Published online Sep 27, 2024. doi: 10.4240/wjgs.v16.i9.2934
Revised: August 20, 2024
Accepted: August 21, 2024
Published online: September 27, 2024
Processing time: 56 Days and 0.4 Hours
Despite significant advancements in the medical treatment of primary hepatocellular carcinoma (PHC) in recent years, enhancing therapeutic effects and im
To investigate the expression levels of serum vascular endothelial growth factor (VEGF) and interleukin (IL)-17 in patients with PHC and evaluate their diagnostic value while exploring their relationship with patients’ clinical characteristics.
The study included 50 patients with confirmed PHC who visited Wuhan Han
Serum VEGF and IL-17 levels were significantly higher in the study group com
Serum VEGF and IL-17 levels were significantly higher in PHC patients compared to healthy individuals. Their levels were closely related to pathological features such as tumor metastasis and clinical TNM stage, and there was a significant positive correlation between VEGF and IL-17. These biomarkers may serve as valuable reference in
Core Tip: The incidence and mortality rates of primary hepatocellular carcinoma (PHC) have been increasing. Despite significant advancements in medical care, improving treatment outcomes and prognosis for liver cancer remains a global challenge. This study measured the levels of vascular endothelial growth factor (VEGF) and interleukin (IL)-17 in the serum of PHC patients using Enzyme-Linked Immunosorbent Assay, with healthy individuals as controls. This study aimed to assess changes in serum VEGF and IL-17 levels and evaluate their diagnostic efficacy for PHC while exploring their correlations with patients’ clinicopathological characteristics. Our findings confirm that VEGF and IL-17 can serve as valuable reference indicators for the early diagnosis and treatment guidance of PHC, offering crucial insights for enhancing early management strategies.
- Citation: Tian Q, Zeng H, Lu QQ, Xie HY, Li Y. Diagnostic value of serum vascular endothelial growth factor and interleukin-17 in primary hepatocellular carcinoma. World J Gastrointest Surg 2024; 16(9): 2934-2941
- URL: https://www.wjgnet.com/1948-9366/full/v16/i9/2934.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i9.2934
Primary hepatocellular carcinoma (PHC) is the fourth leading cause of cancer-related death worldwide[1], and its inci
Serum tumor markers are currently one of the primary modalities for diagnosing PHC, and they have gained in
In this study, serum VEGF and IL-17 Levels in PHC patients were measured using Enzyme-Linked Immunosorbent Assay (ELISA), with healthy individuals serving as controls. The study aimed to investigate changes in serum VEGF and IL-17 levels, assess their diagnostic efficacy for PHC, and explore their relationship with the clinicopathological characteristics of patients. Currently, there is a relative lack of research on the expression of serum VEGF and IL-17 levels in PHC patients, as well as their diagnostic value and relationship with clinicopathological features. The analysis conducted in this study helps to fill this gap and may contribute to improving the overall survival rate and prognosis of liver cancer patients through early detection and diagnosis.
Clinical and pathological data were collected from 50 patients with PHC who were diagnosed at Hanyang Hospital, Affiliated with Wuhan University of Science and Technology, China from January 2021 to January 2022. All participants were clearly diagnosed through histopathology and had not received anti-tumor medication before blood sample collection, including embolization, chemotherapy, radiotherapy, radiofrequency ablation, or anhydrous alcohol injection. These PHC patients comprised the study group. Additionally, 50 healthy individuals from the same period were selected as the control group. There was no statistically significant difference in baseline data between the two groups (P > 0.05; Table 1).
| Data | Control group (n = 50) | Study group (n = 50) | t/χ2 value | P value | |
| Gender, n (%) | Male | 32 (64.0) | 35 (70.0) | 0.407 | 0.523 |
| Female | 18 (36.0) | 15 (30.0) | |||
| Age (year) | 48.22 ± 4.23 | 47.48 ± 3.73 | 0.940 | 0.349 | |
| Height (m) | 1.68 ± 0.16 | 1.67 ± 0.15 | 0.322 | 0.748 | |
| Education level, n (%) | Elementary school and below | 2 (4.0) | 4 (8.0) | 1.940 | 0.585 |
| Junior high school | 11 (22.0) | 10 (20.0) | |||
| High School | 20 (40.0) | 24 (48.0) | |||
| College and above | 17 (34.0) | 12 (24.0) | |||
| Marital status, n (%) | Unmarried | 3 (6.0) | 6 (12.0) | 1.673 | 0.643 |
| Married | 38 (76.0) | 36 (72.0) | |||
| Divorced | 8 (16.0) | 6 (12.0) | |||
| Widowed | 1 (2.0) | 2 (4.0) | |||
| Place of residence, n (%) | City | 28 (56.0) | 31 (62.0) | 0.372 | 0.542 |
| Village or town | 22 (44.0) | 19 (38.0) | |||
The study group met the diagnostic criteria for PHC[12], with a diagnosis confirmed by pathology, and no prior treat
Exclusion criteria included patients under 18 years of age, those with incomplete medical records, those with liver cancer secondary to or combined with other tumors, those with immune diseases such as AIDS or other conditions that could cause elevated study factors, and those with mental illness or serious physical conditions that would prevent cooperation with the test.
Basic demographic characteristics: The name, gender, age, height, contact information, education level, and marital status of participants were collected. Pathological information for the study group included histological classification, staging criteria, tumor diameter, presence of combined cirrhosis, degree of differentiation, and tumor metastasis.
Serum VEGF and IL-17 assay: All participants fasted for 8-12 hours before sample collection (healthy participants fasted randomly in the early morning). A 5 mL blood sample was drawn from the elbow vein into non-anticoagulated tubes in the early morning, and the samples were then separated by high-speed centrifugation at 4000 rpm for 5 minutes after clotting. VEGF and IL-17 levels were measured using an ELISA kit (eBiosciences) following the manufacturers’ in
Observation indexes: (1) Observation and comparison of serum VEGF and IL-17 levels between the study group and the control group; (2) Observation and comparison of serum VEGF and IL-17 levels within the study group, categorized by different pathological characteristics; (3) Analysis of the correlation between serum VEGF and IL-17 levels in the study group; and (4) Analysis of the diagnostic value of serum VEGF and IL-17 levels for PHC.
SPSS 22.0 was used for data analysis. The correlation between serum VEGF and IL-17 Levels was assessed using Pearson correlation analysis, and the diagnostic value of serum VEGF and IL-17 for PHC was evaluated using receiver operating characteristic (ROC) curve analysis. A P value of less than 0.05 was considered statistically significant.
A comparison of general information, including gender, age, height, education level, marital status, and place of resi
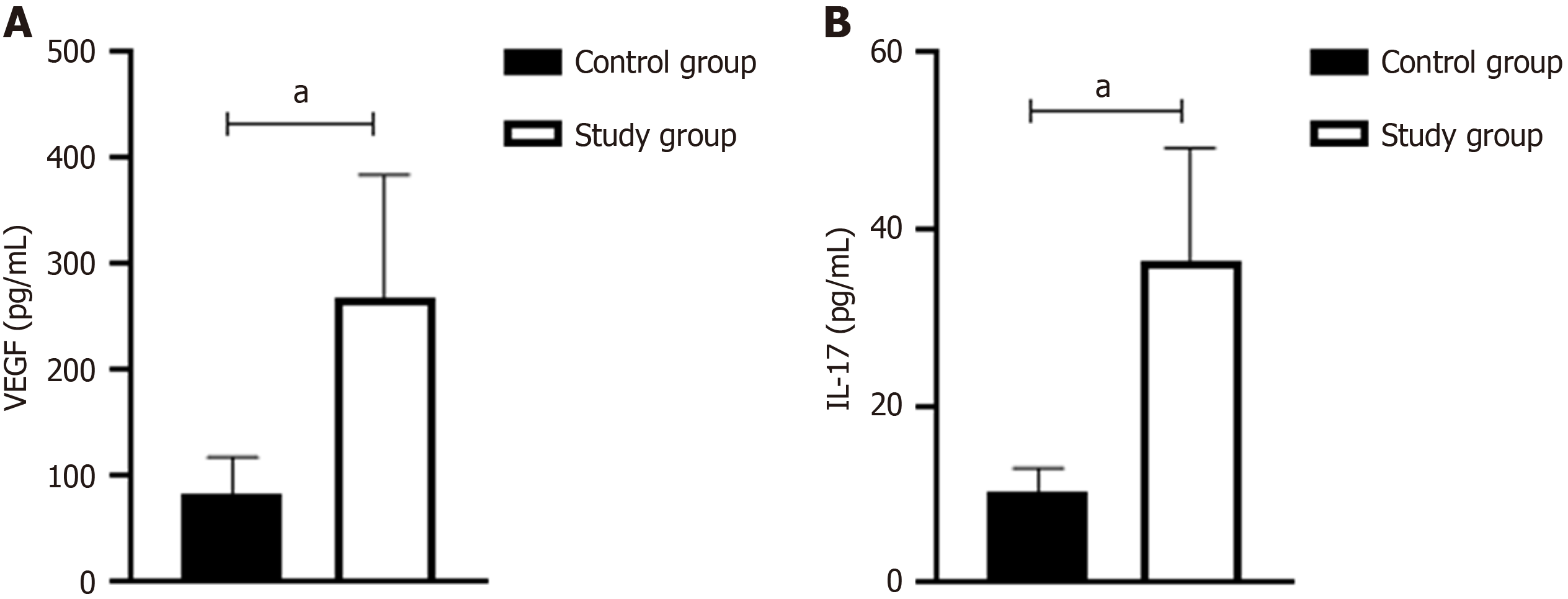
When comparing serum VEGF and IL-17 levels between the control group and the study group, the results showed that both serum VEGF and IL-17 levels were significantly higher in the study group than in the control group (P < 0.05; Figure 1).
The serum VEGF and IL-17 levels were compared across different pathological characteristics within the study groups. The results indicated no significant relationship between gender, age, combined cirrhosis, tumor diameter, or degree of differentiation and the expression of serum VEGF and IL-17 in PHC patients (P > 0.05). However, there was a significant relationship between clinical TNM stage and tumor metastasis with serum VEGF and IL-17 expression (P < 0.05; Table 2).
| Factor | Classification | Cases | VEGF (pg/mL) | P value | IL-17 (pg/mL) | P value |
| Gender | Male | 35 | 264.90 ± 113.04 | 0.800 | 36.15 ± 13.26 | 0.228 |
| Female | 15 | 274.17 ± 128.28 | 37.06 ± 12.07 | |||
| Age | < 50 years old | 36 | 275.39 ± 107.69 | 0.467 | 35.83 ± 11.95 | 0.606 |
| ≥ 50 years old | 14 | 248.37 ± 139.08 | 37.94 ± 15.15 | |||
| Histological classification | Hepatocellular type | 41 | 268.75 ± 109.59 | 0.892 | 37.00 ± 13.15 | 0.504 |
| Cholangiocyte type | 9 | 262.83 ± 152.11 | 33.81 ± 11.35 | |||
| TNM stage | Stage I + Stage II | 19 | 217.72 ± 97.70 | 0.016 | 28.44 ± 11.25 | < 0.001 |
| Stage III + Stage IV | 31 | 298.30 ± 117.96 | 41.32 ± 11.25 | |||
| Tumor diameter | > 5 cm | 21 | 255.59 ± 112.66 | 0.538 | 38.93 ± 12.72 | 0.243 |
| ≤ 5 cm | 29 | 276.44 ± 120.51 | 34.61 ± 12.76 | |||
| Combined cirrhosis | Yes | 28 | 249.73 ± 114.81 | 0.223 | 34.64 ± 12.43 | 0.271 |
| No | 22 | 290.53 ± 117.38 | 38.69 ± 13.18 | |||
| Degree of differentiation | Highly differentiated | 27 | 277.33 ± 111.53 | 0.532 | 37.78 ± 13.15 | 0.418 |
| Low differentiation | 23 | 256.35 ± 123.74 | 34.82 ± 12.47 | |||
| Tumor metastasis | Yes | 24 | 304.50 ± 117.44 | 0.030 | 43.79 ± 10.90 | < 0.001 |
| No | 26 | 233.69 ± 106.95 | 29.91 ± 10.94 |
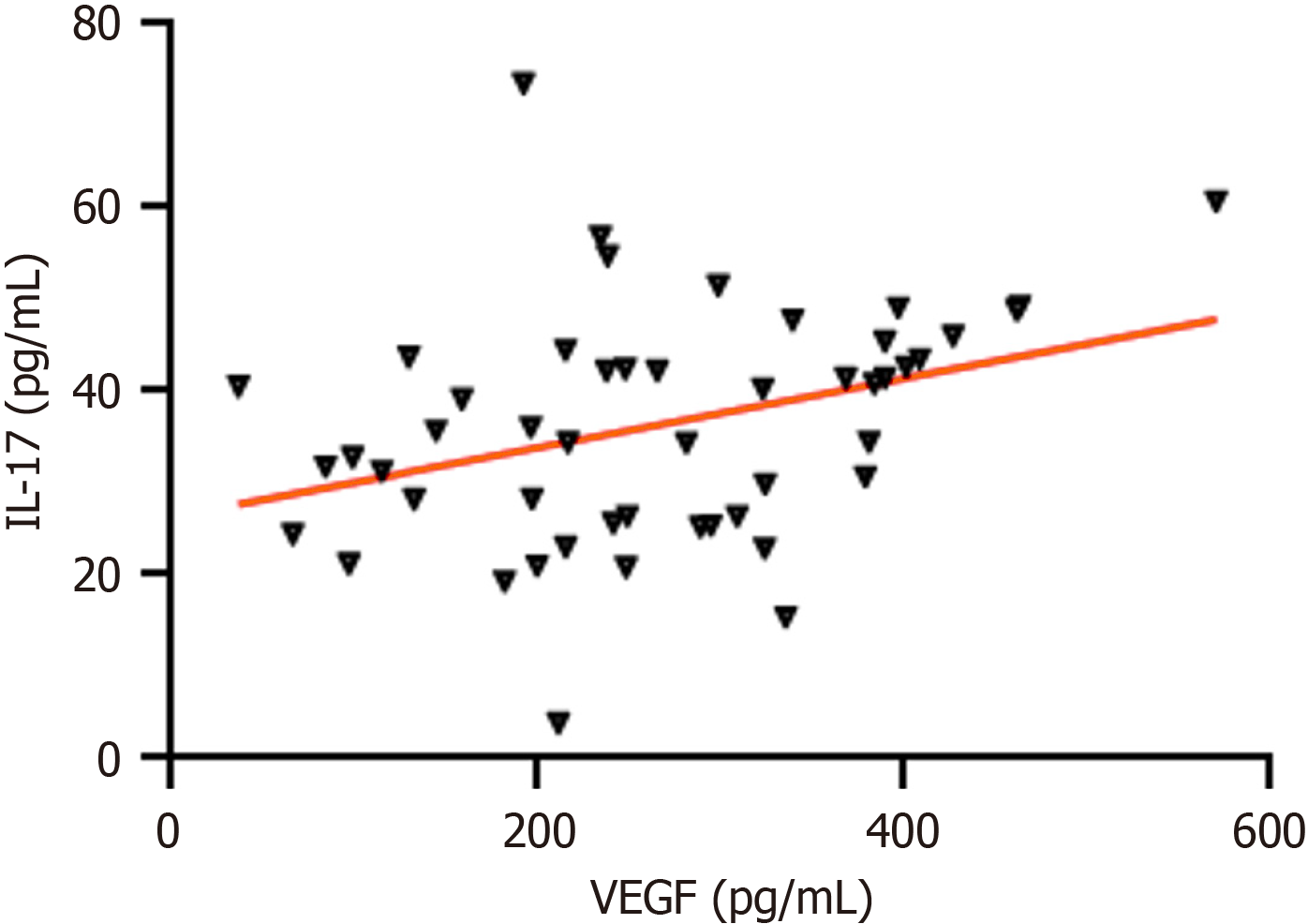
Pearson correlation analysis revealed a positive correlation between serum VEGF and IL-17 levels in PHC patients (r = 0.343, P = 0.015; Figure 2).
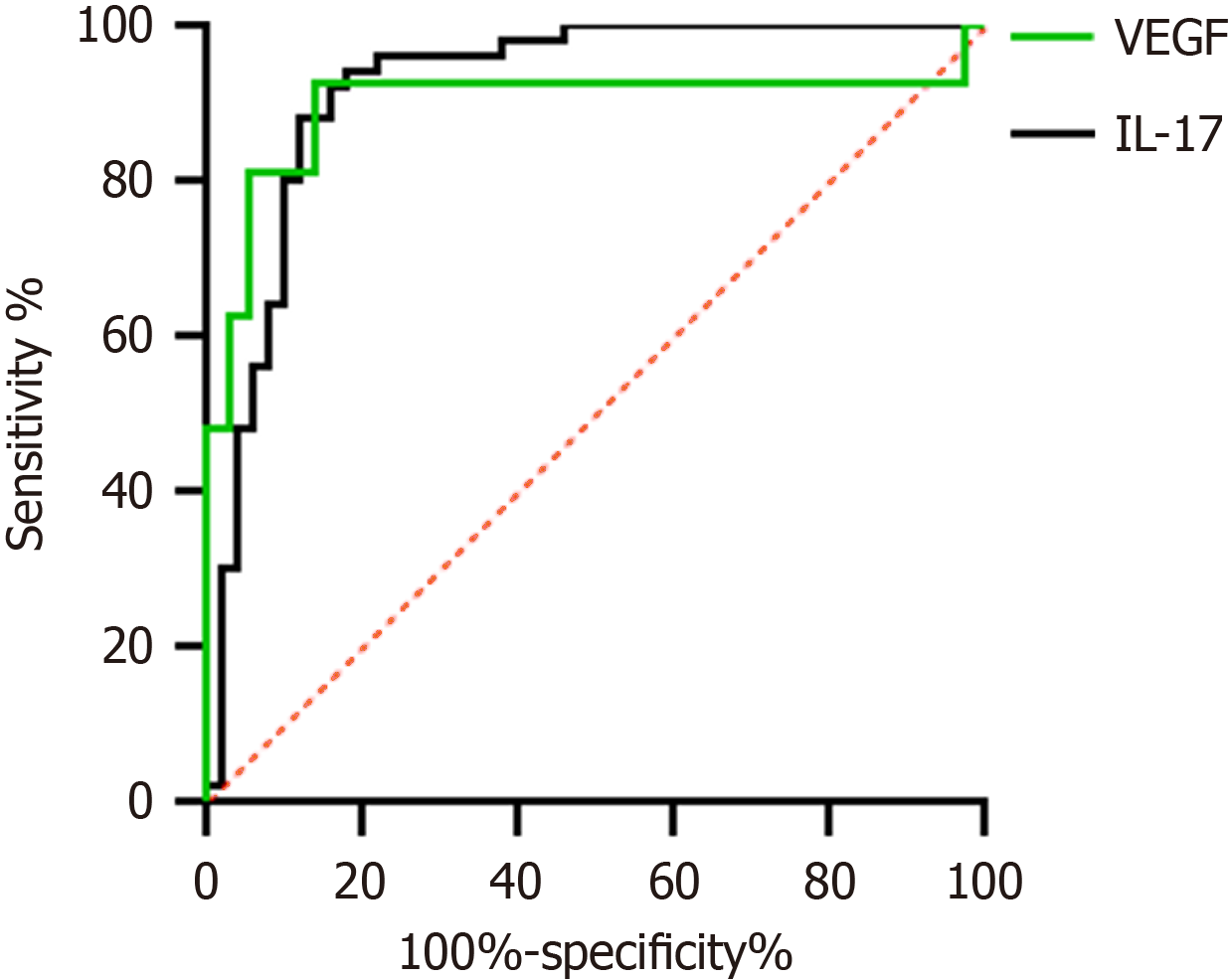
The results of the ROC curve analysis showed that the diagnostic area under the curve for serum VEGF and IL-17 in PHC was 0.919. The sensitivity was 82.0% and 92.6%, and the specificity was 94.4% and 86.5%, respectively. Serum VEGF levels greater than 102.775 pg/mL or IL-17 levels greater than 13.289 pg/mL suggest that the patient is at risk of developing PHC (Table 3, Figure 3).
| Indicator | AUC | 95%CI | Optimal cut-off value | Sensitivity (%) | Specificity (%) |
| VEGF | 0.919 | 0.861-0.977 | 102.775 | 82.00 | 94.40 |
| IL-17 | 0.898 | 0.799-0.997 | 13.289 | 92.60 | 86.50 |
In China, PHC, as one of the most common malignant tumors of the digestive system, ranks just behind gastric and esophageal cancers in incidence[13]. Currently, early surgical treatment is the preferred approach for PHC, but many patients miss the opportunity for surgical resection by the time they present with symptoms. Even when surgical re
The persistent growth and metastasis of PHC tumor cells are closely associated with neovascularization, and VEGF is the most effective pro-angiogenic factor among tumor neovascularization factors. It promotes the proliferation of vascular endothelial cells, increases vascular permeability, and degrades the extravascular matrix, thus providing the foundation for vascular growth and extension[17,18]. Both tumor progression and metastasis are accompanied by abnormalities in VEGF, which is highly expressed in the serum and tissues of PHC patients[19], consistent with the findings of this study. This study further analyzed the relationship between different pathological characteristics and VEGF levels in PHC patients. The results showed no significant differences in serum VEGF levels based on gender, age, tumor diameter, or degree of differentiation. However, serum VEGF levels were significantly higher in patients with clinical TNM stage III + IV and tumor metastasis compared to those with clinical TNM stage I + II and no tumor metastasis (P < 0.05). Therefore, VEGF expression in PHC patients is related to tumor stage and metastasis.
IL-17 is a cytokine produced mainly by Th17 cells and neutrophils, and previous studies have found that IL-17 is involved in the pathophysiological processes of several diseases, including chronic inflammation, tumors, and auto
In this study, the relationship between different pathological characteristics and serum IL-17 levels in patients with PHC was analyzed. The results showed no significant difference in serum IL-17 Levels based on gender, age, tumor diameter, or degree of differentiation (P > 0.05). However, IL-17 levels were significantly higher in patients with tumor metastasis compared to those without metastasis, and serum IL-17 levels were significantly lower in patients with TNM stage I-II than in those with stage III-IV (P < 0.05). Pearson correlation analysis revealed a significant positive correlation between serum VEGF and IL-17 levels in PHC patients (r = 0.343, P = 0.015). This indicates that VEGF and IL-17 have a significant positive synergistic effect in the pathogenesis of PHC, suggesting their importance in the in-depth investigation of the pathogenesis of this cancer and the potential association network of therapeutic targets.
However, several limitations in this study require further consideration. First, the diagnostic value of VEGF and IL-17 in assessing the clinical parameters of PHC patients was not analyzed. Including this analysis would be beneficial for further exploring the potential of VEGF and IL-17 in determining the clinical parameters of PHC patients. Second, this study did not discuss the predictive implications of VEGF and IL-17 for the prognosis of PHC patients. Adding such an analysis could help to complement the predictive potential of these two indicators. Finally, the pathogenesis of VEGF and IL-17 in PHC was not explored through basic research. Therefore, conducting relevant analyses would further our understanding of the underlying mechanisms of PHC. Future research will address these aspects to further advance the clinical application of VEGF and IL-17 in PHC.
In summary, the levels of serum VEGF and IL-17 in PHC patients were significantly higher than those in the healthy population. A close relationship was observed between these markers and specific pathological characteristics, including tumor metastasis and clinical TNM stage. VEGF showed a significant positive correlation with IL-17, indicating that both can serve as reference indicators for the early diagnosis and treatment guidance of PHC.
| 1. | Nakano A, Hirabayashi K, Yamamuro H, Mashiko T, Masuoka Y, Yamamoto S, Ozawa S, Nakagohri T. Combined primary hepatic neuroendocrine carcinoma and hepatocellular carcinoma: case report and literature review. World J Surg Oncol. 2021;19:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Li QJ, He MK, Chen HW, Fang WQ, Zhou YM, Xu L, Wei W, Zhang YJ, Guo Y, Guo RP, Chen MS, Shi M. Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin Versus Transarterial Chemoembolization for Large Hepatocellular Carcinoma: A Randomized Phase III Trial. J Clin Oncol. 2022;40:150-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 242] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 3. | Hack SP, Spahn J, Chen M, Cheng AL, Kaseb A, Kudo M, Lee HC, Yopp A, Chow P, Qin S. IMbrave 050: a Phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation. Future Oncol. 2020;16:975-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 157] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 4. | Lee YT, Fujiwara N, Yang JD, Hoshida Y. Risk stratification and early detection biomarkers for precision HCC screening. Hepatology. 2023;78:319-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 5. | Chi Z, Hong B, Tan S, Wu Y, Li H, Lu CH, Li W. Impact Assessment of heavy metal cations to the characteristics of photosynthetic phycocyanin. J Hazard Mater. 2020;391:122225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Zhou H, Song T. Conversion therapy and maintenance therapy for primary hepatocellular carcinoma. Biosci Trends. 2021;15:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 7. | Dorn AR, Brower A, Turner H, Lapa K. Hypoglycemia and seizures associated with canine primary hepatic neuroendocrine carcinoma. J Vet Diagn Invest. 2021;33:749-752. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Le THV, Kwon SM. Vascular Endothelial Growth Factor Biology and Its Potential as a Therapeutic Target in Rheumatic Diseases. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Khan SZ, Ajmal N, Shaikh R. Diabetic Retinopathy and Vascular Endothelial Growth Factor Gene Insertion/Deletion Polymorphism. Can J Diabetes. 2020;44:287-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Zeng H, Hu L, Xie H, Ma W, Quan S. Polymorphisms of vascular endothelial growth factor and recurrent implantation failure: a systematic review and meta-analysis. Arch Gynecol Obstet. 2021;304:297-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Kumar R, Theiss AL, Venuprasad K. RORγt protein modifications and IL-17-mediated inflammation. Trends Immunol. 2021;42:1037-1050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 103] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 12. | Xu K, Chen Y, Chen M, Zhang W, Wang Y, Ji W, Wang H, Xin X, Feng J, Li Y, Yan L. [Diagnosis and treatment of primary hepatic neuroendocrine carcinoma]. Zhonghua Zhong Liu Za Zhi. 2015;37:451-455. [PubMed] |
| 13. | Tan DJH, Ng CH, Lin SY, Pan XH, Tay P, Lim WH, Teng M, Syn N, Lim G, Yong JN, Quek J, Xiao J, Dan YY, Siddiqui MS, Sanyal AJ, Muthiah MD, Loomba R, Huang DQ. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol. 2022;23:521-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 205] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 14. | Negri F, Gnetti L, Pedrazzi G, Silini EM, Porta C. Sorafenib and hepatocellular carcinoma: is alpha-fetoprotein a biomarker predictive of tumor biology and primary resistance? Future Oncol. 2021;17:3579-3584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Li W, Liu K, Chen Y, Zhu M, Li M. Role of Alpha-Fetoprotein in Hepatocellular Carcinoma Drug Resistance. Curr Med Chem. 2021;28:1126-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Ikeda H, Sato Y, Yoneda N, Harada K, Sasaki M, Kitamura S, Sudo Y, Ooi A, Nakanuma Y. α-Fetoprotein-producing gastric carcinoma and combined hepatocellular and cholangiocarcinoma show similar morphology but different histogenesis with respect to SALL4 expression. Hum Pathol. 2012;43:1955-1963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Dong X, Jin K, Hu X, Du F, Lan H, Han N, Ma Z, Xie B, Cui B, Teng L, Cao F. Antitumor effect of FP3 in combination with cetuximab on patient-derived tumor tissue xenograft models of primary colon carcinoma and related lymphatic and hepatic metastases. Int J Mol Med. 2012;30:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | El-Hanboshy SM, Helmy MW, Abd-Alhaseeb MM. Catalpol synergistically potentiates the anti-tumour effects of regorafenib against hepatocellular carcinoma via dual inhibition of PI3K/Akt/mTOR/NF-κB and VEGF/VEGFR2 signaling pathways. Mol Biol Rep. 2021;48:7233-7242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Jia ZZ, Huang YQ, Feng YL, Jiang GM. [Correlations between serum hypoxia inducible factor-1α, vascular endothelial growth factor and computed tomography perfusion imaging at pre-and post-TACE in patients with primary hepatic carcinoma]. Zhonghua Yi Xue Za Zhi. 2013;93:1472-1475. [PubMed] |
| 20. | Dai CM, Jin S, Zhang JZ. [Effect of Dahuang Zhechong Pills combined with TACE on VEGF, MMP-2, TGF-β1 and immune function of patients with primary liver cancer (blood stasis and collaterals blocking type)]. Zhongguo Zhong Yao Za Zhi. 2021;46:722-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 21. | Qiu W, Wang B, Gao Y, Tian Y, Tian M, Chen Y, Xu L, Yao TP, Li P, Yang P. Targeting Histone Deacetylase 6 Reprograms Interleukin-17-Producing Helper T Cell Pathogenicity and Facilitates Immunotherapies for Hepatocellular Carcinoma. Hepatology. 2020;71:1967-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Hasan I, Gani RA, Lesmana LA, Kresno SB, Pandelaki J, Suwarto S. The Association between Peripheral Th17, Th1, IL-17, and IFN-γ Levels and TACE Response in Patients with Unresectable Hepatocellular Carcinoma with or without Cirrhosis. Acta Med Indones. 2020;52:326-333. [PubMed] |
| 23. | Gasmi I, Machou C, Rodrigues A, Brouillet A, Nguyen TC, Rousseau B, Guillot A, Rodriguez C, Demontant V, Ait-Ahmed Y, Calderaro J, Luciani A, Pawlotsky JM, Lafdil F. Interleukin-17 programs liver progenitor cell transformation into cancer stem cells through miR-122 downregulation with increased risk of primary liver cancer initiation. Int J Biol Sci. 2022;18:1944-1960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 24. | Aboushousha T, Emad M, Rizk G, Ragab K, Hammam O, Fouad R, Helal NS. IL-4, IL-17 and CD163 Immunoexpression and IL-6 Gene Polymorphism in Chronic Hepatitis C Patients and Associated Hepatocellular Carcinoma. Asian Pac J Cancer Prev. 2021;22:1105-1113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |