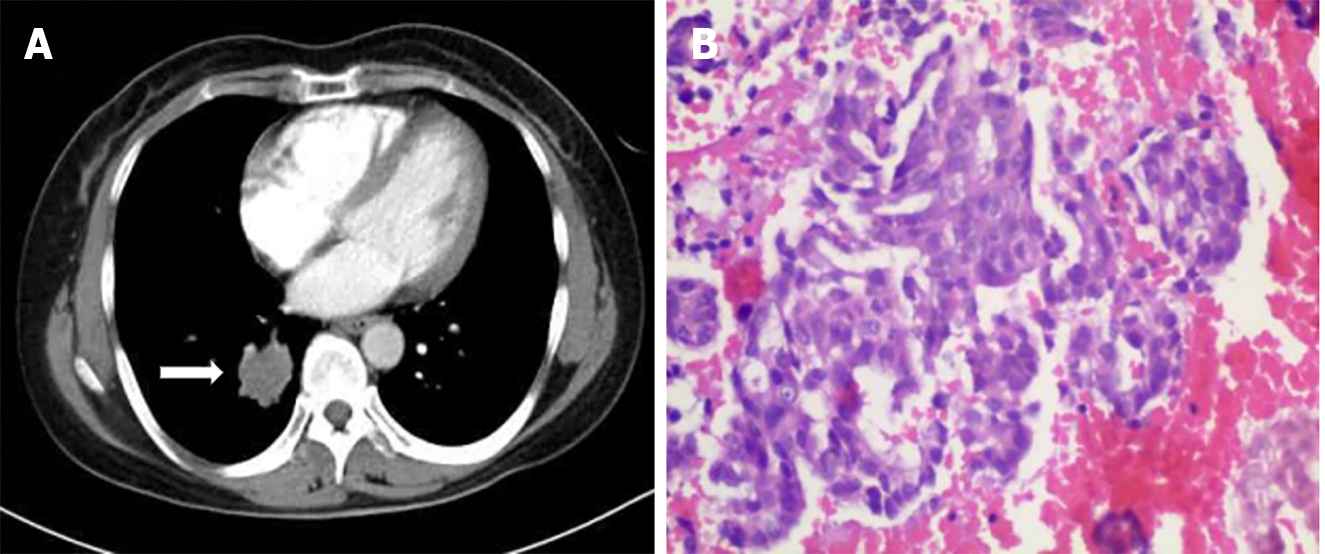
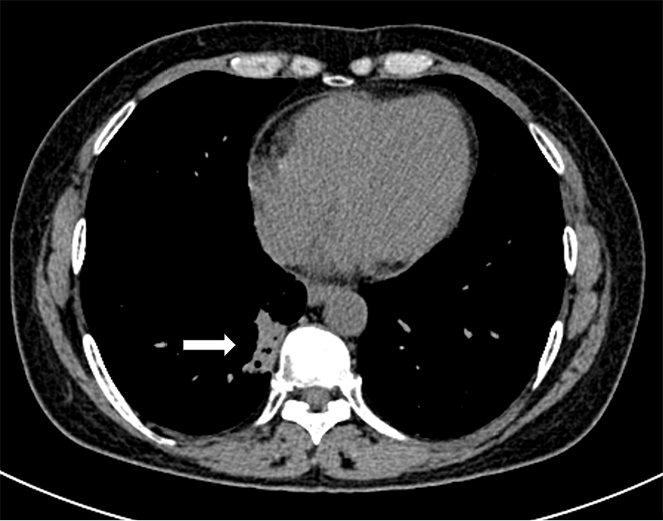
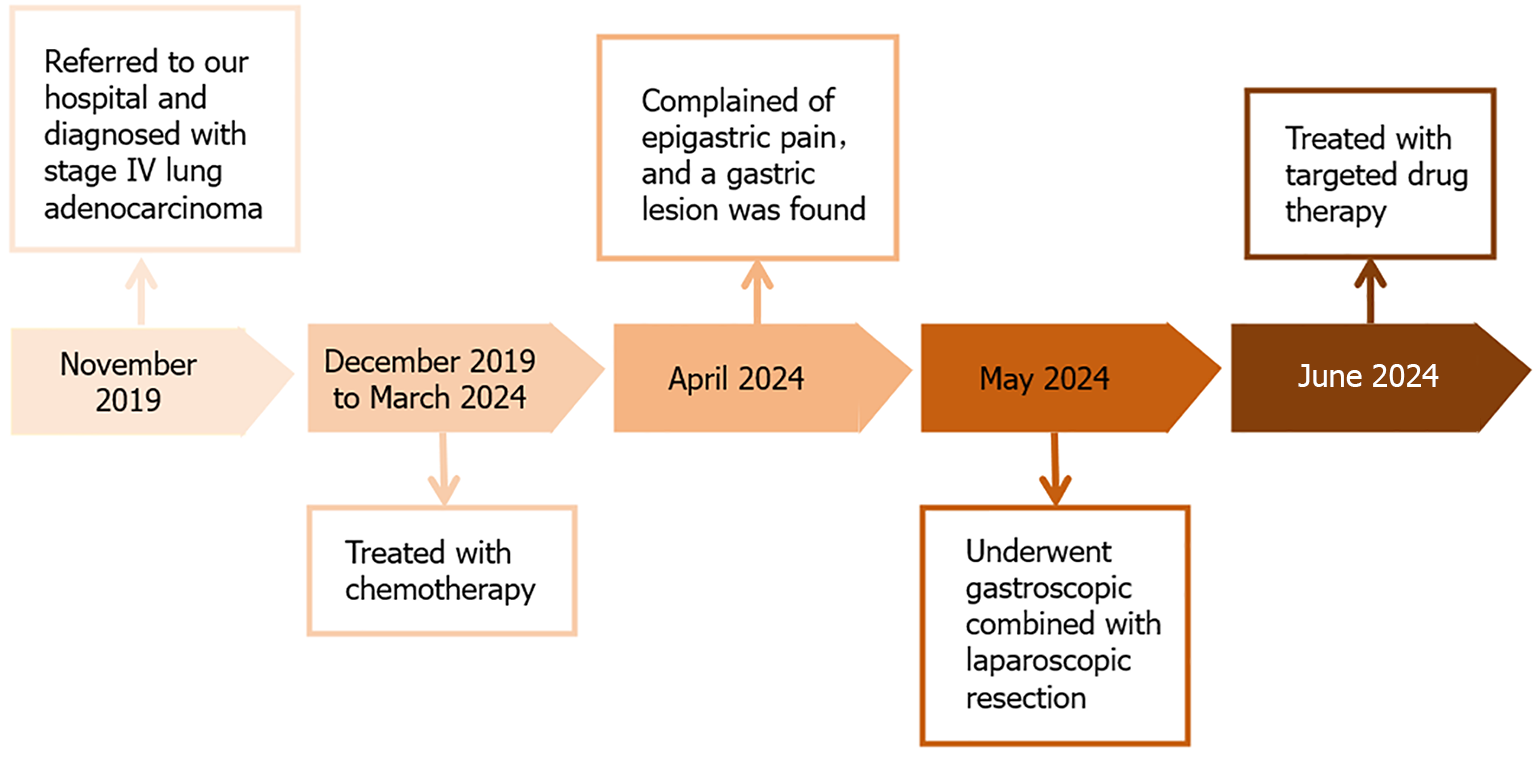
The patient first attended our hospital complaining of a cough in November 2019. An enhanced computed tomography (CT) scan of the chest showed a 30 mm × 26 mm tumor in the right lower lobe (Figure 1A). Bronchoscopy revealed new growths in the posterior basal segment of the right lower lobe. The bronchoscopic biopsy tissue was identified as AC by pathological examination (Figure 1B). Immunohistochemistry (IHC) examination showed that the tumor cells were positive for thyroid transcription factor 1 (TTF-1) and cytokeratin (CK)-7, but negative for CK-5/6. A positron emission tomography-CT scan revealed multiple metastases in the right supraclavicular fossa, hilus pulmonis, mediastinal lymph nodes, and left femur. The patient was diagnosed with stage IV lung AC. A chemotherapy protocol consisting of "sintilimab 200 mg d0 + pemetrexed 800 mg d1 + carboplatin 600 mg d1 q21d" was administered for eight cycles from December 2019 to May 2020, and the patient did not experience obvious adverse effects. CT reexamination showed a reduction in size of the primary lung tumor; therefore, maintenance chemotherapy of "pemetrexed 840 mg d1" was administered for 17 cycles from October 2020 to September 2022. However, CT reexamination showed new brain lesions in April 2023. Considering tumor progression, the chemotherapy protocol of "pemetrexed 840 mg d1 + bevacizumab 500 mg d1 q21d" was administered for 15 cycles from May 2023 to March 2024, and the patient did not experience obvious adverse effects.
The patient's personal and family history was unremarkable.
Physical examination of the abdomen showed no significant abnormalities.
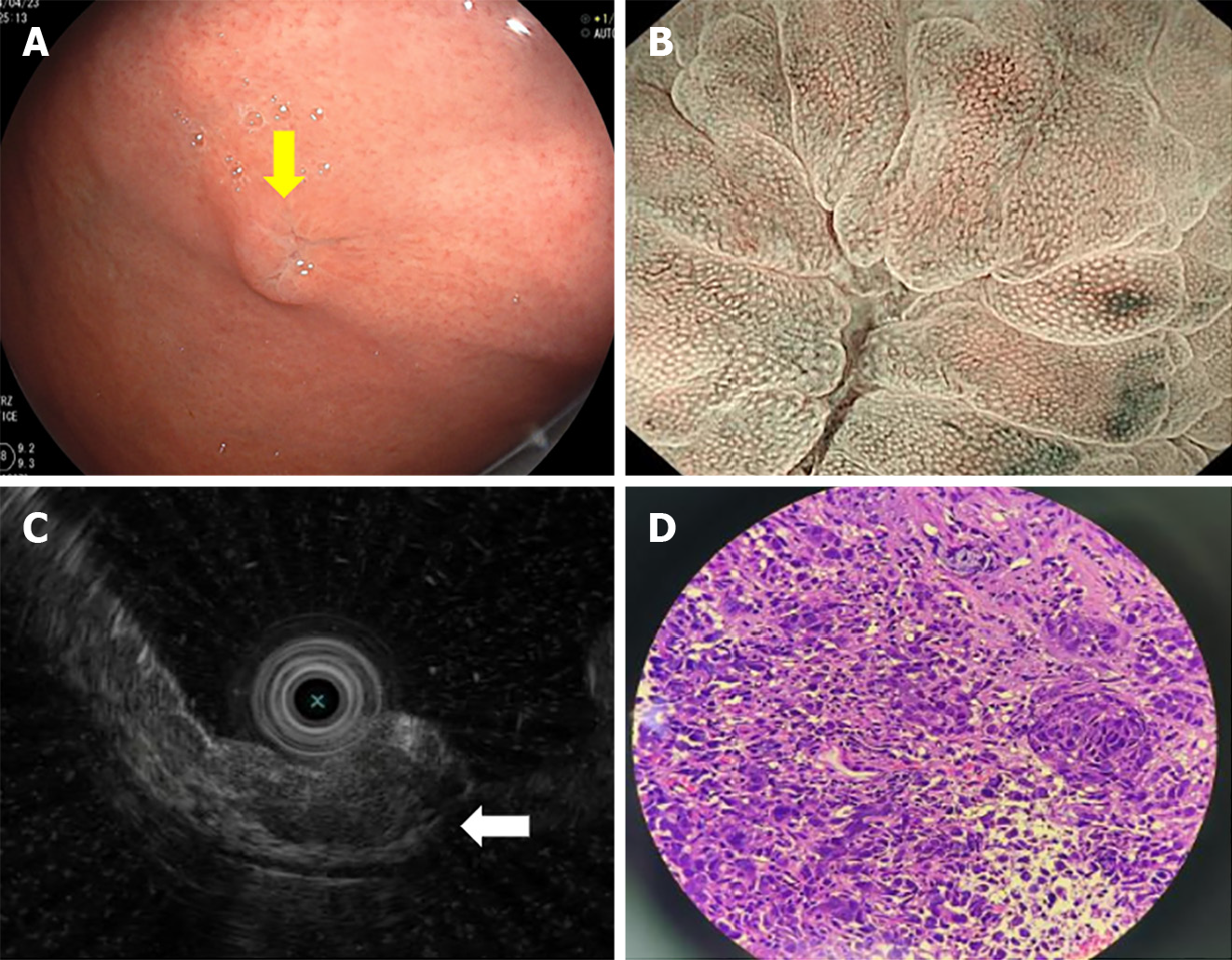
Gastroscopy showed a 10 mm × 8 mm protruding submucosal tumor with central erosion on the gastric fundus (Figure 2A). Magnifying endoscopy showed that the demarcation line of the lesion was not obvious. The microvascular and microsurface patterns were absent on the erosive surface but were still regular on the surrounding mucosa (Figure 2B). On endoscopic ultrasonography, the lesion was a hypoechoic mass with an irregular margin in the submucosal layer, but the submucosal layer had not broken through (Figure 2C).
Pathological examination of gastric biopsies showed poorly differentiated carcinoma (Figure 2D). TTF-1 and novel aspartic proteinase A (Napsin A) were positive, while p40 was negative by IHC.
Multidisciplinary discussions were organized with gastroenterology, gastrointestinal surgery, oncology, pathology, and medical imaging experts.
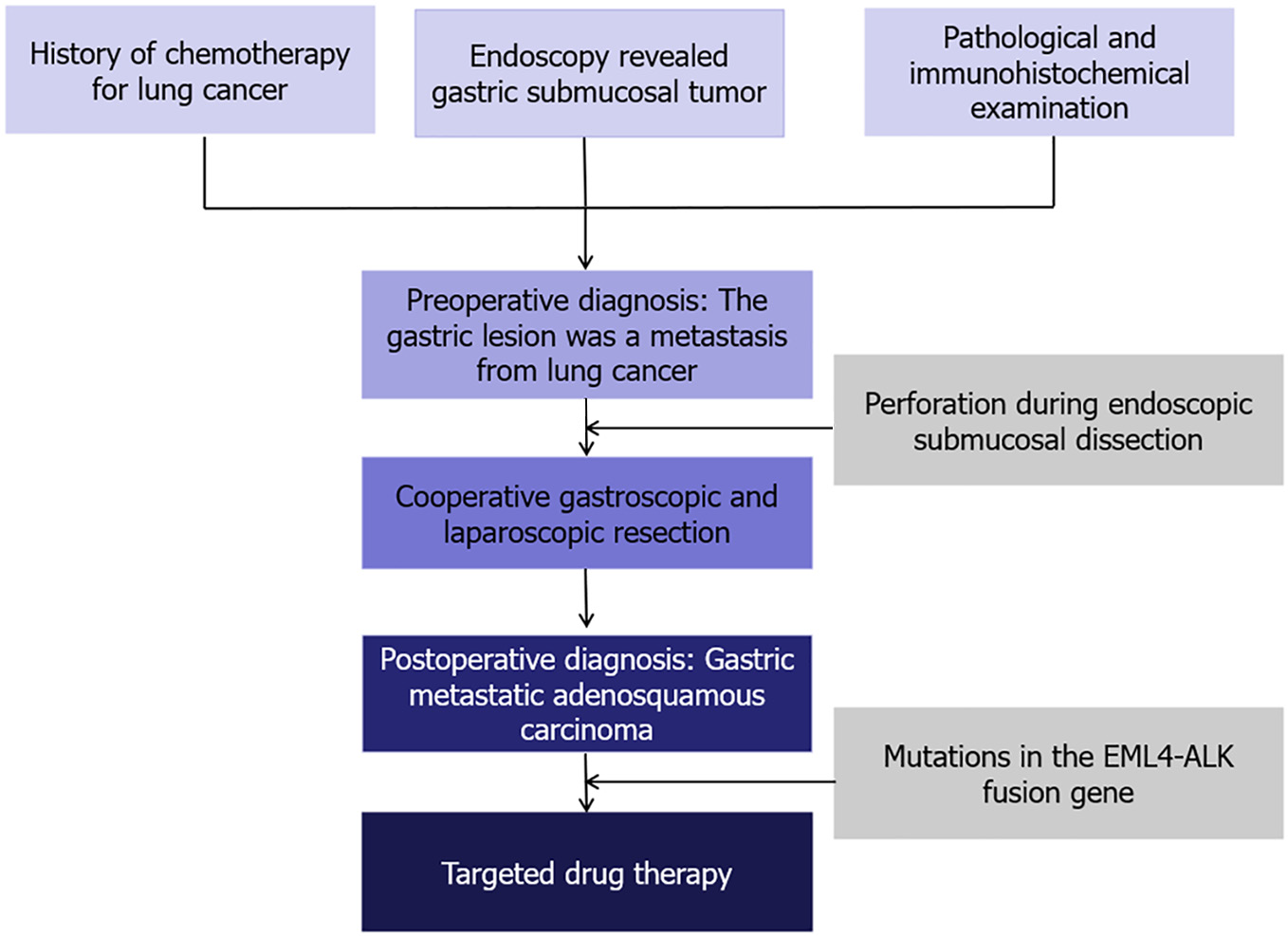
Combined with the patient's medical history, and findings of pathological and immunohistochemical examinations, the gastric lesion was considered to be a metastasis from lung cancer.
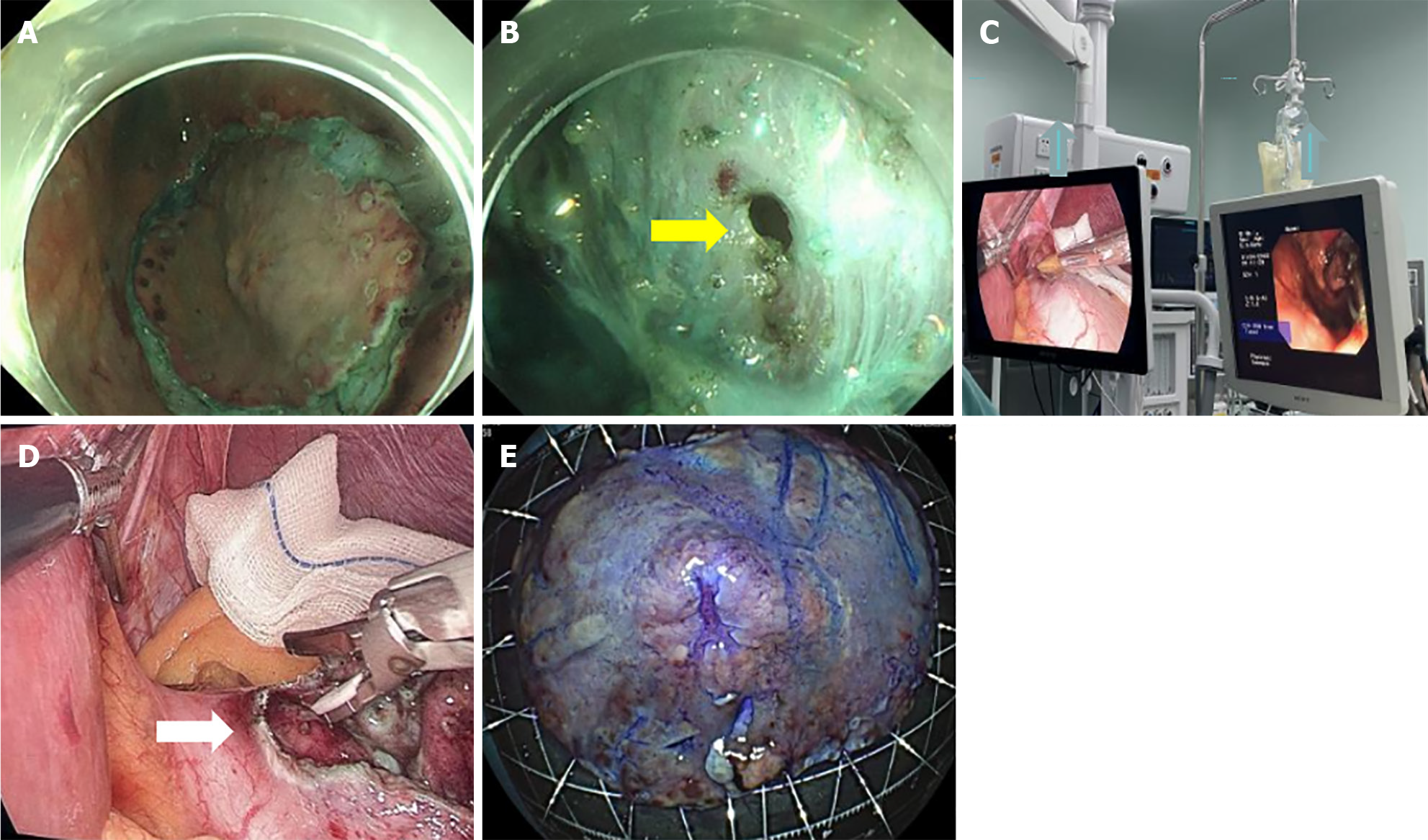
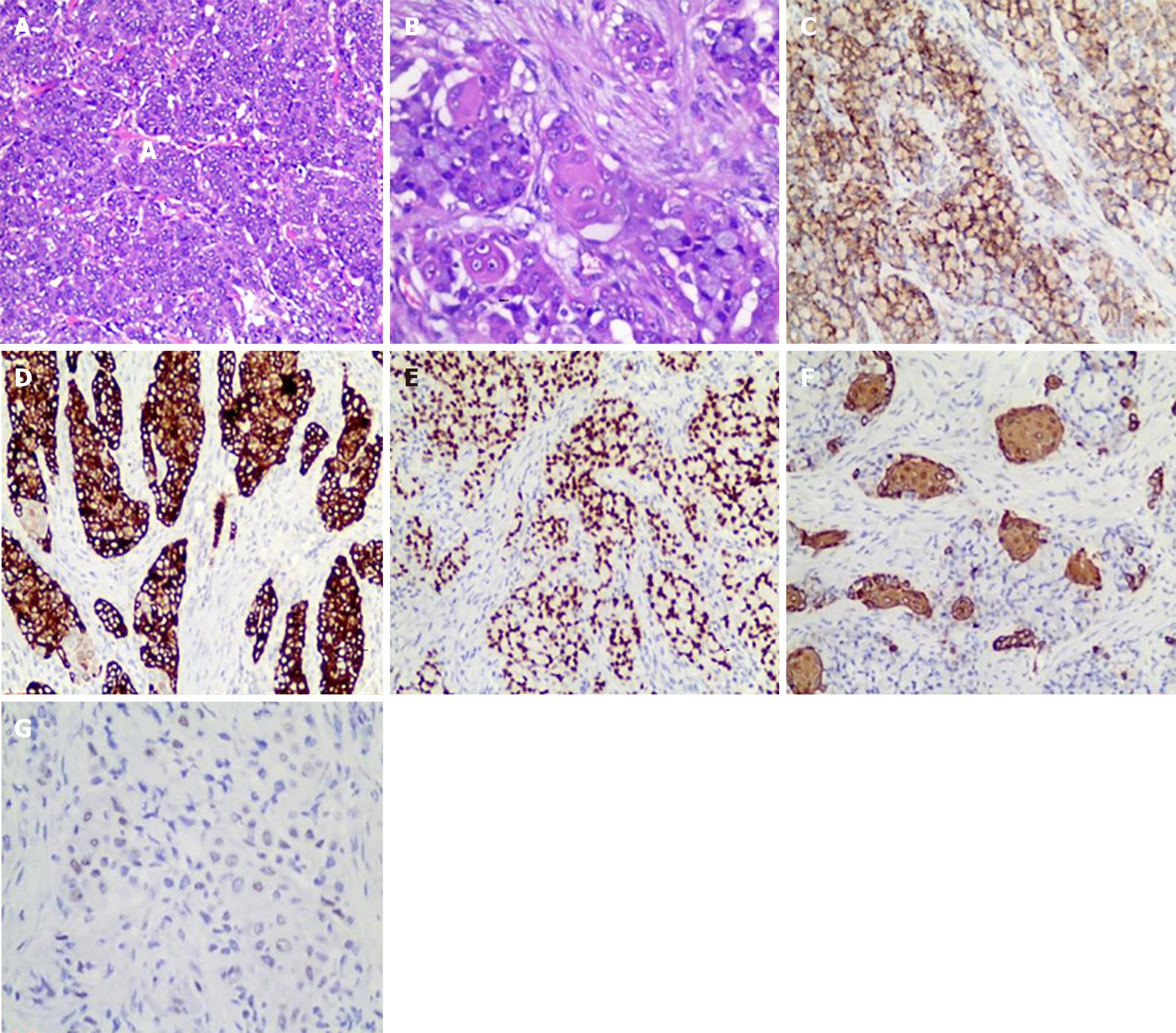
Treatment options were discussed with the patient and her family. The patient opted for endoscopic resection, thus we performed endoscopic submucosal dissection (ESD) after obtaining written informed consent from the patient in May 2024 (Figure 3A). Perforation occurred during ESD (Figure 3B), so the surgical method was adjusted to combined gastroscopic and laparoscopic resection (Figure 3C). The specific steps involved were as follows: First, under laparoscopic monitoring, we performed full-thickness resection of the majority of the gastric wall around the lesion from the perforation site with gastroscopy. Then, the remaining lesion near the cardia was resected under gastroscopic guidance (Figure 3D). The sizes of the resected specimens were 60 mm × 40 mm and 10 mm × 8 mm, respectively (Figure 3E). Histopathological examination revealed adenosquamous carcinoma (ASC) (Figure 4A and B) which was confined to the submucosa with tumor embolus in the vasculature. Napsin A (Figure 4C), CK-7 (Figure 4D), TTF-1 (Figure 4E), CK-5/6 (Figure 4F), p40 (Figure 4G), CD-34, and D2-40 were positive by IHC. Postoperative genetic testing of the gastric specimen revealed mutations in the EML4-ALK fusion gene. The patient was subsequently treated with targeted drug therapy using “brigatinib 180 mg qd”. The patient's assessment, diagnoses and interventions are shown in Figure 5.
Currently, the patient has good performance status with no complaints of discomfort during follow-up. CT reexamination showed a reduction in size of the primary lung tumor on August 6, 2024 (Figure 6). The timeline information in this case is shown in Figure 7.
Primary lung cancer is the leading cause of cancer-related death worldwide[1]. Among the sites of lung cancer metastases, the gastrointestinal tract is exceedingly rare, and its frequency has been reported to range from 0.19% to 5.1%[4-7]. The small intestine is the most common site of gastrointestinal metastasis in advanced lung cancer[8,9]; however, gastric metastasis is rare.
Most patients with gastric metastasis from lung cancer have no specific symptoms, and only a few patients complain of epigastric pain or chronic blood loss. Gastroscopy is helpful for the diagnosis of gastric metastasis. Nemoto et al[10] summarized 16 cases of gastric metastasis from primary lung cancer and found that the metastatic tumor was generally located above the body of the stomach. Most cases of metastases present as submucosal tumors with raised mucosal folds and modest ulcerations towards the top, often referred to as volcano-like ulcers[11].
Histological and immunohistochemical staining can determine the histological type of the lesion and provide important information for distinguishing whether the gastric lesion is a primary lesion or metastasis. Ibrahimli et al[12] summarized 33 cases of lung cancer with gastric metastases and found that among the histological types of primary lung cancers that lead to gastric metastases, AC was the most common diagnosis, followed by small cell lung cancer and squamous cell carcinoma (SCC), but ASC has not been reported. In our case, the histological type of the gastric metastasis was ASC, and this may be the first report of gastric metastatic ASC. ASC is a mixed histologic tumor, as defined by the World Health Organization, and shows components of both SCC and AC, with each comprising at least 10% of the tumor[13]. The incidence of ASC is relatively low, accounting for only 0.4% to 4% of all lung carcinomas[14]. Compared with SCC and AC, ASC exhibits stronger invasiveness, higher malignancy, and a poorer prognosis[15]. Microscopic morphological features have always been the gold standard for tumor classification. Hematoxylin and eosin staining is usually sufficient to distinguish AC from SCC. However, in a few cases, especially when the tumor is poorly differentiated and lacks cytological characteristics, it is difficult to distinguish; thus, IHC is very important. TTF-1, Napsin A, and CK-7 are commonly used as specific immunohistochemical markers to identify pulmonary AC[16-18], while p40 and CK-5/6 are used to identify pulmonary SCC[19,20]. Combined staining of Napsin A, CK-7, TTF-1, CK-5/6 and p40 contributes to the diagnosis of pulmonary ASC.
In our case, the histological types for the lung tumor and the gastric metastasis were AC and ASC, respectively, which are not the same. There are several possible reasons for this as follows: (1) The bronchoscopic biopsy tissue was too small to reflect the whole tumor; and (2) Gene mutation and abnormal differentiation after tumor progression and chemoradiotherapy. Genetic testing of our patient's gastric specimen revealed the presence of the
There are several possible routes by which lung cancer can metastasize to the stomach: (1) Bloodstream and lymphatic metastases. According to the nearly normal overlying mucosa observed during endoscopy in our patient's gastric metastasis lesion, and considering that the tumor was mainly confined to the submucosa with tumor embolus in the vasculature, we consider that the metastasis arose from the bloodstream or lymphatic route; and (2) Implantation metastases. The sputum of lung cancer patients may contain cancer cells, and the patient may swallow sputum with cancer cells into their stomach, initiating metastatic gastric cancer. However, this pathogenesis was not clarified in previous reports.
Gastrointestinal metastasis, as the terminal stage of lung cancer, is always associated with a poor prognosis. In a study of 366 patients with gastrointestinal metastases from lung cancer, more than 50% of patients died within 3 months after the diagnosis of gastrointestinal metastasis from lung cancer[22]. Therefore, treatment strategies should be comprehensive and personalized. Kobashi et al[23] reported gastric perforation due to chemotherapy alone for gastric metastases. Chemotherapy alone may also aggravate necrosis of gastric metastasis and lead to hemorrhage. Taira et al[11] reported two cases of lung cancer with gastric metastases; the patients refused surgical resection and eventually died due to chronic blood loss. In addition, Kim et al[5] reported that a patient with lung cancer and small bowel metastasis survived for more than five years after surgical resection. Therefore, we suggest that surgical resection may be an option for palliation in appropriately selected patients when chemotherapy alone cannot alleviate gastrointestinal symptoms such as recurrent abdominal pain or chronic bleeding. At present, there is no standardized method of surgical resection, which varies according to the size of the tumor, the depth of invasion, and the individual wishes of patients. In our case, the gastric metastasis was an isolated small lesion and confined to the submucosa; laparoscopy could not determine the boundary in the serosa. Moreover, the patient requested initial endoscopic resection as it offered advantages such as less invasive, lower costs, and faster recovery. Therefore, we performed ESD first, but perforation occurred during the procedure. Consequently, the surgical method was adjusted to a combination of gastroscopic and laparoscopic resection, and there are no previous cases of gastric metastasis from lung cancer resected using a combination of gastroscopy and laparoscopy.
Although the patient currently has good performance status, her long-term prognosis still requires further follow-up. We will continue to follow this patient in the future.
In summary, this case provides important insights into the rare pathological type of metastatic gastric ASC. The successful resection of metastatic gastric lesions via a combined gastroscopic and laparoscopic approach offers valuable guidance for the selection of surgical methods in future cases.
Gastric metastasis from lung cancer is rare. However, as a result of significant advances in radiotherapy and chemotherapy, supportive care for lung carcinoma patients, and increasing life span, we may encounter this type of metastatic tumor more frequently in the foreseeable future[9]. Therefore, gastrointestinal symptoms in a known case of lung cancer should raise suspicion of advanced metastatic disease[10]. Endoscopy is an important diagnostic tool. Histological and immunohistochemical staining are useful for diagnosing metastatic lesions and differentiating them from primary lesions. Surgical management may offer access to palliative remedies and provide extended survival with an enhanced quality of life in appropriately selected patients.
We would like to acknowledge the patient and her family for allowing us to use her medical records in this case report and allowing this case to be published.
| 1. | Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2279] [Cited by in RCA: 4680] [Article Influence: 4680.0] [Reference Citation Analysis (3)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13210] [Article Influence: 1467.8] [Reference Citation Analysis (3)] |
| 3. | van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet. 2011;378:1741-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 803] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 4. | Kim YI, Kang BC, Sung SH. Surgically resected gastric metastasis of pulmonary squamous cell carcinoma. World J Gastrointest Surg. 2013;5:278-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Kim MS, Kook EH, Ahn SH, Jeon SY, Yoon JH, Han MS, Kim CH, Lee JC. Gastrointestinal metastasis of lung cancer with special emphasis on a long-term survivor after operation. J Cancer Res Clin Oncol. 2009;135:297-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Green LK. Hematogenous metastases to the stomach. A review of 67 cases. Cancer. 1990;65:1596-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Menuck LS, Amberg JR. Metastatic disease involving the stomach. Am J Dig Dis. 1975;20:903-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 135] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Garwood RA, Sawyer MD, Ledesma EJ, Foley E, Claridge JA. A case and review of bowel perforation secondary to metastatic lung cancer. Am Surg. 2005;71:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Yang CJ, Hwang JJ, Kang WY, Chong IW, Wang TH, Sheu CC, Tsai JR, Huang MS. Gastro-intestinal metastasis of primary lung carcinoma: clinical presentations and outcome. Lung Cancer. 2006;54:319-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 151] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 10. | Nemoto M, Prasoon P, Ichikawa H, Hanyu T, Kano Y, Muneoka Y, Usui K, Hirose Y, Miura K, Shimada Y, Nagahashi M, Sakata J, Ishikawa T, Tsuchida M, Wakai T. Primary lung squamous cell carcinoma and its association with gastric metastasis: A case report and literature review. Thorac Cancer. 2020;11:1708-1711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Taira N, Kawabata T, Gabe A, Furugen T, Ichi T, Kushi K, Yohena T, Kawasaki H, Higuchi D, Chibana K, Fujita K, Nakamoto A, Owan I, Kuba M, Ishikawa K. Analysis of gastrointestinal metastasis of primary lung cancer: Clinical characteristics and prognosis. Oncol Lett. 2017;14:2399-2404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Ibrahimli A, Aliyev A, Majidli A, Kahraman A, Galandarova A, Khalilzade E, Mammadli H, Huseynli K, Assaf K, Kilinc C, Muradov N, Alisan OF, Abdullayev S, Sahin YI, Samadov E. Metastasis to the stomach: a systematic review. F1000Res. 2023;12:1374. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to The 2015 World Health Organization Classification of Tumors of the Lung, Pleura, Thymus, and Heart. J Thorac Oncol. 2015;10:1240-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 1095] [Article Influence: 109.5] [Reference Citation Analysis (0)] |
| 14. | Li C, Lu H. Adenosquamous carcinoma of the lung. Onco Targets Ther. 2018;11:4829-4835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Wang J, Wang Y, Tong M, Pan H, Li D. Research progress of the clinicopathologic features of lung adenosquamous carcinoma. Onco Targets Ther. 2018;11:7011-7017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Zamecnik J, Kodet R. Value of thyroid transcription factor-1 and surfactant apoprotein A in the differential diagnosis of pulmonary carcinomas: a study of 109 cases. Virchows Arch. 2002;440:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 100] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Ordóñez NG. Thyroid transcription factor-1 is a marker of lung and thyroid carcinomas. Adv Anat Pathol. 2000;7:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 161] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 18. | Wang CY, Xu G, Gao C, Wang D. Esophageal metastases from primary lung cancer: a case report. J Med Case Rep. 2021;15:265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Downey P, Cummins R, Moran M, Gulmann C. If it's not CK5/6 positive, TTF-1 negative it's not a squamous cell carcinoma of lung. APMIS. 2008;116:526-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Whithaus K, Fukuoka J, Prihoda TJ, Jagirdar J. Evaluation of napsin A, cytokeratin 5/6, p63, and thyroid transcription factor 1 in adenocarcinoma versus squamous cell carcinoma of the lung. Arch Pathol Lab Med. 2012;136:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Italiano A, Vandenbos FB, Otto J, Mouroux J, Fontaine D, Marcy PY, Cardot N, Thyss A, Pedeutour F. Comparison of the epidermal growth factor receptor gene and protein in primary non-small-cell-lung cancer and metastatic sites: implications for treatment with EGFR-inhibitors. Ann Oncol. 2006;17:981-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Hu Y, Feit N, Huang Y, Xu W, Zheng S, Li X. Gastrointestinal metastasis of primary lung cancer: An analysis of 366 cases. Oncol Lett. 2018;15:9766-9776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Kobashi M, Kanzaki H, Okada H. Perforation of gastric metastasis during chemotherapy with ramucirumab. Curr Probl Cancer. 2021;45:100666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |