Published online Sep 27, 2024. doi: 10.4240/wjgs.v16.i9.2829
Revised: July 11, 2024
Accepted: August 1, 2024
Published online: September 27, 2024
Processing time: 209 Days and 5.9 Hours
Hepatocellular carcinoma (HCC) often presents as unresectable, necessitating effective treatment modalities. Combining transarterial chemoembolization (TACE) with immunotherapy and targeted therapy has shown promise, yet real-world evidence is needed.
To investigate effectiveness and safety of TACE with tislelizumab ± targeted therapy for unresectable HCC in real-world setting.
This retrospective study included patients with unresectable HCC receiving combined treatment of TACE and tislelizumab. The clinical outcomes included progression-free survival (PFS), overall survival (OS), objective response rate (ORR), and disease control rate (DCR). All patients were evaluated according to the mRECIST criteria. The adverse event (AE) was also assessed.
In this study of 56 patients with median follow-up of 10.9 months, 7 had previous immunotherapy. Tislelizumab was administered before TACE in 21 (37.50%) and after in 35 (62.50%) patients, with 91.07% receiving concurrent targeted therapy. Median PFS was 14.0 (95%CI: 7.0-18.00) months, and OS was 28 (95%CI: 2.94-53.05) months. Patients with prior immunotherapy had shorter PFS (6 vs. 18 months, P = 0.006). Overall ORR and DCR were 82.14% and 87.50%. Grade ≥ 3 treatment-related AEs included increased alanine aminotransferase (8.93%), aspartate aminotransferase (10.71%), and total bilirubin (3.57%).
The combination of TACE and tislelizumab, with or without targeted therapy, demonstrated promising efficacy and safety in unresectable HCC, especially in immunotherapy-naive patients, warranting further prospective validation studies.
Core Tip: Combining transarterial chemoembolization with tislelizumab ± targeted therapy shows promise for treating unresectable hepatocellular carcinoma (HCC). In this real-world study of 56 cases, median progression-free survival (PFS) and overall survival were 14.0 and 28 months, respectively. Notably, individuals with prior immunotherapy had shorter PFS. The approach demonstrated high objective response rate and disease control rate, with manageable adverse events. These findings support further investigation and validate the potential of this combination therapy in HCC management, especially in immunotherapy-naive patients.
- Citation: Tan BB, Fu Y, Shao MH, Chen HL, Liu P, Fan C, Zhang H. Combined transarterial chemoembolization and tislelizumab for patients with unresectable hepatocellular carcinoma. World J Gastrointest Surg 2024; 16(9): 2829-2841
- URL: https://www.wjgnet.com/1948-9366/full/v16/i9/2829.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i9.2829
Over the past decade, there has been a concerning increase in the occurrence of liver cancer, with 905677 new cases and 830180 reported deaths in the year 2020[1]. Liver cancer, of which hepatocellular carcinoma (HCC) is the major type, has a poor prognosis[2,3]. The 5-year overall survival (OS) is 20.8% for overall population, 36.1% for patients with localized disease, and 3.1% for patients who presented with metastatic disease, according to data from the SEER programme[4]. The management of HCC is multidisciplinary, encompassing a spectrum of commonly used modalities such as surgery, local ablative therapies, transarterial chemoembolization (TACE), chemotherapy, targeted therapy, radiation therapy, and immunotherapy[2,5]. Despite improvements in treatment strategies and ongoing exploration of new drug, it is imperative to emphasize that the existing clinical requirements for improving prognosis have not been comprehensively fulfilled. A major factor affecting prognosis is that HCC is typically asymptomatic throughout the clinical course of the disease, and many patients are diagnosed when HCC is unresectable, and treatment options are often limited[6]. In such cases, patients with unresectable HCC often confront a complex decision-making task. To obtain better treatment effectiveness, considerations of therapeutic approaches should go beyond just specific treatments and involve a range of methods, such as locoregional and systematic treatments, comprehensive supportive care, and the possibility of participation in innovative clinical trials[2,5].
TACE is an arterially directed therapy that involves chemotherapy injection in the tumor-feeding arteries, followed by embolization[7]. Significant survival benefit has been demonstrated for TACE in treating unresectable HCC as compared with supportive care[8-10], with the 3-year OS rates improved from 3% to 26%[8]. A previous comprehensive systematic review, including 101 articles and 10,108 patients with HCC treated by TACE, revealed an objective response rate (ORR) of 52.5% and one-, two-, three- and five-year OS rates of 70.3%, 51.8%, 40.4%, and 32.4%, respectively[11]. Owing to its effectiveness, TACE has been recommended as the standard treatment for intermediate HCC of Barcelona Clinic Liver Cancer (BCLC) stage B in both EASL and American Association for the Study of Liver Diseases (AASLD) guidelines. Moreover, the application of TACE can be extended for CNLC stages IIb, IIIa, and IIIb HCC cases in specific real-life conditions[12]. While TACE has demonstrated effectiveness, clinical challenges persist. For instance, a subset of patients may develop TACE refractory with ongoing tumor growth or recurrence after TACE interventions[13]. Therefore, investigating innovative TACE-based combination strategies is essential to address these limitations.
Immunotherapy targeting programmed cell death-1 (PD-1) pathway has been recommended in first-line and second-line settings for advanced HCC patients according to recent guideline[14]. Tislelizumab, a novel PD-1 blockade, was approved as single agent for first-line treatment of advanced HCC by NMPA. A phase II trial (RATIONALE 208) of previously treated unresectable HCC showed that tislelizumab induced durable responses[15]. The phase III RATIONALE 301 trial showed that compared with sorafenib, tislelizumab had non-inferior OS benefits but a higher response rate and more durable responses[16]. These encouraging outcomes demonstrate its potential as a valuable therapeutic option in the management of advanced HCC, and further exploration of its effectiveness in combination with other therapies is expecting. Although clinical trials have shown the favorable efficacy of tislelizumab, it is worth noting the challenges in understanding the role of tislelizumab in combination regimens also arise from the lack of real-world research outcomes.
Currently, investigators are actively exploring the integration of TACE with other therapeutic strategies, including targeted therapy, immunotherapy, local ablative techniques, systemic treatments, etc. The combination of immunotherapy with TACE has been suggested[2,17], since synergistic effects with immunotherapy can be achieved by TACE through a dual mechanism which not only induces tumor cell death by starvation but also significantly impacts the tumor immune microenvironment[18-20]. The liver contains immunosuppressive cells that can participate in the immune tolerance toward cancer cells[21], while TACE can induce the release of antigens and inflammatory cytokines, with the potential of turning an immunologically cold tumor into a hot tumor[22,23]. Immunotherapy on top of TACE could further inhibit the immune tolerance and activate the anti-tumor immune response[24]. Hence, the antigens released after TACE can help enhance the immune response, rendering the combination of TACE with immunotherapy a highly promising synergistic treatment approach. Yet the evidence regarding the combination of tislelizumab with TACE remains limited.
This retrospective study aimed to evaluate the clinical benefits of patients with unresectable HCC receiving TACE combined with tislelizumab by investigating the effectiveness and safety in a real-world setting, and to provide valuable insights for optimizing clinical practice.
This retrospective study analyzed data from patients with unresectable HCC who sought treatment at the Hepatobiliary Surgery Department of the First Affiliated Hospital of the Army Medical University. This study was approved by the Ethics Committee of the First Affiliated Hospital of the Army Medical University. The requirement for informed consent was waived due to the retrospective nature of this study.
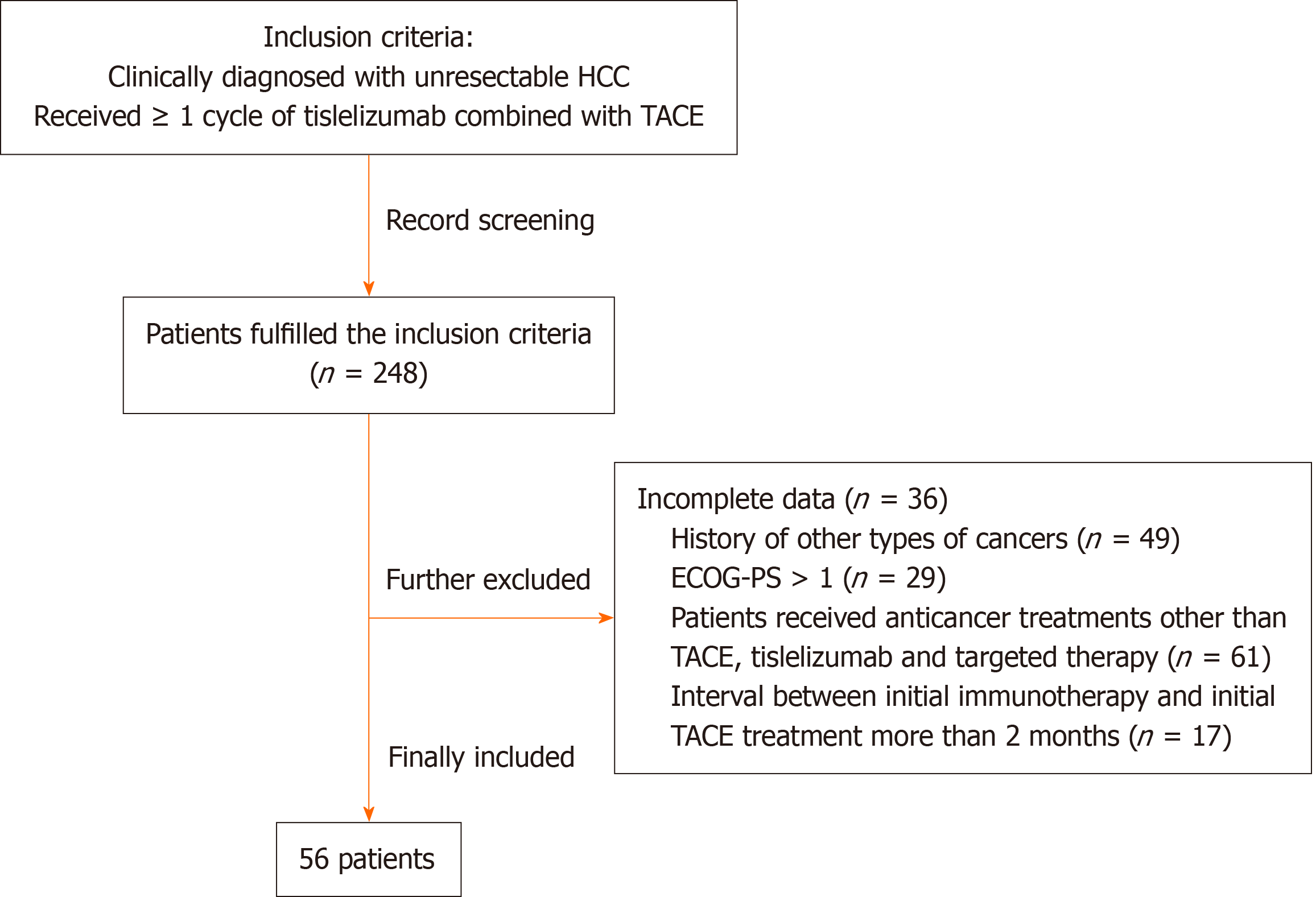
The inclusion criteria were: (1) Clinically diagnosed with unresectable HCC according to the 2005 AASLD criteria[25]; and (2) Received a minimum of one cycle of tislelizumab combined with TACE. The exclusion criteria were: (1) Incomplete data for disease assessment, as determined by the investigators; (2) History of other types of cancers; (3) Eastern Cooperative Oncology Group Performance Status > 1; (4) Patients received anticancer treatments other than TACE, tislelizumab and targeted therapy; or (5) The interval between initial immunotherapy and TACE treatment was more than 2 months.
TACE: The TACE treatment was conducted identically to the procedure outlined in a previous study[7]. In brief, after superselective catheterization of tumor-feeding artery, 3-15 mL iodized oil and 20-60 mg doxorubicin hydrochloride were utilized depending on the tumor size. finally, the tumor-feeding arteries were embolized with selected materials (conventional lipiodol, drug loaded microsphere, or both, based on tumor characteristics).
Tislelizumab: A 250 mL solution of 5% glucose and normal saline is used for intravenous infusion, with 200 mg of tislelizumab. A treatment course comprises 3 weeks, with dynamic monitoring of blood routine, liver and kidney function, coagulation parameters, thyroid function, and cardiac enzyme profiles.
Target therapy: All the patients included were allowed with or without target therapy, and the treatment decisions were made by the investigating physicians in real-world clinical practice.
Follow-up was censored on June 25, 2023. The primary outcome was PFS. Secondary outcomes encompassed ORR, disease control rate (DCR), and OS. Tumor response was evaluated by contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) according to mRECIST criteria. Definition of tumor response was as follows: Complete response (CR): CT or MRI revealing no arterial phase enhancement in the target lesions; partial response (PR): Total diameter of target lesions decreased by ≥ 30% (arterial enhancement); stable disease (SD): Decrease in the total diameter of target lesions (arterial enhancement) not meeting the PR criteria or an elevation not reaching progressive disease (PD); PD: Total diameter of target lesions increased by ≥ 20% (arterial enhancement) or detection of new lesions. ORR = CR + PR/n; DCR = CR + PR + SD/n. OS was the time elapsed between the first TACE or tislelizumab to the date of death or last follow-up. PFS was the time elapsed between the first TACE or tislelizumab and the first tumor progression, death, or last follow-up. Adverse events (AEs) were evaluated per Common Terminology Criteria for Adverse Events (CTCAE) version 5.0[26].
The patient characteristics (including sex, age, staging, treatments, etc.), outcomes, and AEs were collected from the electronic medical records.
For continuous variables, normal distribution was assessed using the Shapiro-Wilk test, which revealed a deviation from normal distribution for all continuous variables. Therefore, these variables were presented as median (P25-P75). Categorical variables were described as number with percentage. Survival analysis was conducted using Kaplan-Meier curves, and comparisons utilized the log-rank test. Two-sided P < 0.05 was deemed statistically significant. Data analysis employed SPSS 26.0 (IBM, Armonk, NY, United States). The figures were generated using MedCalc (MedCalc Software bvba, Ostend, Belgium).
According to the inclusion criteria, a total of 248 patients with unresectable HCC receiving tislelizumab and TACE were initially identified. Of these, 192 patients were sequentially excluded per exclusion criteria (Figure 1). Finally, totally 56 patients were included. The detailed characteristics of the included patients are summarized in Table 1. The 56 patients comprised 50 men, with a median age of 53 years (47.5 to 58 years). Twenty-four patients were treatment-naive. The majority (51/56) of the patients received targeted therapy concurrently. Lenvatinib was the most commonly used targeted agent (36/51), followed by regorafenib (6/51), apatinib (3/51), bevacizumab (3/51), and sorafenib (3/51). The median maximum diameter of tumors was 65 (39-93.5) mm. Vascular invasion and extrahepatic metastasis were present in 21 (37.50%) and seven (12.50%) patients, respectively. BCLC staging was A in four (7.14%), B in 16 (28.57%), and C in 36 (64.29%) patients. The patients underwent a median of 1 (1-2) TACE cycles. A total of 49 (87.50%) were at their first line of tislelizumab. The first cycle of tislelizumab was given before TACE in 21 (37.50%) patients, and after TACE in 35 (62.50%).
| Characteristics | Values |
| Age (years), median (P25-P75) | 53 (47.5-58) |
| < 60, n (%) | 49 (87.50) |
| ≥ 60, n (%) | 7 (12.50) |
| Sex, n (%) | |
| Male | 50 (89.29) |
| Female | 6 (10.71) |
| Viral infection, n (%) | |
| Hepatitis B virus | 42 (75.00) |
| Hepatitis C virus | 3 (5.36) |
| Hepatitis B and C viruses | 11 (19.64) |
| Prior cancer treatment modalities, n (%) | |
| Untreated | 24 (42.86) |
| Surgery | 6 (10.71) |
| Ablation therapy | 3 (5.36) |
| TACE | 23 (41.07) |
| Line of immunotherapy, n (%) | |
| First-line | 49 (87.5.0) |
| Second-line | 5 (8.93) |
| Third-line and beyond | 2 (3.57) |
| Immunotherapy cycles | 4 (3-8.5) |
| ≤ 4 cycles | 29 (51.79) |
| > 4 cycles | 27 (48.21) |
| TACE sessions, median (P25-P75) | 1 (1-2) |
| 1 time, n (%) | 36 (64.29) |
| 2 times, n (%) | 13 (23.21) |
| 3 times or more, n (%) | 7 (12.50) |
| Immunotherapy sequence, n (%) | |
| Before | 21 (37.50) |
| After | 35 (62.50) |
| Concomitant targeted/immunotherapy, n (%) | |
| No | 5 (8.93) |
| Yes | 51 (91.07) |
| Targeted/immunotherapy agents, n (%) | |
| Lenvatinib | 36 (64.29) |
| Apatinib | 3 (5.36) |
| Bevacizumab | 3 (5.36) |
| Regorafenib | 6 (10.71) |
| Sorafenib | 3 (5.36) |
| Targeted therapy duration (months), median (P25-P75) | 5 (3-9) |
| Tumor maximum diameter (mm), median (P25-P75) | 65 (39-93.5) |
| Tumor distribution, n (%) | |
| Unilateral liver | 34 (60.71) |
| Bilateral liver | 22 (39.29) |
| Vascular invasion, n (%) | 21 (37.50) |
| Extrahepatic metastasis, n (%) | 7 (12.50) |
| BCLC staging, n (%) | |
| A | 4 (7.14) |
| B | 16 (28.57) |
| C | 36 (64.29) |
| Child-Pugh score, n (%) | |
| A | 55 (98.21) |
| B | 1 (1.79) |
| ECOG performance status, n (%) | |
| 0 | 54 (96.43) |
| 1 | 2 (3.57) |
| AFP (ng/mL), median (P25-P75) | 227.29 (38.7-9484.5) |
| ALT (IU/L), median (P25-P75) | 38.9 (26.25-60.7) |
| AST (IU/L), median (P25-P75) | 46 (34.25-76.2) |
| Total bilirubin (µmol/L), median (P25-P75) | 16.4 (12.65-23.025) |
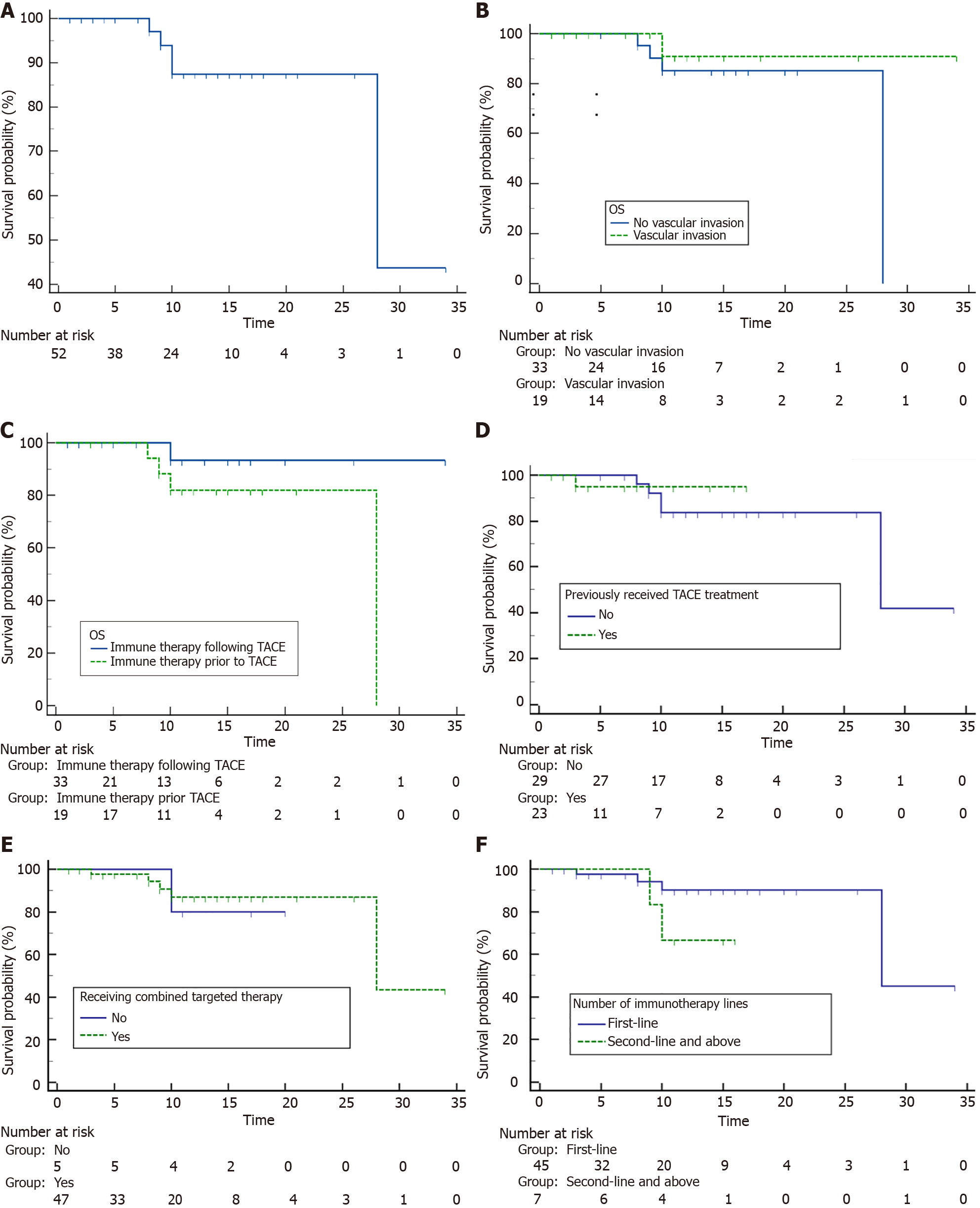
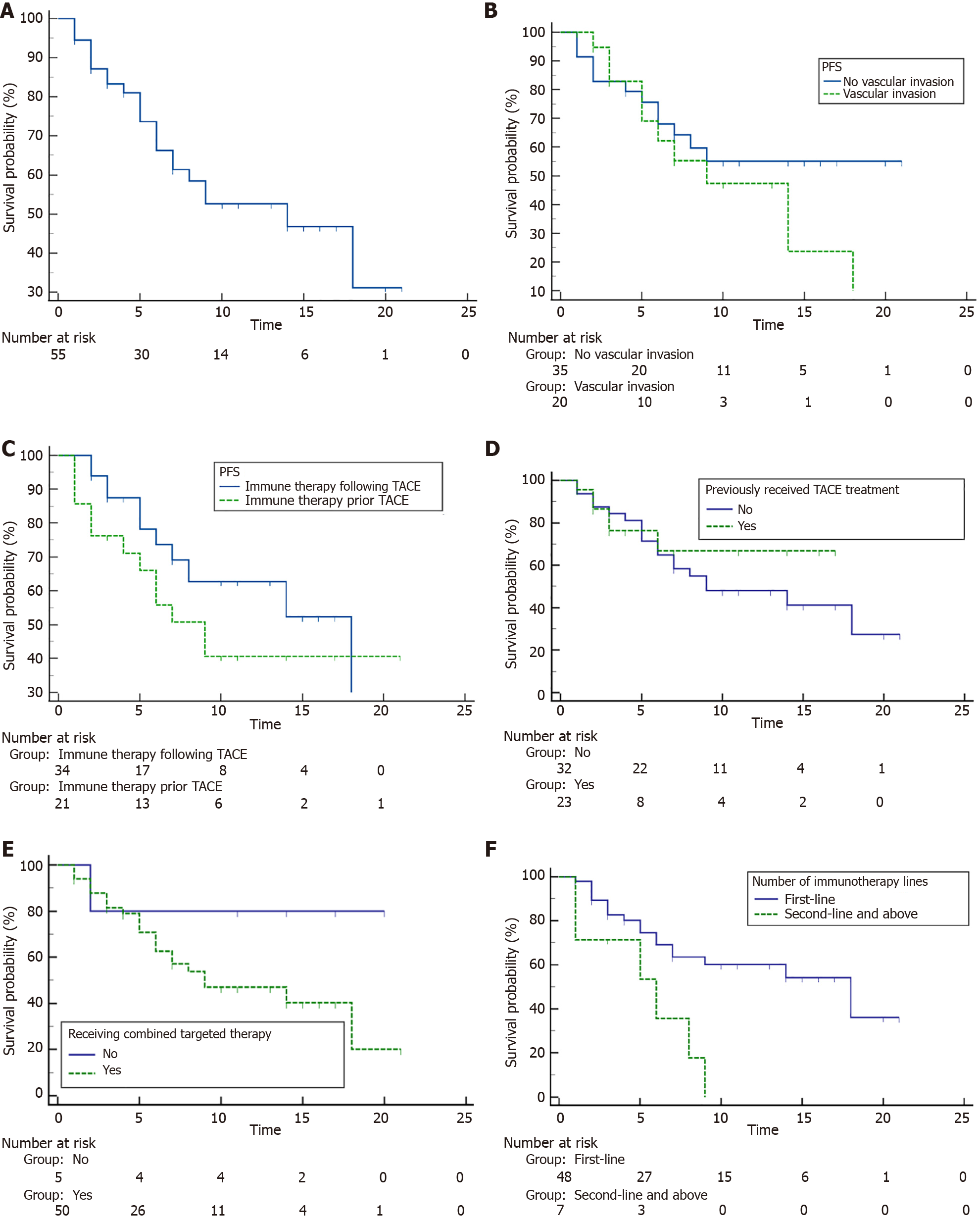
After a median follow-up of 10.9 months, a total of seven patients died and 24 patients showed progressed disease. The 1-year OS rate for all included patients was 87.5% (95%CI: 69.9%-95.1%), and the median OS was 28 (95%CI: 2.9%-53.1%) months. The 6-month and 1-year PFS rates were 67.2% (95%CI: 51.8%-78.7%) and 53.9% (95%CI: 37.9%-67.5%), respectively, with a median PFS of 14.0 (95%CI: 7.0%-18.0%) months.
Given the potential impact of patient characteristics, treatment sequences and previous treatments such as TACE and immunotherapy on prognosis, subgroup analyses for OS and PFS were conducted. Detailed results were summarized in Supplementary Table 1. The median OS was 28 months (95%CI: 28.0%-NA) for patients treated by first-line immunotherapy, but not reached for those at second/third-line. Patients who did not undergo prior TACE exhibited a median OS of 28 (95%CI: 28.0%-NA) months, while those with a history of prior TACE did not reach median OS. There were no significant differences of OS in terms of other factors such as vascular invasion, treatment sequence and concomitant target therapy (P > 0.05 for all, Figure 2 and Supplementary Table 1). As for the PFS in the subgroups shown in Figure 3 and Supplementary Table 1, patients treated by first-line immunotherapy had significantly prolonged median PFS than those with prior immunotherapy (18.0 vs 6.0 months, P = 0.006). Meanwhile, median PFS times were similar in patients who had previously undergone TACE treatment and those who had not (median PFS, not reached vs 9.0 months, P = 0.554). In terms of other factors including vascular invasion, immunotherapy sequence and concomitant target therapy, no significant differences were observed for PFS (P > 0.05 for all).
The best tumor responses of all included patients with unresectable HCC are shown in Table 2. The overall ORR was 82.14% (95%CI, 71.7%-92.3%), and the DCR was 87.50% (95%CI, 78.1%-96%). The ORR and DCR in different subgroups were shown in Table 2. Patients receiving first-line immunotherapy showed notably higher ORR compared to patients receiving second- or third-line immunotherapy (85.7% vs 57.1%), as well as DCR (91.8% vs 57.1%). In subgroups of prior cancer treatment modalities such as TACE, there were no significant differences of ORR and DCR. As for other subgroups, the ORR appeared to be lower in patients with hepatitis C virus infection (66.7%), prior surgery and ablation (70.0%), received two or more TACE sessions (70.0%), received immunotherapy before TACE (71.4%), received targeted therapy with apatinib, bevacizumab, regorafenib, or sorafenib (66.7%), and bilateral liver lesions (63.6%). Consistent variations were observed for the DCR (Table 2).
| Number of patients | ORR (95%CI) | DCR (95%CI) | |
| Total | 56 | 0.821 (0.717-0.923) | 0.875 (0.781-0.96) |
| Age (years) | |||
| < 60 | 49 | 0.816 (0.704-0.929) | 0.878 (0.782-0.973) |
| ≥ 60 | 7 | 0.857 (0.508-1.207) | 0.857 (0.508-1.207) |
| Sex | |||
| Male | 50 | 0.8 (0.685-0.915) | 0.86 (0.76-0.96) |
| Female | 6 | 1 (1-1) | 1 (1-1) |
| Viral infection | |||
| Hepatitis B virus | 42 | 0.81 (0.686-0.933) | 0.833 (0.716-0.951) |
| Hepatitis C virus | 3 | 0.667 (-0.768-2.101) | 1 (1-1) |
| Hepatitis B and C viruses | 11 | 0.909 (0.707-1.112) | 1 (1-1) |
| Prior cancer treatment modalities | |||
| Untreated | 24 | 0.87 (0.721-1.018) | 0.913 (0.788-1.038) |
| Surgery & Ablation therapy | 9 | 0.7 (0.354-1.046) | 0.8 (0.498-1.102) |
| TACE | 23 | 0.87 (0.721-1.018) | 0.913 (0.788-1.038) |
| Line of immunotherapy | |||
| First-line | 49 | 0.857 (0.756-0.959) | 0.918 (0.839-0.998) |
| Second-line and above | 7 | 0.571 (0.077-1.066) | 0.571 (0.077-1.066) |
| Immunotherapy cycles | |||
| ≤ 4 cycles | 29 | 0.862 (0.729-0.996) | 0.897 (0.779-1.014) |
| > 4 cycles | 27 | 0.778 (0.61-0.945) | 0.852 (0.709-0.995) |
| Transarterial chemoembolization sessions, | |||
| 1 time | 36 | 0.889 (0.781-0.997) | 0.917 (0.822-1.012) |
| 2 times and above | 20 | 0.7 (0.48-0.92) | 0.8 (0.608-0.992) |
| Immunotherapy sequence | |||
| Before | 21 | 0.714 (0.504-0.925) | 0.762 (0.563-0.961) |
| After | 35 | 0.886 (0.775-0.997) | 0.943 (0.862-1.024) |
| Concomitant targeted/immunotherapy | |||
| No | 5 | 0.8 (0.245-1.355) | 0.8 (0.245-1.355) |
| Yes | 51 | 0.824 (0.715-0.932) | 0.882 (0.791-0.974) |
| Targeted/immunotherapy agents | |||
| Lenvatinib | 36 | 0.889 (0.781-0.997) | 0.917 (0.822-1.012) |
| Apatinib, bevacizumab, regorafenib, or sorafenib | 15 | 0.667 (0.396-0.937) | 0.8 (0.571-1.029) |
| Tumor distribution | |||
| Unilateral liver | 34 | 0.941 (0.858-1.025) | 0.971 (0.911-1.03) |
| Bilateral liver | 22 | 0.636 (0.418-0.855) | 0.727 (0.525-0.929) |
| Vascular invasion | |||
| Yes | 21 | 0.8 (0.661-0.939) | 0.857 (0.735-0.979) |
| No | 35 | 0.857 (0.694-1.02) | 0.905 (0.768-1.042) |
| Extrahepatic metastasis | |||
| Yes | 7 | 0.816 (0.704-0.929) | 0.878 (0.782-0.973) |
| No | 49 | 0.857 (0.508-1.207) | 0.857 (0.508-1.207) |
| BCLC staging | |||
| A or B | 20 | 0.9 (0.756-1.044) | 0.95 (0.845-1.055) |
| C | 36 | 0.9 (0.756-1.044) | 0.95 (0.845-1.055) |
Table 3 shows the detailed AE profile. Forty-three patients experienced adverse reactions. The most frequently observed AEs of grade ≥ 3 included ALT elevation (5/56, 8.93%), aspartate aminotransferase elevation (6/56, 10.71%), total bilirubin elevation (2/56, 3.57%) and rash (2/56, 3.57%). No grade ≥ 3 itching, vomiting, diarrhea, sensory abnormalities, thyroiditis, abdominal pain, gastric ulcer, or bone marrow suppression was observed. The overall toxicity profile was tolerable and manageable. No death or treatment termination due to AE was recorded.
| n (%) | |
| Elevated ALT | |
| Grade 0-2 | 51 (91.07) |
| Grade ≥ 3 | 5 (8.93) |
| Elevated AST | |
| Grade 0-2 | 50 (89.29) |
| Grade ≥ 3 | 6 (10.71) |
| Elevated total bilirubin | |
| Grade 0-2 | 54 (96.43) |
| Grade ≥ 3 | 2 (3.57) |
| Rash | |
| Grade 0-2 | 54 (96.43) |
| Grade ≥ 3 | 2 (3.57) |
| Itching | |
| Grade 0-2 | 56 (100.00) |
| Grade ≥ 3 | 0 |
| Vomiting | |
| Grade 0-2 | 56 (100.00) |
| Grade ≥ 3 | 0 |
| Diarrhea | |
| Grade 0-2 | 56 (100.00) |
| Grade ≥ 3 | 0 |
| Sensory abnormalities | |
| Grade 0-2 | 56 (100.00) |
| Grade ≥ 3 | 0 |
| Thyroiditis | |
| Grade 0-2 | 56 (100.00) |
| Grade ≥ 3 | 0 |
| Abdominal pain | |
| Grade 0-2 | 56 (100.00) |
| Grade ≥ 3 | 0 |
| Gastric ulcer | |
| Grade 0-2 | 56 (100.00) |
| Grade ≥ 3 | 0 |
| Bone marrow suppression | |
| Grade 0-2 | 56 (100.00) |
| Grade ≥ 3 | 0 |
The findings in this study indicate that the combination of tislelizumab and TACE, either with or without targeted therapy, resulted in favorable treatment effectiveness and well tolerance among individuals with unresectable HCC. These findings hold significance, given that patients in this disease stage typically face a grim prognosis and limited treatment alternatives.
The recent RATIONALE 301 trial showed that compared with sorafenib, tislelizumab resulted in higher ORR (14.3% vs 5.4%) and more durable response, while OS was similar and PFS was longer with sorafenib, but the patients did not undergo TACE[16,27]. Evidence is available regarding the use of TACE and immunotherapy for unresectable HCC. A retrospective real-world study in China (the CHANCE 001 study) showed that compared with TACE monotherapy, TACE plus PD-(L)1 blockade and targeted therapy could significantly improve PFS (9.5 vs 8.0 months), OS (19.2 vs 15.7 months), and ORR (60.1% vs 32.0%) in Chinese patients with advanced HCC[2]. The retrospective study by Zhang et al[17] showed that camrelizumab after TACE could achieve PFS and OS of 6.1 and 13.3 months, respectively, with an ORR of 35.3%. The multicenter retrospective study by Marinelli et al[28] showed that compared with nivolumab alone, nivolumab combined with TACE led to longer PFS (8.8 vs 3.7 months) and OS (35.1 vs 16.6 months). Based on recent reports, the combination of TACE, targeted therapy plus PD-(L)1 inhibitor (such as camrelizumab, sintilimab and pembrolizumab) for unresectable HCC has been associated with a median PFS of approximately 8-13.3 months[29-33] and median OS of 18-24 months[29-31,33]. Numerically, our study observed a more favorable prognosis, which might be attributable to the use of the tislelizumab. This offers an important hint that tislelizumab may provide better therapeutic efficacy and more potent synergistic effect with TACE. However, due to the single-arm design of our study, assessing the superiority of tislelizumab combination therapy upon other regimens is impractical. Therefore, these findings of our study warrant validation in further clinical studies.
In the present study, the PFS and OS were consistent among subgroups based on vascular invasion and timing of tislelizumab in relation to TACE. These results suggest that vascular invasion and the timing of the first cycle of tislelizumab might not have critical influence on clinical outcomes, possibly allowing a wider selection for suitable patients. Still, the analysis of the ORR according to tislelizumab timing might suggest that tislelizumab given before TACE could result in a lower ORR. Yet to be confirmed in future trials, the results are supported by the fact that TACE influences the tumor immune microenvironment in a way that can be used to potentiate the effects of both immunotherapy and TACE[18-20]. Tislelizumab given as the second or above line of treatment also appeared to lead to a lower ORR, suggesting the possibility of resistance to immunotherapy in previously treated tumors. Indeed, in the CHANCE001 study, previous HCC-related therapy was independently associated with poorer OS and PFS[2]. In addition, previous treatments had a significant impact on the effectiveness of tislelizumab in combination with TACE. Patients with prior immunotherapy showed poorer responses and prognosis, especially in PFS. Reattempting immunotherapy post-failure, even in conjunction with TACE, poses significant challenges. Emphasizing the synergy of TACE with immunotherapy in the initial treatment is crucial. Surprisingly, prior TACE did not notably impact treatment effectiveness, suggesting potential restoration of TACE sensitivity by immunotherapy. This observation warrants further exploration, offering insights into overcoming TACE challenges in HCC patients. In the present study, the AE profile appeared tolerable as only a few patients had grade ≥ 3 AEs. Similar results were reported in the combined therapy group of the CHANCE001 study, with 15.8% of grade ≥ 3 AEs[2]. Although safety data from retrospective studies must be taken with caution because of the monitoring of AEs and the risk of underreporting, the frequency of grade ≥ 3 AEs in the present study appears lower than in the IMbrave 150 (61.6%), ORIENT-32 (56%), and RATIONALE 301 trials[16,34,35]. Thus, the present findings suggest an acceptable safety profile for tislelizumab combination therapy. On the other hand, a recent network meta-analysis showed that tislelizumab is the most cost-effective first-line treatment strategy among the commonly used immunotherapy and targeted therapy drugs for unresectable HCC in China[36], which is also, to a certain extent, attributed to its commendable safety profile. However, due to the retrospective nature, this study could not differentiate between TACE- and immunotherapy-related AEs in all patients. Future studies should also consider this issue.
This study had some limitations. It was a retrospective study limited to the data availability. There is a possibility of information bias. In addition, the patients were from a single center, resulting in a small sample size. Finally, clinical trials are necessary to confirm the findings.
In conclusion, combined TACE and tislelizumab with or without target therapy demonstrated favorable treatment response, survival outcomes and well tolerance in patients with unresectable HCC. The results contribute to the groundwork for future clinical trials of tislelizumab combined with TACE. As a new treatment strategy, combination of tislelizumab plus TACE shows promising potential to improve the prognosis of patients with unresectable HCC.
We thank every patient included in this study for their contribution to the research and every author for their hard work.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64542] [Article Influence: 16135.5] [Reference Citation Analysis (176)] |
| 2. | Zhu HD, Li HL, Huang MS, Yang WZ, Yin GW, Zhong BY, Sun JH, Jin ZC, Chen JJ, Ge NJ, Ding WB, Li WH, Huang JH, Mu W, Gu SZ, Li JP, Zhao H, Wen SW, Lei YM, Song YS, Yuan CW, Wang WD, Huang M, Zhao W, Wu JB, Wang S, Zhu X, Han JJ, Ren WX, Lu ZM, Xing WG, Fan Y, Lin HL, Zhang ZS, Xu GH, Hu WH, Tu Q, Su HY, Zheng CS, Chen Y, Zhao XY, Fang ZT, Wang Q, Zhao JW, Xu AB, Xu J, Wu QH, Niu HZ, Wang J, Dai F, Feng DP, Li QD, Shi RS, Li JR, Yang G, Shi HB, Ji JS, Liu YE, Cai Z, Yang P, Zhao Y, Zhu XL, Lu LG, Teng GJ; CHANCE001 Investigators. Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001). Signal Transduct Target Ther. 2023;8:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 153] [Article Influence: 76.5] [Reference Citation Analysis (1)] |
| 3. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3165] [Article Influence: 527.5] [Reference Citation Analysis (37)] |
| 4. | Devarbhavi H, Asrani SK, Arab JP, Nartey YA, Pose E, Kamath PS. Global burden of liver disease: 2023 update. J Hepatol. 2023;79:516-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 834] [Reference Citation Analysis (4)] |
| 5. | Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, Nault JC, Neumann U, Ricke J, Sangro B, Schirmacher P, Verslype C, Zech CJ, Arnold D, Martinelli E; ESMO Guidelines Committee. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv238-iv255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 721] [Article Influence: 103.0] [Reference Citation Analysis (0)] |
| 6. | Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400:1345-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1284] [Cited by in RCA: 1219] [Article Influence: 406.3] [Reference Citation Analysis (41)] |
| 7. | West HJ, Jin JO. Transarterial Chemoembolization. JAMA Oncol. 2015;1:1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 1987] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 9. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, Rodés J, Bruix J; Barcelona Liver Cancer Group. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2611] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 10. | Kong JY, Li SM, Fan HY, Zhang L, Zhao HJ, Li SM. Transarterial chemoembolization extends long-term survival in patients with unresectable hepatocellular carcinoma. Medicine (Baltimore). 2018;97:e11872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology. 2016;64:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 519] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 12. | Xie DY, Zhu K, Ren ZG, Zhou J, Fan J, Gao Q. A review of 2022 Chinese clinical guidelines on the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2023;12:216-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 89] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 13. | Yamanaka K, Hatano E, Kitamura K, Iida T, Ishii T, Machimito T, Taura K, Yasuchika K, Isoda H, Shibata T, Uemoto S. Early evaluation of transcatheter arterial chemoembolization-refractory hepatocellular carcinoma. J Gastroenterol. 2012;47:343-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Zhou J, Sun H, Wang Z, Cong W, Zeng M, Zhou W, Bie P, Liu L, Wen T, Kuang M, Han G, Yan Z, Wang M, Liu R, Lu L, Ren Z, Zeng Z, Liang P, Liang C, Chen M, Yan F, Wang W, Hou J, Ji Y, Yun J, Bai X, Cai D, Chen W, Chen Y, Cheng W, Cheng S, Dai C, Guo W, Guo Y, Hua B, Huang X, Jia W, Li Q, Li T, Li X, Li Y, Li Y, Liang J, Ling C, Liu T, Liu X, Lu S, Lv G, Mao Y, Meng Z, Peng T, Ren W, Shi H, Shi G, Shi M, Song T, Tao K, Wang J, Wang K, Wang L, Wang W, Wang X, Wang Z, Xiang B, Xing B, Xu J, Yang J, Yang J, Yang Y, Yang Y, Ye S, Yin Z, Zeng Y, Zhang B, Zhang B, Zhang L, Zhang S, Zhang T, Zhang Y, Zhao M, Zhao Y, Zheng H, Zhou L, Zhu J, Zhu K, Liu R, Shi Y, Xiao Y, Zhang L, Yang C, Wu Z, Dai Z, Chen M, Cai J, Wang W, Cai X, Li Q, Shen F, Qin S, Teng G, Dong J, Fan J. Guidelines for the Diagnosis and Treatment of Primary Liver Cancer (2022 Edition). Liver Cancer. 2023;12:405-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 194] [Reference Citation Analysis (0)] |
| 15. | Ducreux M, Abou-alfa G, Ren Z, Edeline J, Li Z, Assenat E, Rimassa L, Blanc J, Ross P, Fang W, Hu S, Zhang T, Tran A, Pan H, Yen C, Wu J, Li V, Chica-duque S, Merle P, Cheng A. O-1 Results from a global phase 2 study of tislelizumab, an investigational PD-1 antibody, in patients with unresectable hepatocellular carcinoma. Ann Oncol. 2021;32 Suppl 3:S217. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 16. | Qin S, Kudo M, Meyer T, Bai Y, Guo Y, Meng Z, Satoh T, Marino D, Assenat E, Li S, Chen Y, Boisserie F, Abdrashitov R, Finn RS, Vogel A, Zhu AX. Tislelizumab vs Sorafenib as First-Line Treatment for Unresectable Hepatocellular Carcinoma: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2023;9:1651-1659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 112] [Article Influence: 56.0] [Reference Citation Analysis (1)] |
| 17. | Zhang JX, Chen P, Liu S, Zu QQ, Shi HB, Zhou CG. Safety and Efficacy of Transarterial Chemoembolization and Immune Checkpoint Inhibition with Camrelizumab for Treatment of Unresectable Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2022;9:265-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Brandi N, Renzulli M. The Synergistic Effect of Interventional Locoregional Treatments and Immunotherapy for the Treatment of Hepatocellular Carcinoma. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 19. | Zhang Z, Li C, Liao W, Huang Y, Wang Z. A Combination of Sorafenib, an Immune Checkpoint Inhibitor, TACE and Stereotactic Body Radiation Therapy versus Sorafenib and TACE in Advanced Hepatocellular Carcinoma Accompanied by Portal Vein Tumor Thrombus. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 20. | Zhao M, Huang H, He F, Fu X. Current insights into the hepatic microenvironment and advances in immunotherapy for hepatocellular carcinoma. Front Immunol. 2023;14:1188277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 21. | Ringelhan M, Pfister D, O'Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 735] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 22. | Pinato DJ, Murray SM, Forner A, Kaneko T, Fessas P, Toniutto P, Mínguez B, Cacciato V, Avellini C, Diaz A, Boyton RJ, Altmann DM, Goldin RD, Akarca AU, Marafioti T, Mauri FA, Casagrande E, Grillo F, Giannini E, Bhoori S, Mazzaferro V. Trans-arterial chemoembolization as a loco-regional inducer of immunogenic cell death in hepatocellular carcinoma: implications for immunotherapy. J Immunother Cancer. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 126] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 23. | Tischfield DJ, Gurevich A, Johnson O, Gatmaytan I, Nadolski GJ, Soulen MC, Kaplan DE, Furth E, Hunt SJ, Gade TPF. Transarterial Embolization Modulates the Immune Response within Target and Nontarget Hepatocellular Carcinomas in a Rat Model. Radiology. 2022;303:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 24. | Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW, Yoon JH. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383-91.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 388] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 25. | Murray KF, Carithers RL Jr; AASLD. AASLD practice guidelines: Evaluation of the patient for liver transplantation. Hepatology. 2005;41:1407-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 516] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 26. | Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the Common Terminology Criteria for Adverse Events (CTCAE - Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermosifiliogr (Engl Ed). 2021;112:90-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 404] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 27. | Qin S, Finn RS, Kudo M, Meyer T, Vogel A, Ducreux M, Macarulla TM, Tomasello G, Boisserie F, Hou J, Li X, Song J, Zhu AX. RATIONALE 301 study: tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Future Oncol. 2019;15:1811-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 28. | Marinelli B, Kim E, D'Alessio A, Cedillo M, Sinha I, Debnath N, Kudo M, Nishida N, Saeed A, Hildebrand H, Kaseb AO, Abugabal YI, Pillai A, Huang YH, Khan U, Muzaffar M, Naqash AR, Patel R, Fischman A, Bishay V, Bettinger D, Sung M, Ang C, Schwartz M, Pinato DJ, Marron T. Integrated use of PD-1 inhibition and transarterial chemoembolization for hepatocellular carcinoma: evaluation of safety and efficacy in a retrospective, propensity score-matched study. J Immunother Cancer. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 29. | Jiang N, Zhong B, Huang J, Li W, Zhang S, Zhu X, Ni C, Shen J. Transarterial chemoembolization combined with molecularly targeted agents plus immune checkpoint inhibitors for unresectable hepatocellular carcinoma: a retrospective cohort study. Front Immunol. 2023;14:1205636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 30. | Cao F, Yang Y, Si T, Luo J, Zeng H, Zhang Z, Feng D, Chen Y, Zheng J. The Efficacy of TACE Combined With Lenvatinib Plus Sintilimab in Unresectable Hepatocellular Carcinoma: A Multicenter Retrospective Study. Front Oncol. 2021;11:783480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 31. | Sun B, Zhang L, Sun T, Ren Y, Cao Y, Zhang W, Zhu L, Guo Y, Gui Y, Liu F, Chen L, Xiong F, Zheng C. Safety and efficacy of lenvatinib combined with camrelizumab plus transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: A two-center retrospective study. Front Oncol. 2022;12:982948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 32. | Yang H, Yang T, Qiu G, Liu J. Efficacy and Safety of TACE Combined with Lenvatinib and PD-(L)1 Inhibitor in the Treatment of Unresectable Hepatocellular Carcinoma: A Retrospective Study. J Hepatocell Carcinoma. 2023;10:1435-1443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 33. | Li X, Fu Z, Chen X, Cao K, Zhong J, Liu L, Ding N, Zhang X, Zhai J, Qu Z. Efficacy and Safety of Lenvatinib Combined With PD-1 Inhibitors Plus TACE for Unresectable Hepatocellular Carcinoma Patients in China Real-World. Front Oncol. 2022;12:950266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 34. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4682] [Article Influence: 936.4] [Reference Citation Analysis (2)] |
| 35. | Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, Li Q, Lu Y, Chen Y, Guo Y, Chen Z, Liu B, Jia W, Wu J, Wang J, Shao G, Zhang B, Shan Y, Meng Z, Wu J, Gu S, Yang W, Liu C, Shi X, Gao Z, Yin T, Cui J, Huang M, Xing B, Mao Y, Teng G, Qin Y, Wang J, Xia F, Yin G, Yang Y, Chen M, Wang Y, Zhou H, Fan J; ORIENT-32 study group. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. 2021;22:977-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 667] [Article Influence: 166.8] [Reference Citation Analysis (1)] |
| 36. | Liu K, Zhu Y, Zhu H. Immunotherapy or targeted therapy as the first-line strategies for unresectable hepatocellular carcinoma: A network meta-analysis and cost-effectiveness analysis. Front Immunol. 2022;13:1103055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 16.0] [Reference Citation Analysis (0)] |