Published online Sep 27, 2024. doi: 10.4240/wjgs.v16.i9.2878
Revised: July 21, 2024
Accepted: July 26, 2024
Published online: September 27, 2024
Processing time: 112 Days and 10.2 Hours
Whether patients with diffuse gastric cancer, which is insensitive to chemo
To investigate whether perioperative chemotherapy can improve survival of patients with locally advanced diffuse gastric cancer.
A total of 2684 patients with locally advanced diffuse gastric cancer from 18 population-based cancer registries in the United States were analyzed.
Compared with surgery alone, perioperative chemotherapy improved the prognosis of patients with locally advanced gastric cancer. Before stabilized inverse probability of treatment weighting (IPTW), the median overall survival (OS) times were 40.0 months and 13.0 months (P < 0.001), respectively. After IPTW, the median OS times were 33.0 months and 17.0 months (P < 0.001), respectively. Neoadjuvant chemotherapy did not improve the prognosis of patients with locally advanced gastric cancer compared with adjuvant chemotherapy after IPTW. After IPTW, the median OS times were 38.0 months in the neoadjuvant chemotherapy group and 42.0 months in the adjuvant chemotherapy group (P = 0.472).
Patients with diffuse gastric cancer can benefit from perioperative chemotherapy. There was no significant difference in survival between patients who received neoadjuvant chemotherapy and those who received adjuvant chemotherapy.
Core Tip: Patients with diffuse gastric cancer can benefit from perioperative chemotherapy. As there was no significant difference in survival between patients who received neoadjuvant chemotherapy and those who received adjuvant chemotherapy. Clinicians should personalize the selection of neoadjuvant chemotherapy or adjuvant chemotherapy for different patients.
- Citation: Li ZF, Li Z, Zhang XJ, Sun CY, Fei H, Du CX, Guo CG, Zhao DB. Perioperative chemotherapy improves survival of patients with locally advanced diffuse gastric cancer. World J Gastrointest Surg 2024; 16(9): 2878-2892
- URL: https://www.wjgnet.com/1948-9366/full/v16/i9/2878.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i9.2878
Perioperative chemotherapy is the standard of care for locally advanced gastric cancer, as it can eradicate micrometastasis in addition to aiding in achieving local control to improve patient survival[1]. The clinical tumor stage remains the only tool for guiding treatment[2]. Nevertheless, not all patients benefit from perioperative chemotherapy, and whether they benefit depends on localization, molecular subtype, and also on histological subtype[2].
The Lauren classification, the most useful and widely applied classification system for gastric cancer, was first proposed in 1965 and, stratifies gastric cancer into diffuse, intestinal, and mixed types[3]. The Lauren “diffuse” type corresponds to the World Health Organization category of “poorly cohesive” carcinomas. In reality, the terms “diffuse type”, “poorly cohesive” and “signet ring cell” gastric cancer are often used indiscriminately[4,5]. Although the incidence of gastric cancer overall has declined, the incidence of diffuse gastric cancer is increasing[6]. From 1989 to 2015, the median survival of patients with nonmetastatic intestinal type gastric carcinoma rose from 22.8 to 27.6 months. In contrast, those with diffuse type gastric carcinoma experienced a more modest increase, from 16.8 to 18.0 months[7]. It was found that patients with diffuse type gastric cancer have shorter survival after chemotherapy + surgery than those with intestinal type, as the former has been identified as nonresponsive to chemotherapy[2,5,8]. The histopathologic response rates were 22% for the intestinal type, 21% for the mixed type tumors, and only 9% for the diffuse type[2]. Therefore, it is particularly important to determine whether patients with diffuse gastric cancer could benefit from perioperative chemotherapy. Some studies have reported benefits of perioperative chemotherapy for diffuse gastric cancer[9,10], while others have not[2,11-14]. Some believe that there is no cytostatic effect of chemotherapy on diffuse gastric cancer and that it could even be harmful to those patients[13].
As such, we conducted this retrospective study to define the role of perioperative chemotherapy, and secondly, to determine the priority between neoadjuvant chemotherapy and adjuvant chemotherapy for locally advanced diffuse gastric cancer.
The surveillance, epidemiology, and end results (SEER) database (http://www.seer.cancer.gov) containing cancer data from 18 population-based cancer registries, covering > 25% of the United States population, was surveyed for the retrospective collection of data[15]. The analysis focused on the cohort of patients with diffuse gastric cancer [International Classification of Diseases for Oncology, 3rd Edition (ICD-03) histology codes: 8145/3 (carcinoma diffuse type), 8490/3 (signet ring cell carcinoma), and 8142/3 (linitis plastica)] diagnosed from January 1, 2004 to December 31, 2020. And patient demographic (age, sex, ethnicity), diagnostic (tumor site, tumor grade, tumor stage), treatment (surgery, radiotherapy, chemotherapy), and follow-up information were collected. Patients who had tumor staged as I or IV, who did not undergo gastrectomy, and who underwent perioperative radiotherapy were excluded (Supplementary Figure 1). TNM stage was reevaluated according to the 8th American Joint Committee on Cancer tumor staging definitions for gastric cancer. A harvest of at least 21 Lymph nodes was regarded as D2 gastrectomy[16]. Either neoadjuvant chemotherapy or adjuvant chemotherapy were identified as perioperative chemotherapy. Overall survival (OS) and cancer-specific survival (CSS) were defined from the first date of diagnosis to the time of death or last follow-up visit.
Categorical variables were presented as numbers and percentages and compared between groups using the χ2 test. To account for the selection bias, we used the stabilized inverse probability of treatment weighting (IPTW) method to adjust the observed differences in baseline covariates between groups[17]. Factors associated either with the receipt of chemotherapy or with survival were included in constructing the models, which included age, tumor location, diagnosis period, D2 surgery, T stage, and N stage. OS and CSS were compared using the log-rank test and illustrated with Kaplan–Meier curves. Univariate and multivariate analyses using the Cox proportional hazards model were constructed to identify factors independently associated with prognosis. Hazard ratio and 95% confidence interval were used to estimate survival predictors. R version 4.0.4 and SPSS, version 23 (International Business Machine Corp, Armonk, NY, United States) were used to conduct the statistical analysis. Differences with an P < 0.05 were considered statistically significant.
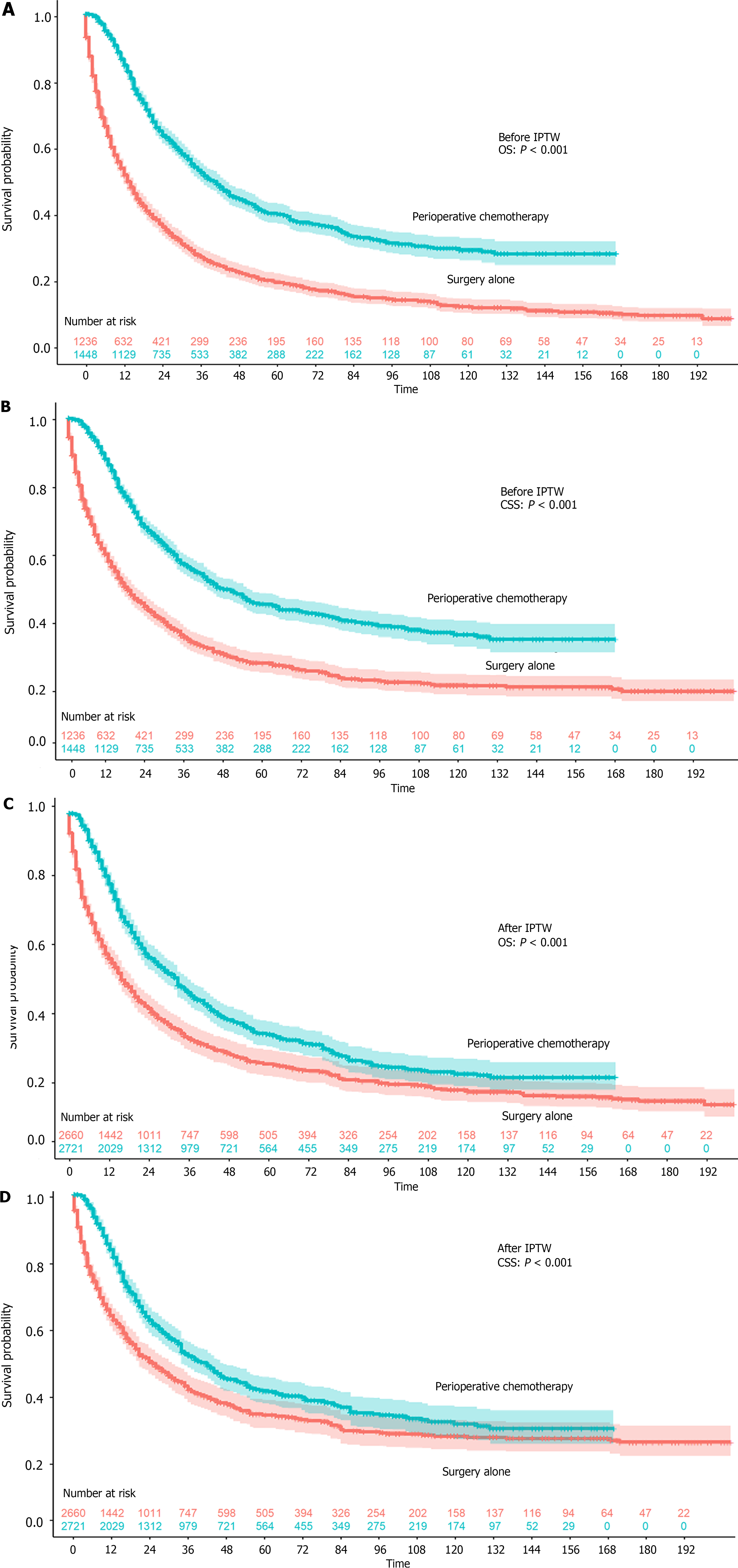
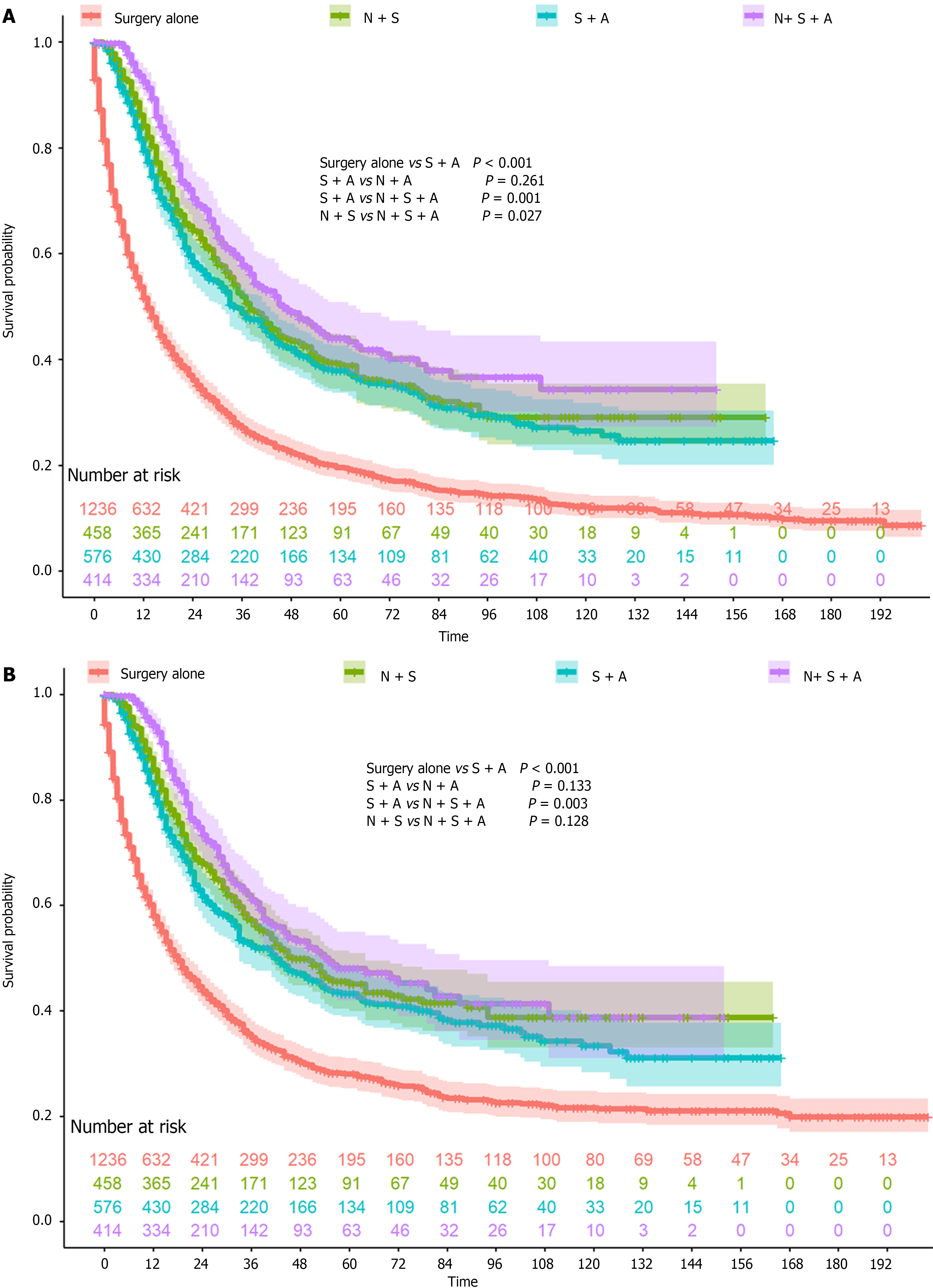
A total of 2684 patients were included in this study, with 1236 who underwent surgery alone and 1448 who underwent perioperative chemotherapy (Table 1). Patients who underwent perioperative chemotherapy were much younger (≤ 45 years old, 16.6% vs 4.8%) and had tumors that were less frequently located in the lower one-third (29.6% vs 36.2%). In addition, more patients underwent perioperative chemotherapy in the past decade (2010-2020), which might be a result of the D2 surgery rate. The OS and CSS rates were both higher in patients who underwent perioperative chemotherapy (both P < 0.001, Figure 1A and B). The 1-year, 3-year, and 5-year OS rates and the 1-year, 3-year, and 5-year CSS rates were 51.6%, 27.0%, and 19.6% and 57.9%, 34.9%, and 28.0%, respectively, in the surgery alone group. The 1-year, 3-year, and 5-year OS rates and 1-year, 3-year, and 5-year CSS rates were 84.6%, 52.3%, and 40.0% and 86.3%, 56.3%, and 45.3%, respectively, in the perioperative chemotherapy group. After IPTW, all covariates were balanced between the two groups, and the OS and CSS were still both higher in patients who underwent perioperative chemotherapy (both P < 0.001, Figure 1C and D). The 1-year, 3-year, and 5-year OS rates and 1-year, 3-year, and 5-year CSS rates were 57.2%, 33.9%, and 26.4% and 62.5%, 41.3%, and 34.3%, respectively, in the surgery alone group. The 1-year, 3-year, and 5-year OS rates and 1-year, 3-year, and 5-year CSS rates were 79.2%, 47.3%, and 35.0% and 81.3%, 51.5%, and 41.2%, respectively, in the perioperative chemotherapy group. As Figure 2 demonstrates that, patients who underwent neoadjuvant chemotherapy or adjuvant chemotherapy both showed a better prognosis than surgery alone.
| Characteristic | Before IPTW | After IPTW | ||||
| Surgery alone | Perioperative chemotherapy (n = 1448) | P value | Surgery alone (n = 2660.3) | Perioperative chemotherapy (n = 2721.4) | P value | |
| Age | < 0.001 | 0.883 | ||||
| ≤ 45 | 59 (4.8) | 241 (16.6) | 316.8 (11.9) | 301.3 (11.1) | ||
| 45-70 | 449 (36.3) | 855 (59.0) | 1268.4 (47.7) | 1312.5 (48.2) | ||
| ≥ 70 | 728 (58.9) | 352 (24.3) | 1075.0 (40.4) | 1107.6 (40.7) | ||
| Sex | 0.111 | 0.191 | ||||
| Male | 634 (51.3) | 697 (48.1) | 1385.5 (52.1) | 1328.0 (48.8) | ||
| Female | 602 (48.7) | 751 (51.9) | 1274.8 (47.9) | 1393.4 (51.2) | ||
| Race | 0.041 | 0.456 | ||||
| White | 828 (67.0) | 915 (63.2) | 1742.1 (65.5) | 1678.2 (61.7) | ||
| Black | 140 (11.3) | 152 (10.5) | 321.1 (12.1) | 335.6 (12.3) | ||
| Asian | 252 (20.4) | 353 (24.4) | 552.6 (20.8) | 654.7 (24.1) | ||
| Other | 16 (1.3) | 28 (1.9) | 44.4 (1.7) | 52.9 (1.9) | ||
| Diagnosis period | < 0.001 | 0.799 | ||||
| 2004-2010 | 693 (56.1) | 275 (19.0) | 980.3 (36.8) | 1019.9 (37.5) | ||
| 2010-2020 | 543 (43.9) | 1173 (81.0) | 1680.0 (63.2) | 1701.6 (62.5) | ||
| Tumor location | 0.001 | 0.994 | ||||
| Upper one-third | 131 (10.6) | 188 (13.0) | 313.3 (11.8) | 315.8 (11.6) | ||
| Middle one-third | 351 (28.4) | 481 (33.2) | 809.9 (30.4) | 838.2 (30.8) | ||
| Lower one-third | 447 (36.2) | 428 (29.6) | 889.2 (33.4) | 916.4 (33.7) | ||
| Over lapping | 156 (12.6) | 196 (13.5) | 351.3 (13.2) | 366.4 (13.5) | ||
| Unknown | 151 (12.2) | 155 (10.7) | 296.5 (11.1) | 284.6 (10.5) | ||
| Histology type | 0.117 | 0.069 | ||||
| Diffuse | 349 (28.2) | 410 (28.3) | 835.0 (31.4) | 716.8 (26.3) | ||
| Signet-ring cell carcinoma | 852 (68.9) | 1014 (70.0) | 1767.7 (66.4) | 1950.4 (71.7) | ||
| Linitis plastica | 35 (2.8) | 24 (1.7) | 57.6 (2.2) | 54.3 (2.0) | ||
| Grade | 0.307 | 0.576 | ||||
| Well/Moderately differentiated | 34 (2.8) | 30 (2.1) | 61.2 (2.3) | 53.6 (2.0) | ||
| Poorly/undifferentiated | 1202 (97.2) | 1418 (97.9) | 2599.1 (97.7) | 2667.8 (98.0) | ||
| T stage | < 0.001 | 0.921 | ||||
| 1 | 76 (6.1) | 86 (5.9) | 184.4 (6.9) | 172.7 (6.3) | ||
| 2 | 73 (5.9) | 160 (11.0) | 206.9 (7.8) | 231.2 (8.5) | ||
| 3 | 562 (45.5) | 678 (46.8) | 1208.2 (45.4) | 1220.7 (44.9) | ||
| 4 | 525 (42.5) | 524 (36.2) | 1060.7 (39.9) | 1096.8 (40.3) | ||
| N stage | 0.084 | 0.821 | ||||
| 0 | 333 (26.9) | 388 (26.8) | 741.1 (27.9) | 706.2 (26.0) | ||
| 1 | 261 (21.1) | 362 (25.0) | 558.6 (21.0) | 566.3 (20.8) | ||
| 2 | 280 (22.7) | 292 (20.2) | 575.2 (21.6) | 619.6 (22.8) | ||
| 3 | 362 (29.3) | 406 (28.0) | 785.4 (29.5) | 829.4 (30.5) | ||
| D2 surgery | < 0.001 | 0.969 | ||||
| No | 914 (73.9) | 667 (46.2) | 1573.7 (59.2) | 1607.3 (59.1) | ||
| Yes | 322 (26.1) | 781 (53.8) | 1086.5 (40.8) | 1114.1 (40.9) | ||
| OS (95%CI) | ||||||
| 1-year | 51.6 (48.9, 54.5) | 84.6 (82.6, 86.5) | 57.2 (53.9, 60.8) | 79.2 (76.0, 82.5) | ||
| 3-year | 27.0 (24.5, 29.6) | 52.3 (49.5, 55.2) | 33.9 (30.4, 37.9) | 47.3 (43.8, 51.1) | ||
| 5-year | 19.6 (17.4, 22.1) | 40.0 (37.1, 43.0) | 26.4 (22.9, 30.4) | 35.0 (31.5, 38.9) | ||
| Median OS (95%CI), month | 13.0 (12.0, 15.0) | 40.0 (36.0, 44.0) | 17.0 (15.0, 20.0) | 33.0 (30.0, 38.0) | ||
| CSS (95%CI) | ||||||
| 1-year | 57.9 (55.1, 60.9) | 86.3 (84.4, 88.1) | 62.5 (59.1, 66.1) | 81.3 (78.1, 84.5) | ||
| 3-year | 34.9 (32.0, 38.0) | 56.3 (53.5, 59.3) | 41.3 (37.4, 45.5) | 51.5 (47.8, 55.4) | ||
| 5-year | 28.0 (25.2, 31.0) | 45.3 (42.3, 48.5) | 34.3 (30.3, 38.7) | 41.2 (37.5, 45.3) | ||
| Median CSS (95%CI), month | 18.0 (16.0, 21.0) | 47.0 (42.0, 54.0) | 24.0 (20.0, 30.0) | 39.0 (33.0, 45.0) | ||
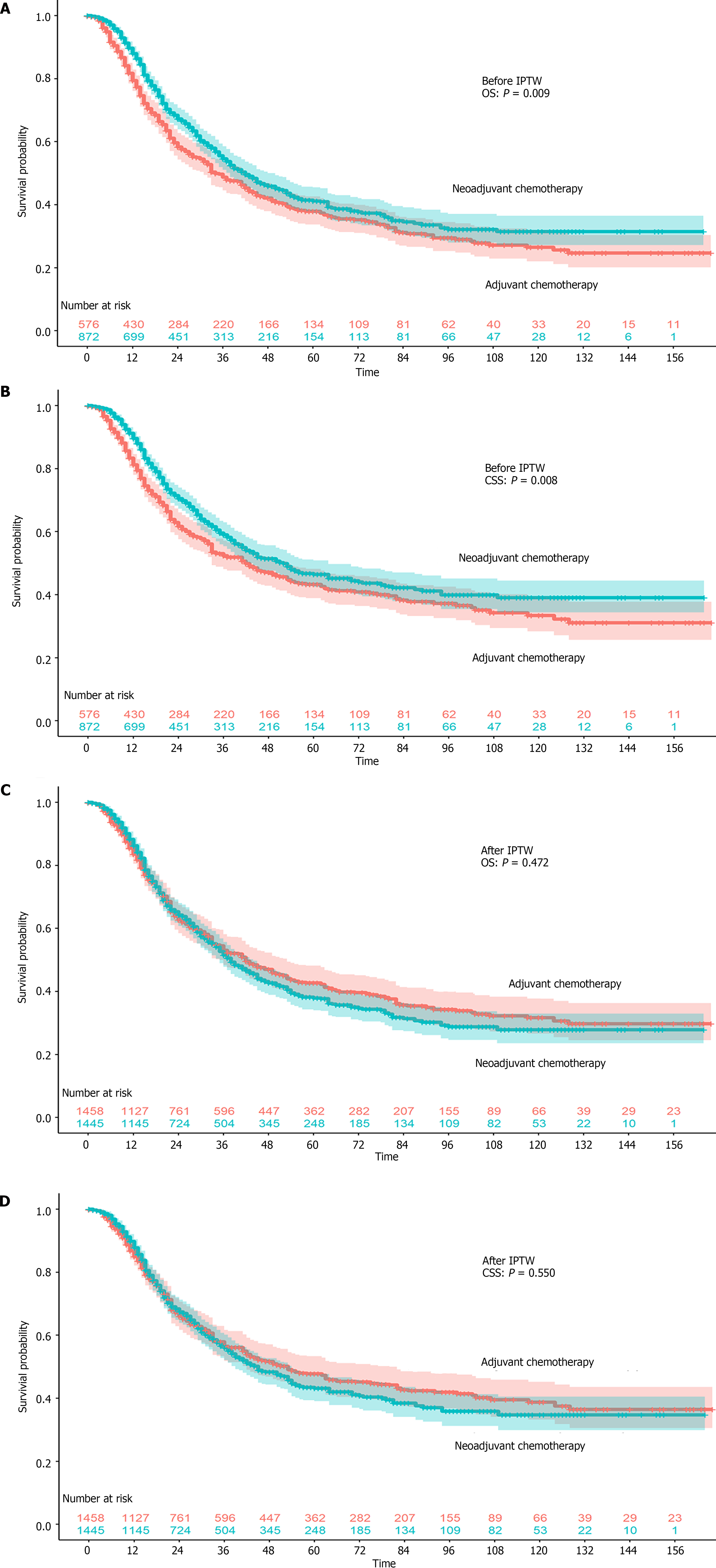
We further clarified whether there is an advantage of neoadjuvant chemotherapy compared with adjuvant chemotherapy. All patients who underwent chemotherapy before surgery were grouped in the neoadjuvant chemotherapy group, regardless of the status of adjuvant chemotherapy (Table 2). Patients who underwent adjuvant chemotherapy were much older (≥ 70 years old 28.0% vs 21.9%). More tumors located in the upper one-third were treated with neoadjuvant chemotherapy (16.4% vs 7.8%). More patients underwent neoadjuvant chemotherapy and D2 gastrectomy in the past 10 years. The OS and CSS rates were both higher in patients who underwent neoadjuvant chemotherapy (OS: P = 0.009; CSS: P = 0.008) (Figure 3A and B). The 1-year, 3-year, and 5-year OS rates and 1-year, 3-year, 5-year CSS rates were 79.4%, 48.7%, and 37.9% and 81.2%, 52.4%, and 43.3%, respectively, in the adjuvant chemotherapy group. The 1-year, 3-year, and 5-year OS rates and 1-year, 3-year, and 5-year CSS rates were 88.0%, 54.6%, and 41.2% and 89.6%, 59.0%, and 46.4%, respectively, in the neoadjuvant chemotherapy group. After IPTW, all covariates were balanced between the two groups, and the OS and CSS rates were not higher any more in patients who underwent neoadjuvant chemotherapy (OS: P = 0.472; CSS: P = 0.550) (Figure 3C and D). The 1-year, 3-year, 5-year OS rates and 1-year, 3-year, 5-year CSS rates were 83.5%, 53.0%, and 42.7% and 85.0%, 56.5%, and 47.8%, respectively, in the adjuvant chemotherapy group. The 1-year, 3-year, 5-year OS rates and 1-year, 3-year, 5-year CSS rates were 86.2%, 51.6%, and 37.8% and 87.8%, 56.0%, and 43.1%, respectively, in the neoadjuvant chemotherapy group.
| Characteristic | Before IPTW | After IPTW | ||||
| Adjuvant chemotherapy (n = 576) | Neoadjuvant chemotherapy (n = 872) | P value | Adjuvant chemotherapy (n = 1458.4) | Neoadjuvant chemotherapy (n = 1445.0) | P value | |
| Age | 0.026 | 0.916 | ||||
| ≤ 45 | 87 (15.1) | 154 (17.7) | 232.9 (16.0) | 243.1 (16.8) | ||
| 45-70 | 328 (56.9) | 527 (60.4) | 882.9 (60.5) | 861.9 (59.7) | ||
| ≥ 70 | 161 (28.0) | 191 (21.9) | 342.6 (23.5) | 339.9 (23.5) | ||
| Sex | 0.347 | 0.823 | ||||
| Male | 268 (46.5) | 429 (49.2) | 694.4 (47.6) | 697.4 (48.3) | ||
| Female | 308 (53.5) | 443 (50.8) | 764.0 (52.4) | 747.6 (51.7) | ||
| Race | 0.170 | 0.314 | ||||
| White | 347 (60.2) | 568 (65.1) | 897.2 (61.5) | 938.7 (65.0) | ||
| Black | 66 (11.5) | 86 (9.9) | 144.3 (9.9) | 140.4 (9.7) | ||
| Asian | 154 (26.7) | 199 (22.8) | 394.7 (27.1) | 332.9 (23.0) | ||
| Other | 9 (1.6) | 19 (2.2) | 22.2 (1.5) | 32.9 (2.3) | ||
| Diagnosis period | < 0.001 | 0.942 | ||||
| 2004-2010 | 151 (26.2) | 124 (14.2) | 270.0 (18.5) | 269.8 (18.7) | ||
| 2010-2020 | 425 (73.8) | 748 (85.8) | 1188.4 (81.5) | 1175.1 (81.3) | ||
| Tumor location | < 0.001 | 0.988 | ||||
| Upper one-third | 45 (7.8) | 143 (16.4) | 202.5 (13.9) | 189.4 (13.1) | ||
| Middle one-third | 198 (34.4) | 283 (32.5) | 500.2 (34.3) | 488.8 (33.8) | ||
| Lower one-third | 198 (34.4) | 230 (26.4) | 417.2 (28.6) | 418.5 (29.0) | ||
| Overlapping | 72 (12.5) | 124 (14.2) | 181.0 (12.4) | 191.2 (13.2) | ||
| Unknown | 63 (10.9) | 92 (10.6) | 157.5 (10.8) | 157.0 (10.9) | ||
| Histology type | 0.753 | 0.999 | ||||
| Diffuse | 159 (27.6) | 251 (28.8) | 400.3 (27.4) | 397.9 (27.5) | ||
| Signet-ring cell carcinoma | 406 (70.5) | 608 (69.7) | 1034.4 (70.9) | 1023.9 (70.9) | ||
| Linitis plastica | 11 (1.9) | 13 (1.5) | 23.7 (1.6) | 23.2 (1.6) | ||
| Grade | 0.179 | 0.056 | ||||
| Well/moderately differentiated | 16 (2.8) | 14 (1.6) | 48.1 (3.3) | 22.2 (1.5) | ||
| Poorly/undifferentiated | 560 (97.2) | 858 (98.4) | 1410.3 (96.7)) | 1422.8 (98.5) | ||
| T stage | < 0.001 | 0.996 | ||||
| 1 | 40 (6.9) | 46 (5.3) | 91.7 (6.3) | 87.8 (6.1) | ||
| 2 | 60 (10.4) | 100 (11.5) | 155.9 (10.7)) | 158.6 (11.0) | ||
| 3 | 223 (38.7) | 455 (52.2) | 683.8 (46.9) | 671.7 (46.5) | ||
| 4 | 253 (43.9) | 271 (31.1) | 527.1 (36.1) | 526.8 (36.5) | ||
| N stage | < 0.001 | 0.963 | ||||
| 0 | 126 (21.9) | 262 (30.0) | 418.2 (28.7) | 395.0 (27.3) | ||
| 1 | 121 (21.0) | 241 (27.6) | 351.0 (24.1) | 356.3 (24.7) | ||
| 2 | 132 (22.9) | 160 (18.3) | 283.7 (19.5) | 282.6 (19.6) | ||
| 3 | 197 (34.2) | 209 (24.0) | 405.6 (27.8) | 411.1 (28.4) | ||
| D2 surgery | < 0.001 | 0.996 | ||||
| No | 310 (53.8) | 357 (40.9) | 672.7 (46.1) | 666.7 (46.1) | ||
| Yes | 266 (46.2) | 515 (59.1) | 785.7 (53.9) | 778.3 (53.9) | ||
| OS (95%CI) | ||||||
| 1-year | 79.4 (76.1, 82.9) | 88.0 (85.8, 90.3) | 83.5 (80.5, 86.6) | 86.2 (83.6, 88.9) | ||
| 3-year | 48.7 (44.5, 53.4) | 54.6 (51.0, 58.5) | 53.0 (48.3, 58.1) | 51.6 (47.8, 55.6) | ||
| 5-year | 37.9 (33.7. 42.6) | 41.2 (37.4, 45.4) | 42.7 (37.9, 48.1) | 37.8 (34.0, 42.2) | ||
| Median OS (95%CI), month | 34.0 (31.0, 42.0) | 42.0 (38.0, 48.0) | 42.0 (35.0, 53.0) | 38.0 (35.0, 44.0) | ||
| CSS (95%CI) | ||||||
| 1-year | 81.2 (78.0, 84.6) | 89.6 (87.5, 91.8) | 85.0 (82.1, 88.0) | 87.8 (85.3, 90.4) | ||
| 3-year | 52.4 (48.1, 57.1) | 59.0 (55.3, 62.9) | 56.5 (51.8, 61.7) | 56.0 (52.1, 60.1) | ||
| 5-year | 43.3 (38.8, 48.2) | 46.4 (42.4, 50.9) | 47.8 (42.8, 53.3) | 43.1 (39.0, 47.4) | ||
| Median CSS (95%CI), month | 42.0 (33.0, 53.0) | 52.0 (43.0, 64.0) | 53.0 (42.0, 80.0) | 45.0 (39.0, 54.0) | ||
We further conducted Cox regression analysis to identify the independent prognostic factors (Table 3). Old age, linitis plastica, and advanced T and N stages were associated with worse prognosis. Asian ethnicity, treatment within the past 10 years, and tumors location in the middle one-third were correlated with a better prognosis. Compared with surgery alone, both neoadjuvant chemotherapy and adjuvant chemotherapy improved the prognosis.
| Characteristic | Univariable analysis HR (95%CI) | P value | Multivariable analysis HR (95%CI) | P value |
| Age | ||||
| ≤ 45 | 1 (Reference) | 1 (Reference) | ||
| 45-70 | 1.435 (1.201-1.716) | < 0.001 | 1.229 (1.026-1.471) | 0.025 |
| ≥ 70 | 2.375 (1.989-2.837) | < 0.001 | 1.791 (1.490-2.153) | < 0.001 |
| Sex | ||||
| Male | 1 (Reference) | |||
| Female | 0.973 (0.886-1.068) | 0.566 | ||
| Race | ||||
| White | 1 (Reference) | 1 (Reference) | ||
| Black | 0.919 (0.792-1.067) | 0.269 | 1.027 (0.883-1.194) | 0.733 |
| Asian | 0.741 (0.657-0.835) | < 0.001 | 0.789 (0.698-0.891) | < 0.001 |
| Other | 0.867 (0.592-1.270) | 0.464 | 1.394 (0.947-2.054) | 0.093 |
| Diagnosis period | ||||
| 2004-2010 | 1 (Reference) | 1 (Reference) | ||
| 2010-2020 | 0.692 (0.629-0.761) | < 0.001 | 0.888 (0.799-0.986) | 0.026 |
| Tumor location | ||||
| Upper one-third | 1 (Reference) | 1 (Reference) | ||
| Middle one-third | 0.813 (0.693-0.955) | 0.012 | 0.838 (0.711-0.987) | 0.034 |
| Lower one-third | 0.996 (0.851-1.165) | 0.956 | 0.954 (0.813-1.120) | 0.568 |
| Overlapping | 1.317 (1.096-1.581) | 0.003 | 1.099 (0.911-1.325) | 0.324 |
| Unknown | 1.436 (1.193-1.729) | < 0.001 | 1.268 (1.049-1.532) | 0.014 |
| Histology type | ||||
| Diffuse | 1 (Reference) | 1 (Reference) | ||
| Signet-ring cell carcinoma | 0.948 (0.853-1.054) | 0.322 | 0.995 (0.894-1.108) | 0.929 |
| Linitis plastica | 1.821 (1.367-2.424) | < 0.001 | 1.514 (1.130-2.029) | 0.006 |
| Grade | ||||
| Well/moderately differentiated | 1 (Reference) | |||
| Poorly/undifferentiated | 0.823 (0.612-1.107) | 0.198 | ||
| T stage | ||||
| 1 | 1 (Reference) | 1 (Reference) | ||
| 2 | 1.171 (0.861-1.593) | 0.314 | 1.449 (1.064-1.975) | 0.019 |
| 3 | 2.376 (1.856-3.041) | < 0.001 | 2.586 (2.012-3.324) | < 0.001 |
| 4 | 3.924 (3.062-5.029) | < 0.001 | 3.852 (2.987-4.967) | < 0.001 |
| N stage | ||||
| 0 | 1 (Reference) | 1 (Reference) | ||
| 1 | 1.230 (1.066-1.419) | 0.005 | 1.534 (1.325-1.777) | < 0.001 |
| 2 | 1.906 (1.660-2.188) | < 0.001 | 2.043 (1.777-2.350) | < 0.001 |
| 3 | 2.496 (2.192-2.841) | < 0.001 | 2.342 (2.049-2.677) | < 0.001 |
| Treatment | ||||
| Surgery alone | 1 (Reference) | 1 (Reference) | ||
| Neoadjuvant chemotherapy | 0.426 (0.380-0.478) | < 0.001 | 0.551 (0.484-0.629) | < 0.001 |
| Adjuvant chemotherapy | 0.519 (0.458-0.587) | < 0.001 | 0.552 (0.484-0.629) | < 0.001 |
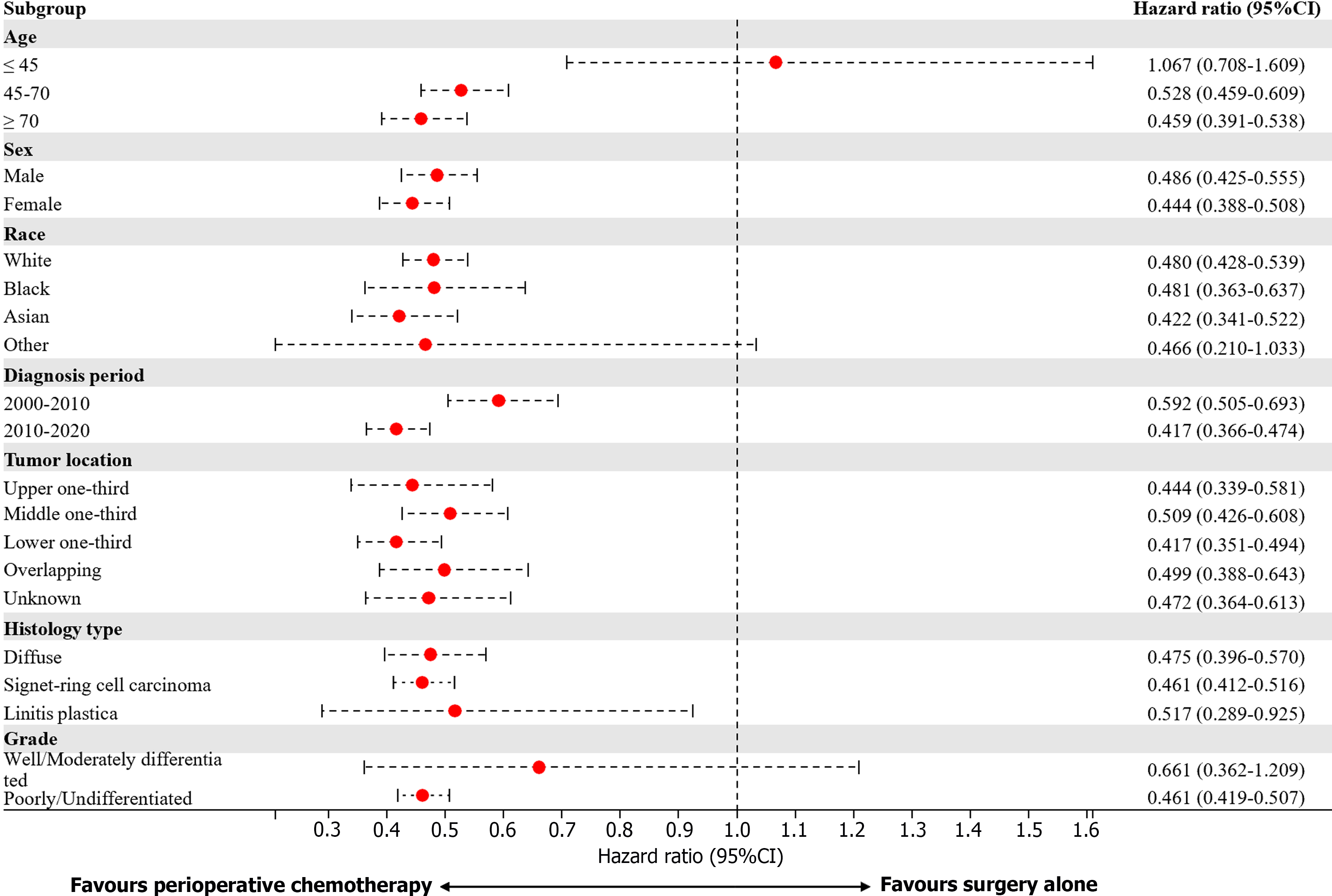
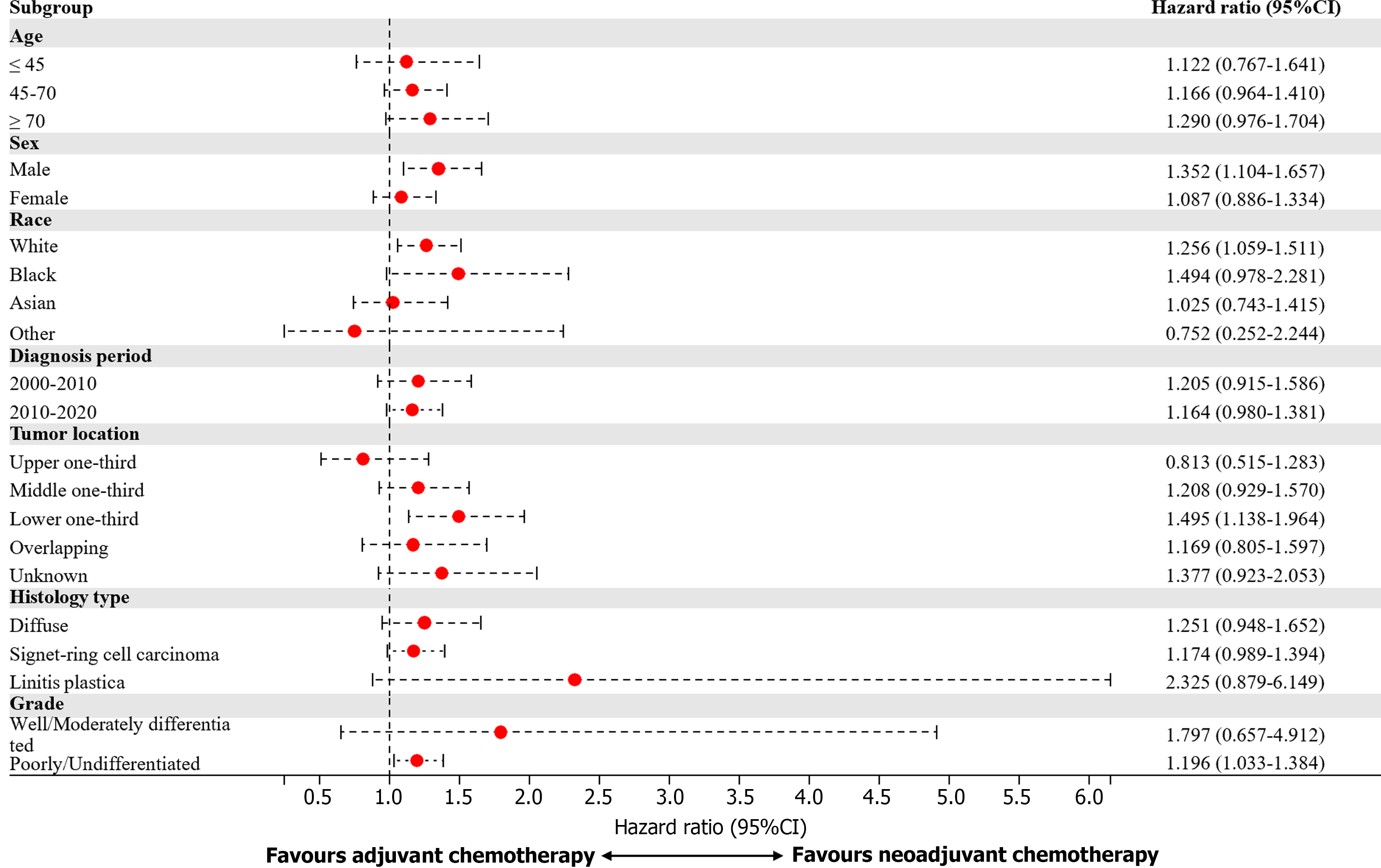
In the subsequent subgroup analysis, the benefits of perioperative chemotherapy differed according to patient age, ethnicity, and tumor grades (Figure 4). Male patients, White patients, patients with tumors in the lower one-third, and patients with poorly differentiated/undifferentiated tumors might benefit more from neoadjuvant chemotherapy than adjuvant chemotherapy (Figure 5).
In our study, we demonstrated that neoadjuvant chemotherapy and adjuvant chemotherapy can both improve the survival of patients with locally advanced diffuse gastric cancer. Neoadjuvant chemotherapy plus surgery was not superior to surgery plus adjuvant chemotherapy.
Schirren et al[2] reported that patients with diffuse type tumors do not show a survival benefit from neoadjuvant chemotherapy. The median survival was 36 months in the surgery-only group compared to 31 months in the neoadjuvant chemotherapy followed by surgery group. Li et al[11] reported that the 5-year OS rates of the neoadjuvant chemotherapy group and surgery-first group were 50% and 64.7% after matching. However, Gertsen et al[9] reported that patients treated with neoadjuvant chemotherapy had a significantly reduced all-cause mortality within 90 days postoperatively and after 90 days compared with patients treated with surgery alone.
The reason for the significant disparities among different studies might be the status of adjuvant chemotherapy. In Schirren’s study, the status of adjuvant chemotherapy was unknown[2]; in Li’s study, more than 70% of patients underwent adjuvant chemotherapy in both groups[11]; in Gertsen’s study, patients in both groups did not undergo adjuvant chemotherapy[9]. Although there are differences among these results, they all showed a certain degree of consistency with our research conclusions, which indicated the importance of adequate perioperative chemotherapy. The JCOG0501 trial could also support this reason. In the phase II trial, for patients with type 4 or large type 3 gastric cancer who underwent 2 circles of neoadjuvant chemotherapy following surgery, the 3-year OS rate was only 24.5%[18]. In their phase III trial, for patients who underwent the same neoadjuvant chemotherapy followed by D2 gastrectomy plus adjuvant chemotherapy with S-1, the 3-year OS rate was 60.9%[19].
Evidence is accruing that diffuse gastric cancer may have possess intrinsic resistance to chemotherapy leading many clinicians to question the advantages of delaying surgery to pursue a neoadjuvant chemotherapy[20]. However, others believe that there may be benefits of preoperative chemotherapy, because postoperative complications after curative surgery for gastric cancer reduce the likelihood of patients receiving adjuvant chemotherapy, which subsequently impacts their overall outcomes negatively[21,22]. We compared neoadjuvant chemotherapy and adjuvant chemotherapy and found no differences. Subgroup analysis indicated that male patients, White patients, and patients with tumors located in the lower one-third might benefit more from neoadjuvant chemotherapy than adjuvant chemotherapy. The outcomes of NCT01717924 will further clarify the whether neoadjuvant chemotherapy or adjuvant chemotherapy is superior[20].
As our study was retrospective and based on a public dataset, there are some limitations. First, patients who were not involved in this study might have experienced rapid disease progression during neoadjuvant chemotherapy and therefore lost the chance to undergo surgery. The selective use of neoadjuvant chemotherapy might have influenced our results. However, as previously reported, although diffuse type gastric cancer is insensitive, only 5% of patients lose the chance to undergo surgery[19]. Second, clinical stage details were lacking and staging was all performed according to pTNM or ypTNM. However, clinical evaluation methods are not accurate enough. cTNM staging does not match pTNM staging in 65.6% of cases, with 50.4% of them being upstaged and 15.2% down staged[23]. In addition, Messager et al[13] reported that neoadjuvant chemotherapy did not lead to tumor downstaging, as indicated by similar pT and pTNM stages observed in both the surgery-only and neoadjuvant chemotherapy groups. Therefore, the ypTNM stage provides similar prognostic information as does the chemo-naive pTNM-stage[24,25]. Furthermore, the patients in the adjuvant chemotherapy group were not influenced by the above limitations. However, there are likely to have been some unidentified and uncorrected biases. For example, patients in the perioperative group always had better economic statuses (data not shown) in the real-world study, let alone physical conditions. In addition, the chemotherapy regimens and cycles were unknown in our study. Nevertheless, this study included a large number of patients and obtained relatively reliable conclusions about the results after IPTW.
Future clinical trials should stratify effect estimations based on histology[26]. There is an immediate requirement for randomized trials specifically focused on diffuse gastric cancer, or stratified according to Lauren classification, to evaluate various therapeutic strategies and/or chemotherapy regimens[13]. Until then, administering perioperative chemotherapy in patients with diffuse type gastric cancer should remain the standard of care[9].
Multimodal therapy including radical gastrectomy and perioperative chemotherapy provides the best long-term survival for patients with diffuse gastric cancer. Clinicians should personalize the selection of neoadjuvant chemotherapy or adjuvant chemotherapy for different patients.
| 1. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4606] [Article Influence: 242.4] [Reference Citation Analysis (0)] |
| 2. | Schirren R, Novotny A, Oesterlin C, Slotta-Huspenina J, Friess H, Reim D. Significance of Lauren Classification in Patients Undergoing Neoadjuvant/Perioperative Chemotherapy for Locally Advanced Gastric or Gastroesophageal Junction Cancers-Analysis from a Large Single Center Cohort in Germany. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Lauren P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attempt at a Histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4011] [Cited by in RCA: 4322] [Article Influence: 149.0] [Reference Citation Analysis (0)] |
| 4. | Mariette C, Carneiro F, Grabsch HI, van der Post RS, Allum W, de Manzoni G; European Chapter of International Gastric Cancer Association. Consensus on the pathological definition and classification of poorly cohesive gastric carcinoma. Gastric Cancer. 2019;22:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (1)] |
| 5. | Heger U, Sisic L, Nienhüser H, Blank S, Hinz U, Haag GM, Ott K, Ulrich A, Büchler MW, Schmidt T. Neoadjuvant Therapy Improves Outcomes in Locally Advanced Signet-Ring-Cell Containing Esophagogastric Adenocarcinomas. Ann Surg Oncol. 2018;25:2418-2427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Ooki A, Yamaguchi K. The dawn of precision medicine in diffuse-type gastric cancer. Ther Adv Med Oncol. 2022;14:17588359221083049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 7. | van der Kaaij RT, Koemans WJ, van Putten M, Snaebjornsson P, Luijten JCHBM, van Dieren JM, Cats A, Lemmens VEPP, Verhoeven RHA, van Sandick JW. A population-based study on intestinal and diffuse type adenocarcinoma of the oesophagus and stomach in the Netherlands between 1989 and 2015. Eur J Cancer. 2020;130:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 8. | Schulz C, Kullmann F, Kunzmann V, Fuchs M, Geissler M, Vehling-Kaiser U, Stauder H, Wein A, Al-Batran SE, Kubin T, Schäfer C, Stintzing S, Giessen C, Modest DP, Ridwelski K, Heinemann V. NeoFLOT: Multicenter phase II study of perioperative chemotherapy in resectable adenocarcinoma of the gastroesophageal junction or gastric adenocarcinoma-Very good response predominantly in patients with intestinal type tumors. Int J Cancer. 2015;137:678-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 9. | Gertsen EC, van der Veen A, Brenkman HJF, Brosens LAA, van der Post RS, Verhoeven RHA, Luijten JCHBM, Vissers PAJ, Vegt E, van Hillegersberg R, Siersema PD, Ruurda JP. Multimodal Therapy Versus Primary Surgery for Gastric and Gastroesophageal Junction Diffuse Type Carcinoma, with a Focus on Signet Ring Cell Carcinoma: A Nationwide Study. Ann Surg Oncol. 2024;31:1760-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Ikoma N, Agnes A, Chen HC, Wang X, Blum MM, Das P, Minsky B, Estrella JS, Mansfield P, Ajani JA, Badgwell BD. Linitis Plastica: a Distinct Type of Gastric Cancer. J Gastrointest Surg. 2020;24:1018-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Li Y, Ma FH, Xue LY, Tian YT. Neoadjuvant chemotherapy vs upfront surgery for gastric signet ring cell carcinoma: A retrospective, propensity score-matched study. World J Gastroenterol. 2020;26:818-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 12. | Wang K, Li E, Busuttil RA, Kong JC, Pattison S, Sung JJY, Yu J, El-Omar EM, Simpson JA, Boussioutas A. A cohort study and meta-analysis of the evidence for consideration of Lauren subtype when prescribing adjuvant or palliative chemotherapy for gastric cancer. Ther Adv Med Oncol. 2020;12:1758835920930359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Messager M, Lefevre JH, Pichot-Delahaye V, Souadka A, Piessen G, Mariette C; FREGAT working group - FRENCH. The impact of perioperative chemotherapy on survival in patients with gastric signet ring cell adenocarcinoma: a multicenter comparative study. Ann Surg. 2011;254:684-93; discussion 693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 14. | Biondi A, Agnes A, Del Coco F, Pozzo C, Strippoli A, D'Ugo D, Persiani R. Preoperative therapy and long-term survival in gastric cancer: One size does not fit all. Surg Oncol. 2018;27:575-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Heger U, Blank S, Wiecha C, Langer R, Weichert W, Lordick F, Bruckner T, Dobritz M, Burian M, Springfeld C, Grenacher L, Siewert JR, Büchler M, Ott K. Is preoperative chemotherapy followed by surgery the appropriate treatment for signet ring cell containing adenocarcinomas of the esophagogastric junction and stomach? Ann Surg Oncol. 2014;21:1739-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Lu J, Wang W, Zheng CH, Fang C, Li P, Xie JW, Wang JB, Lin JX, Chen QY, Cao LL, Lin M, Huang CM, Zhou ZW. Influence of Total Lymph Node Count on Staging and Survival After Gastrectomy for Gastric Cancer: An Analysis From a Two-Institution Database in China. Ann Surg Oncol. 2017;24:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Li Z, Ren H, Wang T, Zhang X, Zhao L, Sun C, Niu P, Guo C, Chen Y, Zhao D. Resection of the Primary Tumor Improves the Survival of Patients With Stage IV Gastric Neuroendocrine Carcinoma. Front Oncol. 2022;12:930491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Iwasaki Y, Sasako M, Yamamoto S, Nakamura K, Sano T, Katai H, Tsujinaka T, Nashimoto A, Fukushima N, Tsuburaya A; Gastric Cancer Surgical Study Group of Japan Clinical Oncology Group. Phase II study of preoperative chemotherapy with S-1 and cisplatin followed by gastrectomy for clinically resectable type 4 and large type 3 gastric cancers (JCOG0210). J Surg Oncol. 2013;107:741-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Iwasaki Y, Terashima M, Mizusawa J, Katayama H, Nakamura K, Katai H, Yoshikawa T, Ito S, Kaji M, Kimura Y, Hirao M, Yamada M, Kurita A, Takagi M, Lee SW, Takagane A, Yabusaki H, Hihara J, Boku N, Sano T, Sasako M. Gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer (JCOG0501): an open-label, phase 3, randomized controlled trial. Gastric Cancer. 2021;24:492-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 103] [Article Influence: 25.8] [Reference Citation Analysis (1)] |
| 20. | Piessen G, Messager M, Le Malicot K, Robb WB, Di Fiore F, Guilbert M, Moreau M, Christophe V, Adenis A, Mariette C. Phase II/III multicentre randomised controlled trial evaluating a strategy of primary surgery and adjuvant chemotherapy versus peri-operative chemotherapy for resectable gastric signet ring cell adenocarcinomas - PRODIGE 19 - FFCD1103 - ADCI002. BMC Cancer. 2013;13:281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Papaxoinis G, Kamposioras K, Weaver JMJ, Kordatou Z, Stamatopoulou S, Germetaki T, Nasralla M, Owen-Holt V, Anthoney A, Mansoor W. The Role of Continuing Perioperative Chemotherapy Post Surgery in Patients with Esophageal or Gastroesophageal Junction Adenocarcinoma: a Multicenter Cohort Study. J Gastrointest Surg. 2019;23:1729-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Sisic L, Blank S, Nienhüser H, Haag GM, Jäger D, Bruckner T, Ott K, Schmidt T, Ulrich A. The postoperative part of perioperative chemotherapy fails to provide a survival benefit in completely resected esophagogastric adenocarcinoma. Surg Oncol. 2020;33:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Papageorge MV, de Geus SWL, Zheng J, Woods AP, Ng SC, Cassidy MR, McAneny D, Tseng JF, Sachs TE. The Discordance of Clinical and Pathologic Staging in Locally Advanced Gastric Adenocarcinoma. J Gastrointest Surg. 2021;25:1363-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Sandø AD, Grønbech JE, Bringeland EA. Does the ypTNM-stage adequately predict long-term survival rates in gastric cancer patients receiving neoadjuvant chemotherapy followed by radical resection? Acta Oncol. 2023;62:1846-1853. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Dimpel R, Novotny A, Slotta-Huspenina J, Langer R, Friess H, Reim D. UICC Staging after Neoadjuvant/Perioperative Chemotherapy Reveals No Significant Survival Differences Compared to Primary Surgery for Locally Advanced Gastric Cancer. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Jiménez Fonseca P, Carmona-Bayonas A, Hernández R, Custodio A, Cano JM, Lacalle A, Echavarria I, Macias I, Mangas M, Visa L, Buxo E, Álvarez Manceñido F, Viudez A, Pericay C, Azkarate A, Ramchandani A, López C, Martinez de Castro E, Fernández Montes A, Longo F, Sánchez Bayona R, Limón ML, Diaz-Serrano A, Martin Carnicero A, Arias D, Cerdà P, Rivera F, Vieitez JM, Sánchez Cánovas M, Garrido M, Gallego J. Lauren subtypes of advanced gastric cancer influence survival and response to chemotherapy: real-world data from the AGAMENON National Cancer Registry. Br J Cancer. 2017;117:775-782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |