Published online Sep 27, 2024. doi: 10.4240/wjgs.v16.i9.2860
Revised: July 29, 2024
Accepted: August 2, 2024
Published online: September 27, 2024
Processing time: 131 Days and 7.1 Hours
Changes in alkaline phosphatase (ALP) and γ-glutamyltransferase (GGT) levels in patients with primary liver cancer (PLC) after radiofrequency ablation (RFA). Hepatocellular carcinoma is a malignant tumor with high incidence worldwide. As a common local treatment, RFA has attracted much attention for its efficacy and influence on liver function.
To investigate the effect of serum ALP and GGT levels on the prognosis of patients with PLC treated by RFA.
The preoperative clinical data of 165 patients who were pathologically or clinically diagnosed with PLC and who received RFA in our hospital between October 2018 and June 2023 were collected. The chi-square test was used to compare the data between groups. The Kaplan-Meier method and Cox regression were used to analyze the associations between serum ALP and GGT levels and overall survival, progression-free survival (PFS) and clinical characteristics of patients before treatment.
The 1-year survival rates of patients with normal (≤ 135 U/L) and abnormal (> 135 U/L) serum ALP before treatment were 91% and 79%, respectively; the 2-year survival rates were 90% and 68%, respectively; and the 5-year survival rates were 35% and 18%, respectively. The difference between the two groups was statistically significant (P = 0.01). Before treatment, the 1-year survival rates of patients with normal serum GGT levels (≤ 45 U/L) and abnormal serum GGT levels (> 45 U/L) were 95% and 87%, the 2-year survival rates were 85% and 71%, and the 5-year survival rates were 37% and 21%, respectively. The difference between the two groups was statistically significant (P < 0.001). Serum ALP [hazard ratio (HR) = 1.766, 95% confidence interval (95%CI): 1.068-2.921, P = 0.027] and GGT (HR = 2. 312, 95%CI: 1.367-3.912, P = 0.002) is closely related to the overall survival of PLC patients after RF ablation and is an independent prognostic factor. The 1-year PFS rates were 72% and 50%, the 2-year PFS rates were 52% and 21%, and the 5-year PFS rates were 14% and 3%, respectively. The difference between the two groups was statistically significant (P < 0001). The 1-year PFS rates were 81% and 56% in patients with normal and abnormal serum GGT levels before treatment, respectively; the 2-year PFS rates were 62% and 35%, respectively; and the 5-year PFS rates were 18% and 7%, respectively, with statistical significance between the two groups (P < 0.001). The serum ALP concentration (HR = 1. 653, 95%CI: 1.001-2.729, P = 0.049) and GGT (HR =
Serum ALP and GGT levels before treatment can be used to predict the prognosis of patients with PLC after RFA, and they have certain guiding significance for the long-term survival of patients with PLC after radiofrequency therapy.
Core Tip: Clinical data of patients with primary liver cancer (PLC) treated with radiofrequency ablation (RFA) were retrospectively analyzed to evaluate the changes in alkaline phosphatase (ALP) and γ-glutamyltransferase (GGT) levels before and after treatment. The subjects of this study were PLC patients undergoing RFA. By collecting ALP and GGT detection results at different time points before and after treatment, the dynamic trend and clinical significance of these indicators were analyzed.
- Citation: Huang WY, Zheng S, Zhu D, Zeng YL, Yang J, Zeng XL, Liu P, Zhang SL, Yuan M, Wang ZX. Analysis of alkaline phosphatase and γ-glutamyltransferase after radiofrequency ablation of primary liver cancer: A retrospective study. World J Gastrointest Surg 2024; 16(9): 2860-2869
- URL: https://www.wjgnet.com/1948-9366/full/v16/i9/2860.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i9.2860
Primary liver cancer (PLC) is a common malignant tumor worldwide, with a high incidence and poor prognosis in China and the Asia-Pacific region[1]. At present, it is believed that the prognosis of PLC patients is not only related to tumor size, tumor number, and the presence of metastasis but also closely related to liver function[2-4]. In clinical practice, liver function is also an important factor affecting the choice of treatment plan; therefore, before the treatment of patients with PLC, it is necessary not only to conduct a comprehensive oncological evaluation but also to conduct a comprehensive evaluation of liver function, which can aid in understanding the pre- and postconditions of patients to a certain extent and providing acupuncture treatment[5]. In recent years, there have been reports in the literature that relevant indicators reflecting liver function, such as alkaline phosphatase (ALP) and γ-glutamyltransferase (GGT), can be applied to the combined diagnosis of PLC, but there are few reports[6-8] on the relationship between these indicators and prognosis.
The incidence and mortality of PLC continue to increase worldwide, and most cases of this tumor are diagnosed at an advanced stage, limiting treatment options[9]. radiofrequency ablation (RFA), a minimally invasive treatment, is widely recognized for its efficacy and safety in treating PLC, especially in patients with early-stage liver cancer. RFA directly destroys tumor cells through the thermal effect generated by high-frequency current, locally controls the lesion, and retains more liver function, thus becoming an important part of the comprehensive treatment of liver cancer. However, although the efficacy of RFA has been recognized clinically, its impact on patient prognosis and its association with biochemical indicators still need to be further studied[10]. ALP and GGT are two biochemical markers commonly used in liver diseases, and their levels generally reflect pathological changes and the functional status of the liver[11-13]. The pattern of changes in these indicators after RFA may reveal the progression of liver recovery, the activity of residual tumors, and the long-term prognosis of patients[14].
In this study, we systematically analyzed the levels of ALP and GGT in patients with PLC after RFA treatment to explore the correlation between these biochemical markers and patient prognosis[15]. The aim of this study was to provide a more accurate prognostic assessment tool for clinical treatment to improve the quality of life and overall survival rate of patients with PLC. We retrospectively analyzed the medical records of a large number of patients with PLC, summarized the changes in ALP and GGT levels before and after RFA treatment, and attempted to establish a correlation model between these parameters and treatment response, recurrence and survival.
Our study evaluated the value of dynamic changes in ALP and GGT for predicting disease progression and evaluating treatment outcomes and explored whether these changes can be used as a reference for adjusting treatment strategies and guiding the frequency and timing of follow-up. In addition, we explored the associations of ALP and GGT with liver cancer pathologic features and their potential use as noninvasive biomarkers for monitoring liver function, predicting recurrence, and assessing patient prognosis.
The clinical data of patients with PLC admitted to our hospital between October 2018 and June 2023 were retrospectively analyzed.
(1) Patients with a pathological diagnosis of PLC; (2) At least one RFA procedure, all of which involved complete ablation; (3) ALP and GGT test results before treatment; (4) Aged 18-80 years; and (5) Complete follow-up data.
(1) A history of other malignant tumors; and (2) Age < 18 years or > 80 years. The follow-up was conducted by telephone inquiry and hospitalization data survey, and the follow-up was conducted once every 3 months. The follow-up ranged from 3 to 54 months, with a median follow-up of 18 months, ending in October 2023.
All patients underwent liver function tests (with an automatic biochemical analyzer (7600-210 Hitachi)) before treatment. Five milliliters of blood was collected on an empty stomach in the morning within 1 week before treatment. The analysis indices included aspartate aminotransferase (AST), alanine aminotransferase (ALT), ALP, and GGT. Serum Alb (Bromocresol green colorimetric method), TBil (2,4-dichloroaniline diazo method), and cholinesterase (hydroxylamine ferric chloride method) were used. In this study, AST > 35 U/L, ALT > 40 U/L, ALP > 135 U/L, GGT > 45 U/L, and TBil > 30 μmol/L were defined as elevated levels, serum Alb < 40 g/L, and cholinesterase < 4300 U/L to reduce.
SPSS 23 was used. Statistical software for data analysis. The χ2 test was used to compare the data between groups. The Kaplan-Meier method and log-rank test were used for survival analysis and comparison. Survival risk factors were analyzed by a Cox proportional hazards regression model, and P < 0.05 was considered to indicate statistical significance.
The data of 165 patients with PLC who underwent RFA were collected. There were 126 males and 39 females. The patients ranged in age from 38 to 80 years, with a median age of 57 years. Postoperative pathology revealed 163 patients with hepatocellular carcinoma and 2 patients with cholangiocarcinoma. The clinical data of the patients and the level of liver function before RFA are shown in Table 1. The recurrence rate was 39.79% at 1 year, 58.79% at 2 years, and 76.97% at 5 years after RFA.
| Clinical data | Number of cases (%) |
| Age | |
| < 60 years old | 92 (55.8) |
| ≥ 60 years old | 73 (44.2) |
| Gender | |
| Male | 126 (76.4) |
| Female | 39 (23.6) |
| Viral hepatitis | |
| Hepatitis B | 119 (72.2) |
| Hepatitis C | 37 (22.4) |
| Hepatitis B comorbidities | 3 (1.8) |
| Hepatitis C | |
| None | 6 (3.6) |
| Child-Pugh | |
| A-level | 136 (82.4) |
| B/C level | 27/2 (16.4/1.2) |
| Tumor staging | |
| 0/A/B | 5/62/65 (3.0/37.6/39.4) |
| C/D | 30/3 (18.2/1.8) |
| Tumor size | |
| < 5 cm | 119 (72.1) |
| ≥ 5 cm | 46 (27.9) |
| Number of tumors | |
| Single shot | 86 (52.1) |
| Multiple occurrences | 79 (47.9) |
| Lymph node metastasis | |
| Yes | 23 (13.9) |
| No | 142 (86.1) |
| Distant metastasis | |
| Yes | 11 (6.7) |
| No | 154 (93.3) |
| AST | |
| ≤ 35 L/I | 63 (38.2) |
| > 35 U/I | 102 (61.8) |
| ALT | |
| ≤ 40 I/I | 49 (29.7) |
| > 40 U/L | 116 (70.3) |
| ALP | |
| ≤ 135 U/I | 127 (77.0) |
| > 135 L/I | 38 (23.0) |
| GGT | |
| ≤ 45 U/I | 65 (39.4) |
| > 45 L/I | 100 (60.6) |
| Cholinesterase | |
| ≥ 4300 IL/I | 100 (60.6) |
| < 4300 IL/I | 65 (39.4) |
| TBil | |
| ≤ 30 μmol/L | 136 (82.4) |
| > 30 μmol/L | 29 (17.6) |
| Alb | |
| ≥ 40 g/L | 52 (31.5) |
| < 40 g/L | 113 (68.5) |
| Hepatic encephalopathy | |
| Yes | 7 (4.2) |
| No | 158 (95.8) |
| Ascites | |
| Yes | 38 (23.0) |
| No | 127 (77.0) |
| Portal vein cancer thrombus | |
| Yes | 8 (4.8) |
| No | 157 (95.2) |
Univariate analysis revealed statistically significant differences in overall survival between patients with abnormal serum ALP, GGT, ALT and TBil before treatment and those with normal liver function indices (P < 0.05).
Multivariate analysis revealed that serum ALP [hazard ratio (HR) = 1.766, 95% confidence interval (95%CI) before treatment: 1.068-2.921] and GGT (HR = 2.312, 95%CI: 1.367-3.912) levels were closely correlated with overall survival in PLC patients after RFA and were found to be independent prognostic factors (Table 2).
| Project | Single factor analysis (95%CI) | P value | Multivariate analysis (95%CI) | P value |
| ALP (> 135 U/L vs ≤ 135 U/L | 1.988 (1.313-3.008) | 0.001 | 1.766 (1.068-2.921) | 0.027 |
| GGT (> 45 U/L vs ≤ 45 U/L) | 2.816 (1.741-4.556) | 0.001 | 2.312 (1.367-3.912) | 0.002 |
| Cholinesterase (< 4300 U/L vs ≥ 4300 U/L) | 1.948 (1.328-2.857) | 0.001 | 1.312 (0.815-2.113) | 0.264 |
| ALT (> 40 U/L vs ≤ 40 U/L) | 1.716 (1.125-2.617) | 0.012 | 1.531 (0.933-2.511) | 0.092 |
| AST (> 35 U/L vs ≤ 35 U/L) | 1.479 (0.937-2.334) | 0.093 | 0.819 (0.483-1.390) | 0.460 |
| TBil (> 30 μmol/L vs ≤ 30 μmol/L) | 2.098 (1.338-3.288) | 0.001 | 1.202 (0.689-2.096) | 0.518 |
| Alb (< 40 g/L vs ≥ 40 g/L) | 1.274 (0.830-1.956) | 0.269 | 0.814 (0.502-1.319) | 0.403 |
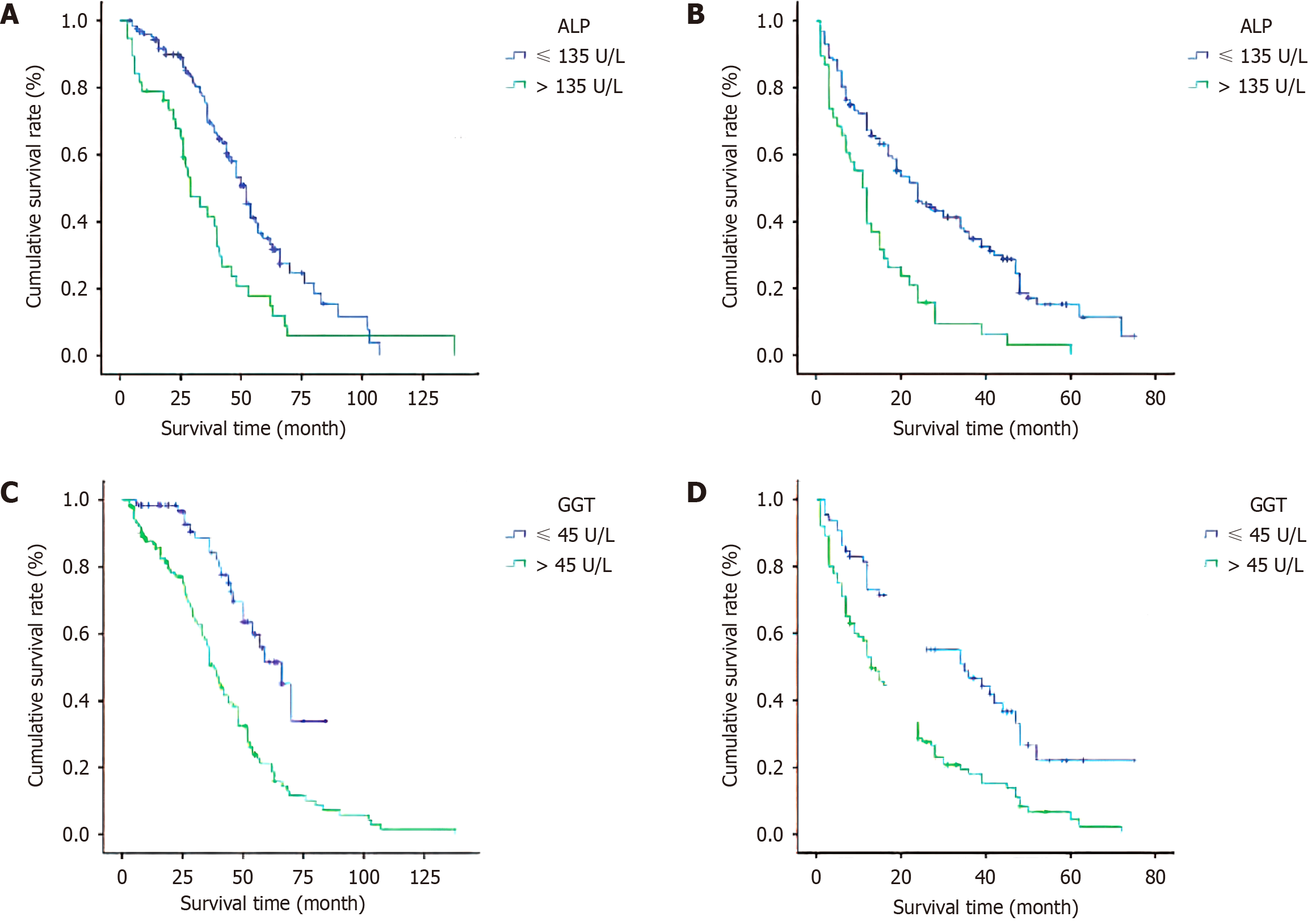
Before treatment, the 1-year survival rates of patients with normal and abnormal serum ALP levels were 91% and 79%, respectively; the 2-year survival rates were 90% and 68%, respectively; and the 5-year survival rates were 35% and 18%, respectively (P = 0.01; Figure 1A). The 1-year survival rates of patients with normal and abnormal serum GGT levels before treatment were 95% and 87%, the 2-year survival rates were 85% and 71%, and the 5-year survival rates were 37% and 21%, respectively (P < 0.001; Figure 1B).
Univariate analysis revealed statistically significant differences in progression-free survival (PFS) between patients with abnormal serum ALP, GGT and TBil levels before treatment and those with normal liver function indices (P values < 0.05). Multifactor analysis revealed that serum ALP and GGT levels before treatment were closely correlated with PFS after RFA in patients with PLC (Table 3).
| Project | Single factor analysis (95%CI) | P value | Multivariate analysis (95%CI) | P value |
| ALP (> 135 U/L vs ≤ 135 U/L) | 2.269 (1.536-3.352) | < 0.001 | 1.653 (1.001-2.729) | 0.049 |
| GGT (> 45 U/L vs ≤ 45 U/L | 2.147 (1.470-3.134 | < 0.001 | 1.949 (1.296-2.930) | 0.001 |
| Cholinesterase (< 4300 U/L vs ≥ 4300 U/L) | 1.343 (0.945-1.910) | 0.100 | 0.956 (0.623-1.469) | 0.839 |
| ALT (> 40 U/L vs ≤ 40 U/L) | 1.121 (0.782-1.609 | 0.534 | 1.046 (0.689-1.588) | 0.834 |
| AST (> 35 U/L vs ≤ 35 U/L) | 1.103 (0.747-1.629 | 0.621 | 0.776 (0.487-1.237) | 0.286 |
| TBil (> 30 μmol/L vs ≤ 30 μmol/L) | 2.020 (1.329-3.072) | 0.001 | 1.335 (0.765-2.329) | 0.309 |
| Alb (< 40 g/L vs ≥ 40 g/L) | 1.464 (0.994-2.156) | 0.054 | 1.119 (0.727-1.722) | 0.60 g |
The 1-year PFS rates were 72% and 50%, the 2-year PFS rates were 52% and 21%, and the 5-year PFS rates were 14% and 3%, respectively, in patients with normal and abnormal serum ALP levels before treatment. The difference was statistically significant (P < 0. 001; Figure 1C). In patients with normal and abnormal serum GGT levels, the 1-year PFS rates were 81% and 56%, the 2-year PFS rates were 62% and 35%, and the 5-year PFS rates were 18% and 7%, respectively, with statistically significant differences (P < 0.001; Figure 1D).
Before treatment, patients with elevated serum ALP levels were compared with those with normal ALP levels, and there were statistically significant differences in sex, Child-Pugh grade and the presence of ascites (all P values < 0.05). Before treatment, patients with elevated serum GGT levels were compared with those with normal GGT levels, and there were statistically significant differences in the Child-Pugh grade, tumor stage, tumor size, and incidence of hepatic encephalopathy (all P values < 0.05; Table 4).
| Project | ALP ≤ 135 U/L (n = 127) | ALP > 135 U/L (n = 38) | χ2 | P value | GGT ≤ 45 U/L (n = 65) | GGT > 45 U/L (n = 100) | χ2 | P value |
| Age (< 60 years vs ≥ 60 years) | 68/59 | 24/14 | 1.096 | 0.295 | 32/33 | 60/40 | 1.852 | 0.174 |
| Gender (female vs male) | 102/25 | 24/14 | 4.770 | 0.029 | 49/16 | 77/23 | 0.057 | 0.811 |
| Tumor staging (0/A/B vs C/D) | 103/24 | 29/9 | 0.419 | 0.518 | 58/7 | 74/26 | 6.188 | 0.013 |
| Child-Puch grading (A vs B/C) | 118/9 | 18/20 | 41.881 | < 0.001 | 50/62 | 74/26 | 12.436 | < 0.001 |
| Tumor size (< 5 cm vs ≥ 5 cm) | 92/35 | 27/11 | 0.028 | 0.867 | 54/11 | 65/35 | 6.402 | 0.011 |
| Number of tumors (single vs multiple) | 66/61 | 20/18 | 0.005 | 0.943 | 38/27 | 48/52 | 1.728 | 0.189 |
| Lymph node metastasis (Y vs N) | 16/111 | 7/31 | 0.827 | 0.363 | 5/60 | 18/82 | 3.489 | 0.062 |
| Remote metastasis (Y vs N) | 10/117 | 1/37 | 1.292 | 0.256 | 3/62 | 8/92 | 0.725 | 0.394 |
| Hepatic encephalopathy (Y vs N) | 4/123 | 3/35 | 1.621 | 0.203 | 0/65 | 7/93 | 4.752 | 0.029 |
| Ascites (Y vs N) | 22/105 | 16/22 | 10.134 | 0.001 | 12/53 | 26/74 | 1.263 | 0.26 |
| Portal vein cancer thrombus (Y vs N) | 6/121 | 2/36 | 0.018 | 0.892 | 1/64 | 7/93 | 2.547 | 0.111 |
At present, the liver function test is one of the most commonly used tests to assess the metabolic reserve function of the liver[16-18]. It is convenient and fast, and AST, ALT, ALP, GGT, Alb, TBil and cholinesterase are closely related to the synthesis and metabolic function of the liver[19]. This study confirmed that serum ALP and GGT levels have certain predictive value for PFS and overall survival after RFA for PLC patients and are indicators of poor prognosis in patients with PLC.
Serum ALP and GGT levels have always been considered to have certain diagnostic value for malignant tumors, and it has been found in recent years that increases in serum ALP and GGT levels increase the risk of malignant tumors[20-22]. Serum GGT is mainly derived from the liver, is produced by hepatocyte mitochondria and is excreted by the biliary tract[23]. It is mainly distributed in hepatocyte plasma and the intrahepatic bile duct epithelium. Relevant studies[24-26] have suggested that the serum GGT level is positively correlated with the incidence of malignant tumors. Serum ALP is a hydrolase synthesized and secreted by liver cells and is excreted by the biliary tract; it is widely distributed in the human liver, bone, intestine, kidney, placenta and other tissues[27]. Abnormal ALP is more common in patients with liver and biliary diseases and bone diseases. Some studies[28-30] have suggested that ALP activity in the tumor nucleus is increased. Another study[31] also proposed that the serum ALP level in patients with malignant tumors is a predictor of bone metastasis. Increase in the serum ALP concentration in patients with PLC may be related to biliary tract inflammation affecting liver function. At present, there are few reports[32-34] on the relationships between serum ALP and GGT levels and the prognosis of patients with malignant tumors. In a prognostic scoring system for liver cancer patients created, a serum ALP concentration > 200 U/L was calculated as 3 points, indicating that the ALP concentration has certain predictive value for liver cancer prognosis. Moreover, a small number of reports have confirmed that the serum GGT level is correlated with the prognosis of patients with colorectal cancer and esophageal cancer.
Through multifactor analysis, this study revealed that serum ALP and GGT levels are independent risk factors affecting the prognosis of patients with PLC and are indicators of poor prognosis[35]. Serum GGT levels are strongly correlated with poor overall survival and PFS in patients with PLC, which is consistent with the results of previous relevant studies[36-38]. At the same time, the relationships between serum ALP and GGT levels and the clinical characteristics of patients with PLC were analyzed, and the results showed that patients with high serum ALP and GGT levels had short overall survival, high mortality and poor prognosis[39]. Further stratified analysis revealed that the proportion of male patients with elevated serum ALP levels was high (36.84%), the Child-Pugh classification of liver function was poor (B/C 52.63%), and the incidence of ascites was high (42.11%)[40]. Among the patients with elevated serum GGT levels, the Child-Pugh grade of liver function was poor (26.00% B/C grade), the tumor stage was late (27% C/D stage), the number of patients with tumors ≥ 5 cm was greater than that of the patients (35%), and the incidence of hepatic encephalopathy was greater (7%). These findings indicate that serum ALP and GGT levels are not only independent prognostic factors after RF ablation for PLC patients but are also are correlated with Child-Pugh grade and liver function indicators in PLC patients. There have been few studies[41,42] on the relationships between serum ALP and GGT levels and relevant clinical features of patients with PLC. The results of this study can guide the prognosis evaluation and treatment plan selection of clinical patients to a certain extent.
At the same time, there are still some limitations in this study: The included patients were all from the same center, and the incidence of hepatitis was high, which may have had a certain impact on the results. This was a retrospective study, and future studies will continue to explore whether a model can be established to organically integrate relevant indicators to more accurately predict the prognosis of patients with PLC.
Through retrospective analysis of ALP and GGT after RFA in patients with PLC, this study revealed that RFA had a significant impact on ALP and ALT levels. ALP levels decreased significantly after treatment, while ALT levels showed unstable changes, increasing in some patients and decreasing in some patients. This suggests that RFA may have some effect on liver function, but the specific mechanism still needs to be further studied. In summary, RFA can be an effective treatment for PLC, but its effects on liver function still need to be carefully evaluated in clinical practice.
| 1. | Izzo F, Granata V, Grassi R, Fusco R, Palaia R, Delrio P, Carrafiello G, Azoulay D, Petrillo A, Curley SA. Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist. 2019;24:e990-e1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 360] [Article Influence: 60.0] [Reference Citation Analysis (1)] |
| 2. | Chen Z, Wang J, Lin Y. Comparison of the efficacy and safety of repeated hepatectomy and radiofrequency ablation in the treatment of primary recurrent liver cancer: a meta-analysis. World J Surg Oncol. 2022;20:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 3. | Abdlaty R, Abbass MA, Awadallah AM. Radiofrequency ablation for liver: Comparison between expert eye and hyperspectral imaging assessment. Photodiagnosis Photodyn Ther. 2022;37:102699. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Tomita K, Matsui Y, Uka M, Umakoshi N, Kawabata T, Munetomo K, Nagata S, Iguchi T, Hiraki T. Evidence on percutaneous radiofrequency and microwave ablation for liver metastases over the last decade. Jpn J Radiol. 2022;40:1035-1045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 5. | Akhan O, Akçalar S, Ünal E, Metin Y, Çiftçi T, Akıncı D. Radiofrequency Ablation for Colorectal Cancer Liver Metastases: Outcomes and Prognostic Factors Associated with Survival. Turk J Gastroenterol. 2023;34:645-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | Han Y, Yan D, Xu F, Li X, Cai JQ. Radiofrequency Ablation versus Liver Resection for Colorectal Cancer Liver Metastasis: An Updated Systematic Review and Meta-analysis. Chin Med J (Engl). 2016;129:2983-2990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Tago T, Katsumata K, Udou R, Kasahara K, Mazaki J, Kuwabara H, Enomoto M, Ishizaki T, Nagakawa Y, Sugimoto K, Itoi T, Tsuchida A. Significance of Radiofrequency Ablation for Unresectable Colorectal Cancer With Liver Metastases. Anticancer Res. 2021;41:5539-5547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Bosi C, Rimini M, Casadei-Gardini A, Giorgio Ercolani. Understanding the causes of recurrent HCC after liver resection and radiofrequency ablation. Expert Rev Anticancer Ther. 2023;23:503-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 9. | Ceppa EP, Collings AT, Abdalla M, Onkendi E, Nelson DW, Ozair A, Miraflor E, Rahman F, Whiteside J, Shah MM, Ayloo S, Dirks R, Kumar SS, Ansari MT, Sucandy I, Ali K, Douglas S, Polanco PM, Vreeland TJ, Buell J, Abou-Setta AM, Awad Z, Kwon CH, Martinie JB, Sbrana F, Pryor A, Slater BJ, Richardson W, Jeyarajah R, Alseidi A. SAGES/AHPBA guidelines for the use of microwave and radiofrequency liver ablation for the surgical treatment of hepatocellular carcinoma or colorectal liver metastases less than 5 cm. Surg Endosc. 2023;37:8991-9000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 10. | Wu L, Zhong Y, Wu D, Xu P, Ruan X, Yan J, Liu J, Li X. Immunomodulatory Factor TIM3 of Cytolytic Active Genes Affected the Survival and Prognosis of Lung Adenocarcinoma Patients by Multi-Omics Analysis. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 63] [Reference Citation Analysis (0)] |
| 11. | Wu L, Zheng Y, Liu J, Luo R, Wu D, Xu P, Wu D, Li X. Comprehensive evaluation of the efficacy and safety of LPV/r drugs in the treatment of SARS and MERS to provide potential treatment options for COVID-19. Aging (Albany NY). 2021;13:10833-10852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 12. | Tang K, Zhang B, Dong L, Wang L, Tang Z. Radiofrequency ablation versus traditional liver resection and chemotherapy for liver metastases from gastric cancer. J Int Med Res. 2020;48:300060520940509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Schullian P, Johnston E, Laimer G, Putzer D, Eberle G, Scharll Y, Ianetti-Hackl C, Bale R. Stereotactic Radiofrequency Ablation of Breast Cancer Liver Metastases: Short- and Long-Term Results with Predicting Factors for Survival. Cardiovasc Intervent Radiol. 2021;44:1184-1193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Wu L, Liu Q, Ruan X, Luan X, Zhong Y, Liu J, Yan J, Li X. Multiple Omics Analysis of the Role of RBM10 Gene Instability in Immune Regulation and Drug Sensitivity in Patients with Lung Adenocarcinoma (LUAD). Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 15. | Tan C, Fisher OM, Huang L, Alzahrani N, Liauw W, Glenn D, Morris DL. Comparison of Microwave and Radiofrequency Ablation in the Treatment of Pulmonary Metastasis of Colorectal Cancer. Anticancer Res. 2022;42:4563-4571. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Covey AM, Sofocleous CT. Radiofrequency ablation as a treatment strategy for liver metastases from breast cancer. Semin Intervent Radiol. 2008;25:406-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Spiliotis AE, Gäbelein G, Holländer S, Scherber PR, Glanemann M, Patel B. Microwave ablation compared with radiofrequency ablation for the treatment of liver cancer: a systematic review and meta-analysis. Radiol Oncol. 2021;55:247-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Yu J, Kim DH, Lee J, Shin YM, Kim JH, Yoon SM, Jung J, Kim JC, Yu CS, Lim SB, Park IJ, Kim TW, Hong YS, Kim SY, Kim JE, Park JH, Kim SY. Radiofrequency Ablation versus Stereotactic Body Radiation Therapy in the Treatment of Colorectal Cancer Liver Metastases. Cancer Res Treat. 2022;54:850-859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Wu L, Zheng Y, Ruan X, Wu D, Xu P, Liu J, Wu D, Li X. Long-chain noncoding ribonucleic acids affect the survival and prognosis of patients with esophageal adenocarcinoma through the autophagy pathway: construction of a prognostic model. Anticancer Drugs. 2022;33:e590-e603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 20. | Tang Y, Zhong H, Wang Y, Wu J, Zheng J. Efficacy of microwave ablation versus radiofrequency ablation in the treatment of colorectal liver metastases: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2023;47:102182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Li R, An C, Wang S, Wang G, Zhao L, Yu Y, Wang L. A heuristic method for rapid and automatic radiofrequency ablation planning of liver tumors. Int J Comput Assist Radiol Surg. 2023;18:2213-2221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Nieuwenhuizen S, Dijkstra M, Puijk RS, Geboers B, Ruarus AH, Schouten EA, Nielsen K, de Vries JJJ, Bruynzeel AME, Scheffer HJ, van den Tol MP, Haasbeek CJA, Meijerink MR. Microwave Ablation, Radiofrequency Ablation, Irreversible Electroporation, and Stereotactic Ablative Body Radiotherapy for Intermediate Size (3-5 cm) Unresectable Colorectal Liver Metastases: a Systematic Review and Meta-analysis. Curr Oncol Rep. 2022;24:793-808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 23. | Fang Z, Wei H, Zhang H, Moser MAJ, Zhang W, Qian Z, Zhang B. Radiofrequency ablation for liver tumors abutting complex blood vessel structures: treatment protocol optimization using response surface method and computer modeling. Int J Hyperthermia. 2022;39:733-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 24. | Bai L, Wang X, Shi S, Gao J, Li X, Wang Y, Jiang M, Zheng C, Liu H. Evaluation of 3D-CEUS in the Recurrence of Liver Cancer after Radiofrequency Ablation. J Healthc Eng. 2021;2021:3123553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Wu L, Zhong Y, Yu X, Wu D, Xu P, Lv L, Ruan X, Liu Q, Feng Y, Liu J, Li X. Selective poly adenylation predicts the efficacy of immunotherapy in patients with lung adenocarcinoma by multiple omics research. Anticancer Drugs. 2022;33:943-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 26. | Minami Y, Nishida N, Kudo M. Radiofrequency ablation of liver metastasis: potential impact on immune checkpoint inhibitor therapy. Eur Radiol. 2019;29:5045-5051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 27. | Yap S, Ooi EH, Foo JJ, Ooi ET. Bipolar radiofrequency ablation treatment of liver cancer employing monopolar needles: A comprehensive investigation on the efficacy of time-based switching. Comput Biol Med. 2021;131:104273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Zhang L, Qiao L, Zhang M, Xue Y, Zhang X, Gao X. Comparison of prognosis among patients with colorectal cancer liver metastases treated by surgical resection, radiofrequency ablation and HIFU: A protocol for network meta-analysis. Medicine (Baltimore). 2022;101:e27915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 29. | Wu L, Li H, Liu Y, Fan Z, Xu J, Li N, Qian X, Lin Z, Li X, Yan J. Research progress of 3D-bioprinted functional pancreas and in vitro tumor models. Int J Bioprinting. 2024;10:1256. [DOI] [Full Text] |
| 30. | Yang S, Zhang Z, Su T, Chen Q, Wang H, Jin L. Comparison of quantitative volumetric analysis and linear measurement for predicting the survival of Barcelona Clinic Liver Cancer 0- and A stage hepatocellular carcinoma after radiofrequency ablation. Diagn Interv Radiol. 2023;29:450-459. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 31. | Wu L, Li X, Qian X, Wang S, Liu J, Yan J. Lipid Nanoparticle (LNP) Delivery Carrier-Assisted Targeted Controlled Release mRNA Vaccines in Tumor Immunity. Vaccines (Basel). 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 32. | Huang Y, Xu L, Huang M, Jiang L, Xu M. Repeat hepatic resection combined with intraoperative radiofrequency ablation versus repeat hepatic resection alone for recurrent and multiple hepatocellular carcinoma patients meeting the Barcelona Clinic Liver Cancer stage A: A propensity score-matched analysis. Cancer Med. 2023;12:9213-9227. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 33. | Jiao XL, Li SC, Hao L, Wang TG, Chen JF. Cost-benefit analysis of hepatic resection, radiofrequency ablation and liver transplantation in small hepatocellular carcinoma. Expert Rev Pharmacoecon Outcomes Res. 2022;22:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 34. | Rim CH, Lee HY, Kim JS, Kim H. Radiofrequency ablation and stereotactic body radiotherapy for hepatocellular carcinoma: should they clash or reconcile? Int J Radiat Biol. 2021;97:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Wu L, Chen X, Zeng Q, Lai Z, Fan Z, Ruan X, Li X, Yan J. NR5A2 gene affects the overall survival of LUAD patients by regulating the activity of CSCs through SNP pathway by OCLR algorithm and immune score. Heliyon. 2024;10:e28282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 36. | Chow R, Simone CB 2nd, Jairam MP, Swaminath A, Boldt G, Lock M. Radiofrequency ablation vs radiation therapy vs transarterial chemoembolization vs yttrium 90 for local treatment of liver cancer - a systematic review and network meta-analysis of survival data. Acta Oncol. 2022;61:484-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Yap S, Ooi EH, Foo JJ, Ooi ET. Comparisons between impedance-based and time-based switching bipolar radiofrequency ablation for the treatment of liver cancer. Comput Biol Med. 2021;134:104488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Schullian P, Laimer G, Johnston E, Putzer D, Eberle G, Scharll Y, Widmann G, Kolbitsch C, Bale R. Technical efficacy and local recurrence after stereotactic radiofrequency ablation of 2653 liver tumors: a 15-year single-center experience with evaluation of prognostic factors. Int J Hyperthermia. 2022;39:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 39. | Perez-Caballero L, Torres-Sanchez S, Bravo L, Mico JA, Berrocoso E. Fluoxetine: a case history of its discovery and preclinical development. Expert Opin Drug Discov. 2014;9:567-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 40. | Luo B, Liu L, Bi J, Bao S, Zhang Y. Role of the pre- to postoperative alpha-fetoprotein ratio in the prognostic evaluation of liver cancer after radiofrequency ablation. Int J Biol Markers. 2022;37:306-313. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 41. | Helton WS. Minimizing complications with radiofrequency ablation for liver cancer: the importance of properly controlled clinical trials and standardized reporting. Ann Surg. 2004;239:459-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Guo C, Liang H, Yuan W, Qin Y. Analysis on the value of soluble intercellular adhesion molecule-1 (sICAM-1), alpha fetoprotein (AFP), and aspartate aminotransferase/platelet ratio index (APRI) in predicting the prognostic survival of patients with primary liver cancer after radiofrequency ablation. Ann Palliat Med. 2021;10:4760-4767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |