Published online Sep 27, 2024. doi: 10.4240/wjgs.v16.i9.2748
Revised: June 6, 2024
Accepted: June 24, 2024
Published online: September 27, 2024
Processing time: 233 Days and 14.8 Hours
Alveolar echinococcosis (AE) primarily manifests in the liver and exhibits characteristics resembling those of slow-growing malignant tumours. Untreated Echinococcus multilocularis infection can be lethal. By infiltrating the vascular systems, biliary tracts, and the hilum of the liver, it might lead to various problems. Due to its ability to infiltrate neighbouring tissues or metastasize to distant organs, AE can often be mistaken for malignancies. We present a concise overview of the epi
Core Tip: Ultrasound can be utilized as a screening and first imaging technique in alveolar echinococcosis (AE). It is also capable of offering direction for interventional procedures. For the purpose of planning and supervising procedures as well as diag
- Citation: Aydin S, Irgul B, Memis KB, Kızılgoz V, Kantarci M. Characteristics of the imaging diagnosis of alveolar echinococcosis. World J Gastrointest Surg 2024; 16(9): 2748-2754
- URL: https://www.wjgnet.com/1948-9366/full/v16/i9/2748.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i9.2748
The zoonotic disease echinococcosis is brought on by tapeworms, or cestodes, belonging to the Echinococcus genus. The definitive host and the intermediate host are two distinct species that are involved in the life cycle of Echinococcus. Humans are accidental intermediary hosts in this life cycle; they are not a natural part of it. Humans can contract alveolar echinococcosis (AE) from Echinococcus multilocularis (E. multilocularis) and cystic echinococcosis (CE) from Echinococcus granulus. If not treated properly, CE and AE are both hazardous and serious illnesses with significant mortality and a dismal prognosis, particularly for AE[1-3].
The peak incidence of AE occurs in people between the ages of 35 and 65, and it affects both men and women equally. A widespread worldwide ailment, AE is most prevalent in the northern hemisphere, particularly in China, Japan, Alaska, North America, and Central and Eastern Europe, Turkey, and Russia[4].
The adult stage (cestode) of E. multilocularis differs from other Echinococcus species by having unique morphological characteristics, an average of five segments, and a maximum size of 4.5 mm[5].
E. multilocularis spreads by a sylvatic cycle and is occasionally associated with domestic cats and dogs through infected small mammals. Small mammals, mainly rodents, are intermediate hosts in this cycle[1,6,7].
The fox releases the adult parasite's eggs into the surroundings, and the cycle is then completed when the intermediate host consumes tainted food. After breaking through the intestinal wall, the eggs enter the portal and lymphatic systems and spread to several organs. In people who are unintentional hosts and natural intermediate hosts, the parasite matures to the metacestode stage[1,6,7].
The liver is where the metasestode stage usually takes place. Metastases can extend from the liver to nearby locations (abdomen, retroperitoneum, etc.) or distant organs (lungs, brain, bones, etc.) later in the illness. It results in infiltrative mass lesions in the liver with many millimetric to three-centimeter-diameter vesicles inside of them[6-8].
The first stage is always asymptomatic and may progress or occur on its own. The incubation period is estimated to last anywhere from five to fifteen years. When the metacestode infiltrates more areas of the liver or interferes with critical functions, symptoms in the progressive phase start to show[1,6,7].
Abdominal pain, jaundice, hepatomegaly, weight loss, anaemia, and occasionally fever are among the initial signs. Severe liver dysfunction-often linked to portal hypertension-defines the advanced stage. The illness can last for a few weeks or for years. Untreated or inadequately managed AE patients may have extremely high mortality rates[6].
Medical history, clinical observations, radiologic imaging techniques, laboratory analyses, and histopathologic con
The kind of organ implicated and the degree of invasion influence the clinical symptoms. The main signs of liver involvement are jaundice and epigastric discomfort, although malaise and weight loss might also appear. Portal hyper
An international standard for evaluating diagnostic performance and treatment results, the "PNM" categorization system was developed by the World Health Organization's Informal Working Group on Echinococcosis. According to the PNM categorization method, there is a parasite mass in the hepatic artery (P), the neighbouring organs are affected (N), and distant sites are affected (M). The PNM classification system aims to raise the standard of care and enable uniform out
| PNM system for classification of human alveolar echinococcosis | Number of patients | |
| P | Hepatic localization of the primary lesion | |
| PX | Primary lesion cannot be assessed | - |
| P0 | No detectable liver lesion | - |
| P1 | Peripheral lesions without proximal vascular and/or biliary involvement | 14 (22.9) |
| P2 | Central lesions with proximal vascular and/or biliary involvement of one lobe | 32 (52.4) |
| P3 | Central lesions with hilar vascular and biliary involvement of both lobes and/or withinvolvement of two hepatic veins | 11 (18) |
| P4 | Any lesion with extension along the portal vein, inferior vena cava, or hepatic arteries and thebiliary tree | 4 (6.5) |
| N | Extra hepatic involvement of neighboring organs or tissues | |
| NX | Cannot be evaluated | - |
| N0 | No regional involvement | 55 (90.1) |
| N1 | Regional involvement of contiguous organs or tissues | 6 (9.8) |
| M | Absence or presence of distant metastases | |
| MX | Not completely evaluated | - |
| M0 | No metastasis | 57 (93.4) |
| M1 | Metastasis present | 4 (6.5) |
Computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound (US) are imaging modalities that can help with the diagnosis, morphology, and treatment choices of AE lesions. The initial stage in the screening process for routine follow-up imaging in AE is ultrasonography. Additionally, the main diagnostic tool is US, and in 95% of cases, a particular serology verifies the diagnosis. Non-contrast CT scans make typical AE calcifications easily visible, but MRI is a better way to detect intra- and extrahepatic invasion, multi-vesicular AE lesions, and necrosis[11-14].
Seventy percent of patients have the typical US appearance: The right lobe of the liver is where AE lesions are primarily found, and they are frequently big (mean diameter of 3 cm). Patches of hyperechogenic (fibrous tissue) and hypoechogenic ("active" parasite tissue) tissue are juxtaposed within the lesion, which has uneven borders and a varied content. It often has calcifications in it that sonography may easily find. A few less common appearances are solitary pseudo-cysts with extensive necrosis, tiny calcified lesions, and numerous clustered hyperechoic nodules resembling hemangiomas. Moreover, US can clarify vascular and biliary involvement: It is evident that there are dilatations of the intrahepatic bile ducts and that parasite tissue has penetrated the walls of the portal, hepatic, and inferior vena cava[11-14].
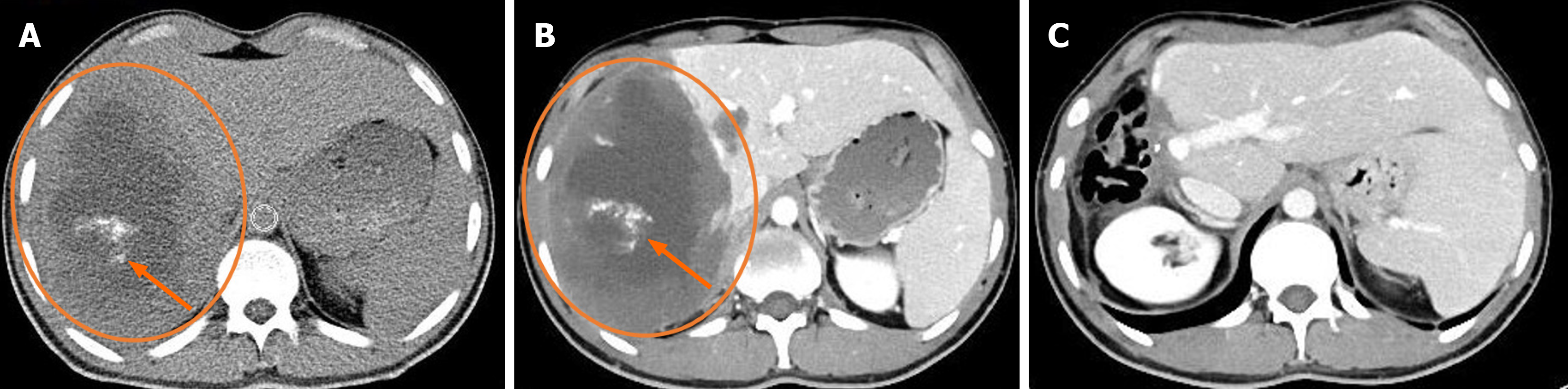
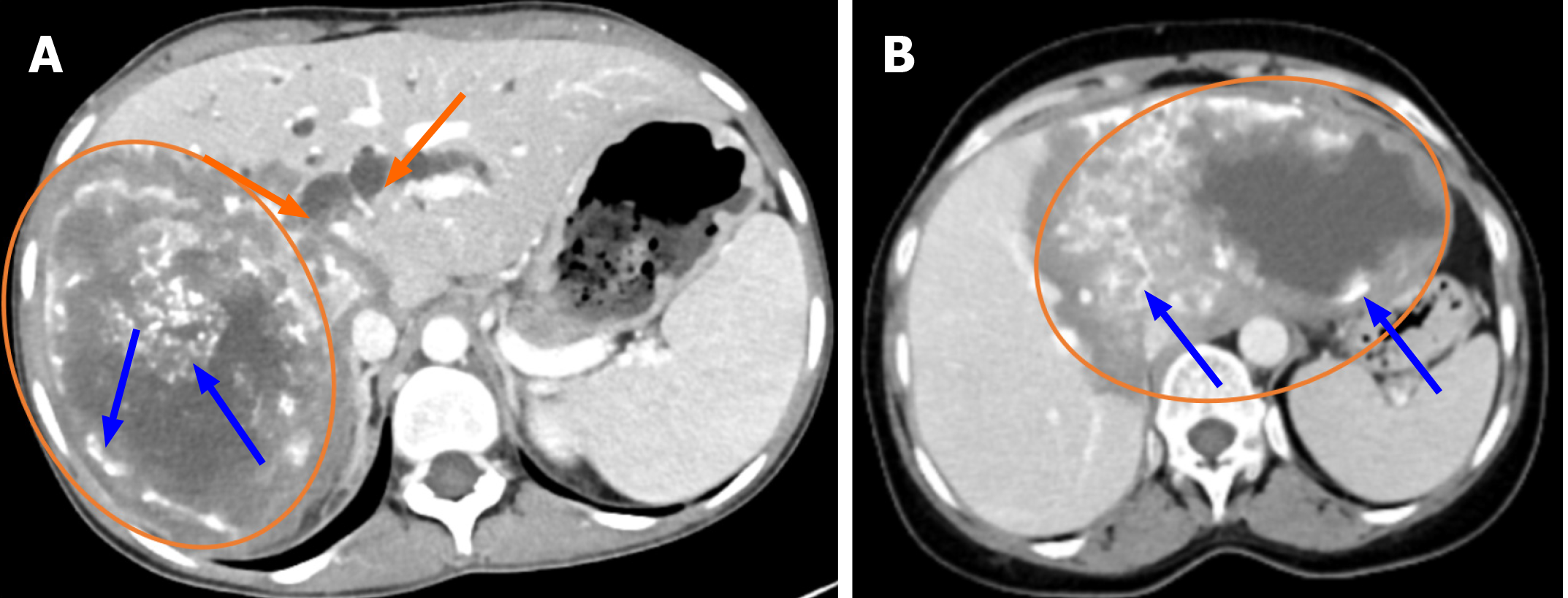
The main imaging technique for characterising lesions, identifying their anatomical location and extent, and spotting common calcifications is CT. It is helpful in assessing how vascular structures and the biliary tract relate to liver lesions. It is also possible to show involvement of extrahepatic organs. This is crucial for figuring out the P, N, and M phases of lesions as well as their resectability[6,11]. AE lesions show up on non-contrast CT as irregularly shaped tumor-like masses with diverse internal architecture and numerous dispersed calcific foci. Significant intra-lesion contrast enhancement is absent on contrast-enhanced CT scans; however, peripheral fibroinflammatory tissue in the delayed phase may exhibit modest contrast enhancement. Occasionally, hypodense necrotic regions may resemble sizable cystic cavities in the lesion's centre. The afflicted liver lobe may exhibit atrophy and capsular retraction as a result of vascular and biliary involvement[6,11]. According to reports, the CT characteristics of AE lesions can be used to differentiate between hepatic metastases and primary neoplasms such as cholangiocarcinoma, biliary cystadenoma, and biliary cystadenocarcinoma[6,15]. On the other hand, CT characteristics including hypoatenuation, calcification, and lack of contrast enhancement in a hepatic lesion frequently aid in classifying it as an adverse event (Figures 1 and 2).
An further three-phase or non-contrast CT scan is necessary to ascertain the existence of calcification and contrast enhancement when a suspected hepatic AE lesion is seen on a contrast-enhanced CT scan. Costs associated with imaging and radiation exposure will rise as a result. With dual-energy CT (DECT) and virtual non-enhanced (VNE) pictures, an unenhanced series of routine contrast-enhanced images can be produced with a much lower radiation dosage[11,16].
In the assessment of liver AE, VNE pictures offered excellent agreement and equivalent diagnostic confidence to non-contrast CT images, according to a study by Kantarci et al[17]. This implies that non-contrast CT scans might be replaced with VNE images, which would result in a large radiation dose decrease.
In most low-resource nations or locations, CT remains the primary modality for morphologic imaging assessment of AE lesions despite its primary drawback-radiation-because it clearly outperforms MRI in displaying calcifications, particularly in tiny clusters. When it comes to identifying the quantity, size, and location of liver lesions, CT is superior than US because it enables a thorough preoperative assessment of vascular, biliary, and extrahepatic dissemination[6,11-14,17].
When it comes to identifying pathognomonic calcifications for AE, MRI is less effective than CT. On T1-weighted and T2-weighted images, calcifications that show up as hyperdense lesions on CT may have variable signal characteristics. The most effective imaging technique for identifying the various elements of a parasitic lesion and demonstrating the involvement of biliary and vascular structures is MRI. Preoperative MRIs should be performed since they are excellent at showing how an organ has spread to nearby organs. An irregularly shaped, infiltrative mass lesion with internal heterogeneity and central necrotic regions is the characteristic MRI finding of AE in the liver. These display signal characteristics that are hypo-, iso-, or hyper-intense on T1-weighted images and hypo-, iso-, or hyper-intense on T2-weighted images. It is unexpected to see adipose tissue and bleeding within the lesion. AE lesions are hypovascular masses that, when they enlarge, create diffuse zones of necrosis due to insufficient vascular supply. One crucial diagnostic characteristic of these lesions is the lack of contrast uptake in the majority of the mass once intravenous contrast is administered. On T1-weighted images augmented with gadolinium, there is a little enhancement of peripheral contrast. This aug
Solid and cystic components can be found in AE lesions. Large irregular cystic patches suggest liquefaction necrosis, while little smooth cysts reflect metasestodal vesicles. Cystic regions within masses are hyperintense and best visualised on T2-weighted images. When it comes to identifying AE lesions that most accurately represent parasite cystic for
| Kodama types | Mean apparent diffusion coefficient value (× 10-3 mm2/s) |
| Type 1 | 1.92 ± 1.01 |
| Type 2 | 1.78 ± 0.86 |
| Type 3 | 1.57 ± 0.11 |
| Type 4 | 1.15 ± 0.21 |
| Type 5 | 1.9 ± 0.18 |
Information regarding the connection between AE lesions and the biliary tract can be obtained using magnetic reso
In a research by Kantarci et al[19], 61 patients had 232 AE lesions. 190 lesions were found on ultrasonography in this investigation. Eighty-one percent of the lesions were located in the liver's right lobe, with a mean size of 3 cm. On ultrasonography, the lesions were shown as mass lesions with many dispersed calcific foci and a mixed heterogeneous echogenic pattern with irregular contours. In this study, 232 lesions were reported to have been discriminated on cross-sectional tests, while 42 Lesions that were not distinguishable on ultrasonography were visible on CT and MR exams. According to these findings, US may be utilised as a preliminary investigative technique to identify AE; CT and MR are more beneficial imaging modalities.
Diffusion weighted imaging (DWI) has been shown to be a viable new imaging technique for the characterization of liver lesions. Malignant lesions have reduced intercellular space and increased cellularity, which limits the diffusion of water molecules. This observation, though, is not anticipated in AE lesions. An important diagnostic technique for localised liver lesions is the apparent diffusion coefficient (ADC). ADC values are higher in DWI pictures acquired at
Before a diagnosis, there is usually an asymptomatic period of several years because of the cysts' slow development pace. A severe case of the disease nearly invariably involves invasion of the bile and artery walls, and the clinical ap
Complete removal of the tumor is frequently unachievable, even though radical liver resection is the preferred course of action to prevent palliative surgical treatments. One potential course of action for treating patients in need of life-saving care is liver transplantation. However, individuals with residual or metastatic AE should not have a liver transplant as it is not always possible[23,24].
Three categories exist for AE lesions: Mixed, pseudocystic, and solid. Pseudocystic and mixed forms of percutaneous cyst drainage as well as any form of percutaneous bile drainage as a palliative measure for bile stasis can be carried out[25].
Cyst development has the potential to squeeze or obstruct the biliary and circulatory systems. Rapid cyst growth is primarily caused by two factors: Infection and cyst necrosis. Necrosis in the centre region of the lesion is frequently the consequence of inadequate vascularization of the mass. Increased intracystic pressure and mass effect caused by necrosis can result in cysto-biliary fistulas, peritoneal and/or pleural cavity rupture, or bile stasis. These issues are reduced by catheter drainage of necrotic material at a decreased intracystic pressure[26].
A significant side effect of AE is cyst infection, which can manifest clinically as septicemia and cholangitis, which might resemble a liver abscess. Until the situation is suitable for major surgery, catheter drainage of infected cysts, such as liver abscesses, should be carried out. Transcatheter draining of a potentially fatal bacterial or fungal infection within the cyst can be done as a bridge operation in symptomatic patients before a curative surgical surgery, as surgical therapy is contraindicated in cases with acutely infected cysts. Both the abscess-related symptoms and the biliary tree and artery compression symptoms can be relieved by catheter drainage[26,27].
Radiological interventions are quite helpful in cases of infectious symptoms of cholangitis or centro-parasitic abscess. When systemic antibiotics are taken in conjunction with percutaneous draining of enormous centro-parasitic abscesses, the patient's clinical status is much improved. It is particularly helpful for older patients who are not candidates for a partial hepatectomy. When parasite tissue invasion of the biliary tree results in cholangitis and a fibro-inflammatory reaction, radiological interventional methods are also highly beneficial[28].
The main causes of biliary obstruction symptoms are either direct invasion of the major bile ducts or the mass effect of hepatic alveolar echinococcus. In addition, pigment stones building up above a parasitic biliary stenosis or a parasite mass connecting to the bile ducts may cause cholelithia symptoms[27]. Liver tissue is extensively destroyed and liver function declines more quickly in cases of biliary obstruction and cholangitis. In these cases, we opt for cyst draining if the patient has a sizable necrotic mass in order to release pressure on the main arteries and biliary network. Cyst draining alone may, in some situations, ease biliary stasis symptoms because the cyst shrinks. The bile content of the cyst can be drained simultaneously with the cyst content without the need for further biliary drainage if biliary invasion and large cysto-biliary fistulas are present. If cyst drainage is the only treatment that these patients benefit from, this method might help them avoid using many catheters and might be adequate till surgery. Catheterization of one or both sides of the biliary tree should relieve symptoms of biliary stasis if patients have not shown enough response with cyst draining[29].
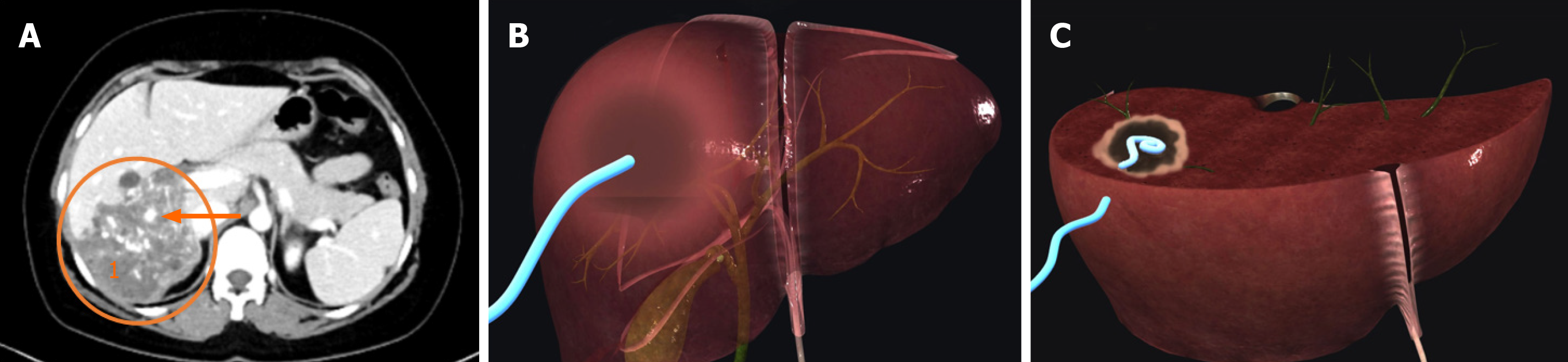
Percutaneous draining of cyst contents and/or bile ducts utilising a minimally invasive approach was found to be very helpful in a study by Muhammedoğlu et al[29] (Figure 3). Even if the mass goes away with long-term treatment, indivi
The only available treatment for patients who are not candidates for surgery is percutaneous cyst drainage or percutaneous biliary drainage combined with the medication albendazole to prevent reinfection, ease compressive symptoms, and protect the patient against rupture. If necessary, percutaneous biliary drainage or cyst draining can be paired with biliary stenting. The optimal course of treatment can be identified with the aid of thorough CT or MRI tests for extrahepatic involvement[6,29].
Inexperienced physicians, particularly in non-endemic locations, may mistakenly identify AE as a metastatic malignant tumor. Familiarity with the imaging findings of this condition is crucial for the radiologist. US can be used as an initial imaging modality and for screening purposes. Additionally, it has the capability to provide guidance for interventional operations. CT and MRI are crucial in diagnosing, planning surgeries, and monitoring patients with AE. Interventional procedures are particularly significant in both diagnosis and treatment of complex patients. Radiology plays a crucial role in providing guidance to clinicians for timely diagnosis and suitable therapy.
| 1. | Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004;17:107-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1187] [Article Influence: 56.5] [Reference Citation Analysis (1)] |
| 2. | Romig T, Deplazes P, Jenkins D, Giraudoux P, Massolo A, Craig PS, Wassermann M, Takahashi K, de la Rue M. Ecology and Life Cycle Patterns of Echinococcus Species. Adv Parasitol. 2017;95:213-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 301] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 3. | Kern P, Menezes da Silva A, Akhan O, Müllhaupt B, Vizcaychipi KA, Budke C, Vuitton DA. The Echinococcoses: Diagnosis, Clinical Management and Burden of Disease. Adv Parasitol. 2017;96:259-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 326] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 4. | Deplazes P, Rinaldi L, Alvarez Rojas CA, Torgerson PR, Harandi MF, Romig T, Antolova D, Schurer JM, Lahmar S, Cringoli G, Magambo J, Thompson RC, Jenkins EJ. Global Distribution of Alveolar and Cystic Echinococcosis. Adv Parasitol. 2017;95:315-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 640] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 5. | Thompson RC. Biology and Systematics of Echinococcus. Adv Parasitol. 2017;95:65-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 237] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 6. | Kantarci M, Bayraktutan U, Karabulut N, Aydinli B, Ogul H, Yuce I, Calik M, Eren S, Atamanalp SS, Oto A. Alveolar echinococcosis: spectrum of findings at cross-sectional imaging. Radiographics. 2012;32:2053-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet. 2003;362:1295-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 717] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 8. | Eckert J, Deplazes P, Kern P. Alveolar echinococcosis (Echinococcus multilocularis). Oxford Med Online. 2011;. [DOI] [Full Text] |
| 9. | Bulakçı M, Kartal MG, Yılmaz S, Yılmaz E, Yılmaz R, Şahin D, Aşık M, Erol OB. Multimodality imaging in diagnosis and management of alveolar echinococcosis: an update. Diagn Interv Radiol. 2016;22:247-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Bresson-Hadni S, Spahr L, Chappuis F. Hepatic Alveolar Echinococcosis. Semin Liver Dis. 2021;41:393-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Liu W, Delabrousse É, Blagosklonov O, Wang J, Zeng H, Jiang Y, Wang J, Qin Y, Vuitton DA, Wen H. Innovation in hepatic alveolar echinococcosis imaging: best use of old tools, and necessary evaluation of new ones. Parasite. 2014;21:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Czermak BV, Unsinn KM, Gotwald T, Waldenberger P, Freund MC, Bale RJ, Vogel W, Jaschke WR. Echinococcus multilocularis revisited. AJR Am J Roentgenol. 2001;176:1207-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Macpherson CN, Bartholomot B, Frider B. Application of ultrasound in diagnosis, treatment, epidemiology, public health and control of Echinococcus granulosus and E. multilocularis. Parasitology. 2003;127 Suppl:S21-S35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Bresson-Hadni S, Delabrousse E, Blagosklonov O, Bartholomot B, Koch S, Miguet JP, Mantion GA, Vuitton DA. Imaging aspects and non-surgical interventional treatment in human alveolar echinococcosis. Parasitol Int. 2006;55 Suppl:S267-S272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Bulakci M, Yilmaz E, Cengel F, Gocmez A, Kartal MG, Isik EG, Celenk E, Yegen G, Salmaslioglu A. Disseminated alveolar hydatid disease resembling a metastatic malignancy: a diagnostic challenge-a report of two cases. Case Rep Radiol. 2014;2014:638375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Walter SS, Schneeweiß S, Maurer M, Kraus MS, Wichmann JL, Bongers MN, Lescan M, Bamberg F, Othman AE. Virtual non-enhanced dual-energy CT reconstruction may replace true non-enhanced CT scans in the setting of suspected active hemorrhage. Eur J Radiol. 2018;109:218-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Kantarcı M, Aydın S, Kahraman A, Oğul H, İrgül B, Levent A. Virtual non-enhanced dual-energy computed tomography reconstruction: a candidate to replace true non-enhanced computed tomography scans in the setting of suspected liver alveolar echinococcosis. Diagn Interv Radiol. 2023;29:736-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 18. | Kodama Y, Fujita N, Shimizu T, Endo H, Nambu T, Sato N, Todo S, Miyasaka K. Alveolar echinococcosis: MR findings in the liver. Radiology. 2003;228:172-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Kantarci M, Aydin S, Eren S, Ogul H, Akhan O. Imaging Aspects of Hepatic Alveolar Echinococcosis: Retrospective Findings of a Surgical Center in Turkey. Pathogens. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Gourtsoyianni S, Papanikolaou N, Yarmenitis S, Maris T, Karantanas A, Gourtsoyiannis N. Respiratory gated diffusion-weighted imaging of the liver: value of apparent diffusion coefficient measurements in the differentiation between most commonly encountered benign and malignant focal liver lesions. Eur Radiol. 2008;18:486-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 21. | Bruegel M, Holzapfel K, Gaa J, Woertler K, Waldt S, Kiefer B, Stemmer A, Ganter C, Rummeny EJ. Characterization of focal liver lesions by ADC measurements using a respiratory triggered diffusion-weighted single-shot echo-planar MR imaging technique. Eur Radiol. 2008;18:477-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 317] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 22. | Piarroux M, Piarroux R, Giorgi R, Knapp J, Bardonnet K, Sudre B, Watelet J, Dumortier J, Gérard A, Beytout J, Abergel A, Mantion G, Vuitton DA, Bresson-Hadni S. Clinical features and evolution of alveolar echinococcosis in France from 1982 to 2007: results of a survey in 387 patients. J Hepatol. 2011;55:1025-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 23. | Kawamura N, Kamiyama T, Sato N, Nakanishi K, Yokoo H, Kamachi H, Tahara M, Yamaga S, Matsushita M, Todo S. Long-term results of hepatectomy for patients with alveolar echinococcosis: a single-center experience. J Am Coll Surg. 2011;212:804-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Vuitton DA, Bresson-Hadni S, Giraudoux P, Bartholomot B, Laplante JJ, Delabrousse E, Blagosklonov O, Mantion G. [Alveolar echinococcosis: from an incurable rural disease to a controlled urban infection]. Presse Med. 2010;39:216-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Liu YH, Wang XG, Gao JS, Qingyao Y, Horton J. Continuous albendazole therapy in alveolar echinococcosis: long-term follow-up observation of 20 cases. Trans R Soc Trop Med Hyg. 2009;103:768-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Matsumoto J, Yagi K. Experimental studies on Echinococcus multilocularis in Japan, focusing on biohazardous stages of the parasite. Exp Parasitol. 2008;119:534-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Bresson-Hadni S, Miguet JP, Mantion G, Giraudoux P, Vuitton DA. [Alveolar echinococcosis: a disease comparable to a slow growing cancer]. Bull Acad Natl Med. 2008;192:1131-8; discussion 1139. [PubMed] |
| 28. | Vuitton DA, Mantion G, Million L, Bresson-Hadni S. [Échinococcose alvéolaire]. Rev Prat. 2020;70:754-764. [PubMed] |
| 29. | Muhammedoğlu B, Pircanoğlu EM, Pişkin E, Torun S, Karadağ M, Topuz S, Köktaş S. Treatment of Hepatic Hydatid Disease: Role of Surgery, ERCP, and Percutaneous Drainage: A Retrospective Study. Surg Laparosc Endosc Percutan Tech. 2020;31:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |