Published online Aug 27, 2024. doi: 10.4240/wjgs.v16.i8.2630
Revised: May 22, 2024
Accepted: July 11, 2024
Published online: August 27, 2024
Processing time: 137 Days and 1.6 Hours
The incidence and mortality rates of primary hepatocellular carcinoma (HCC) are high, and the conventional treatment is radiofrequency ablation (RFA) with transcatheter arterial chemoembolization (TACE); however, the 3-year survival rate is still low. Further, there are no visual methods to effectively predict their prognosis.
To explore the factors influencing the prognosis of HCC after RFA and TACE and develop a nomogram prediction model.
Clinical and follow-up information of 150 patients with HCC treated using RFA and TACE in the Hangzhou Linping Hospital of Traditional Chinese Medicine from May 2020 to December 2022 was retrospectively collected and recorded. We examined their prognostic factors using multivariate logistic regression and created a nomogram prognosis prediction model using the R software (version 4.1.2). Internal verification was performed using the bootstrapping technique. The prognostic efficacy of the nomogram prediction model was evaluated using the concordance index (CI), calibration curve, and receiver operating characteristic curve.
Of the 150 patients treated with RFA and TACE, 92 (61.33%) developed recurrence and metastasis. Logistic regression analysis identified six variables, and a predictive model was created. The internal validation results of the model showed a CI of 0.882. The correction curve trend of the prognosis prediction model was always near the diagonal, and the mean absolute error before and after internal validation was 0.021. The area under the curve of the prediction model after internal verification was 0.882 [95% confidence interval (95%CI): 0.820-0.945], with a specificity of 0.828 and sensitivity of 0.656. According to the Hosmer-Lemeshow test, χ2 = 3.552 and P = 0.895. The predictive model demonstrated a satisfactory calibration, and the decision curve analysis demonstrated its clinical applicability.
The prognosis of patients with HCC after RFA and TACE is affected by several factors. The developed prediction model based on the influencing parameters shows a good prognosis predictive efficacy.
Core Tip: The incidence and mortality rates of primary hepatocellular carcinoma (HCC) are alarming. Even after radiofrequency ablation (RFA) and transcatheter arterial chemoembolization (TACE), the survival rate of patients is still low. Thus, the risk of poor prognosis needs to be accurately predicted. We analyzed the clinical and follow-up data of 150 patients with HCC and solved the problem of poor prognosis assessment by explaining the relationship between the independent influencing factors of HCC and the prognosis of the patients. Subsequently, a predictive nomogram model was developed for determining the prognosis of patients with HCC after RFA and TACE.
- Citation: Shen HH, Hong YR, Xu W, Chen L, Chen JM, Yang ZG, Chen CH. Nomogram predicting the prognosis of primary liver cancer after radiofrequency ablation combined with transcatheter arterial chemoembolization. World J Gastrointest Surg 2024; 16(8): 2630-2639
- URL: https://www.wjgnet.com/1948-9366/full/v16/i8/2630.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i8.2630
Primary hepatocellular carcinoma (HCC) is a frequently occurring tumor of the digestive system with a high and annually increasing morbidity and mortality rate[1]. Most patients with HCC have missed the optimal time for resection once diagnosed[2]. In such cases, radiofrequency ablation (RFA) and transcatheter arterial chemoembolization (TACE) are often used. However, some patients have a poor prognosis. Therefore, improving the postoperative survival time and quality of patients with HCC is one of the key topics in clinical research. If the risk of poor prognosis (recurrence and metastasis) can be accurately predicted after surgery, targeted intervention can be provided. This has important clinical significance for improving the patient’s prognosis and quality of life. Recently, the parameters influencing the prognosis of patients with HCC treated with RFA and TACE have been the subject of extensive research; however, there are no visual methods to effectively predict their prognosis[3]. A nomogram, which visualizes the results of logistic or COX regression analysis, shows the quantitative relationship between multiple predictors, and enables clinicians to assess prognostic risk visually, has been successfully applied to a range of diseases[4].
Therefore, this study aimed to effectively identify individuals with a high risk of recurrence and metastasis, achieve early intervention, and improve prognosis by constructing a nomogram prediction model using the examined parameters that affect the prognosis of patients with HCC treated with RFA and TACE.
The clinical and follow-up data of 150 patients with HCC treated with RFA and TACE in the Hangzhou Linping Hospital of Traditional Chinese Medicine from May 2020 to December 2022 were retrospectively collected. Inclusion criteria included: (1) Magnetic resonance imaging (MRI) or CT, pathological biopsy, and laboratory examination in line with the diagnostic criteria for HCC in the “Guidelines for the Diagnosis and Treatment of Primary Liver Cancer (2011 Edition)”[5]; (2) Patient underwent RFA with TACE treatment; (3) Child-Pugh classification of liver function was A and B; and (4) Clinical data including medical records, laboratory examination, and follow-up were complete. The exclusion criteria were as follows: (1) Abnormal coagulation function and multiple organ failure; (2) Other malignant tumors; (3) Gastrointestinal bleeding; and (4) Hepatic encephalopathy or refractory ascites. The same team of medical professionals performed all operations, and all were successful. The study was approved by the Ethics Committee of the Hangzhou Linping Hospital of Traditional Chinese Medicine, which waived the requirement to obtain informed consent.
TACE was initially administered to all patients, followed by RFA 2-3 weeks later. The patients underwent routine preoperative blood tests, liver and kidney function tests, imaging examinations, and alpha-fetoprotein (AFP) examinations with tumor location, shape, size, and number detection to determine the treatment plan. The Seldinger method was used to puncture the femoral arteries. During the fluoroscopy of digital subtraction angiography (DSA), the catheter was selectively placed into the arteries supplying the tumor, and chemotherapy drugs (50 mg/m2 of lobaplatin for injection and 0.5-1.0 g/m2 of floxuridine) were administered. Pirarubicin hydrochloride (20-40 mg/m2) and lipiodol (5-25 mL) were used as appropriate for embolization, according to the tumor volume and embolization condition during tumor surgery and liver function, respectively. Under DSA fluoroscopy, the catheter was slowly injected into the feeding artery for embolization. Symptomatic treatments for liver protection and analgesia were provided postoperatively. RFA patients fasted for 4-6 hours before treatment, general intravenous anesthesia, CT positioning, and determination of the puncture point and needle insertion direction and angle. The radiofrequency electrode needle was inserted into the tumor center from the positioning point, and after confirming the correct position, it was opened to start the RFA treatment. A single-positioning multi-point puncture technique was used for treatment, and the temperature during treatment was 95-110 °C. When the tumor diameter was < 3 cm, the treatment time was controlled at approximately 5 minutes; when it was between 3 and 4 cm, the treatment time was controlled at approximately 10 minutes; and when it was > 4 cm, the treatment time was controlled at approximately 15 minutes. To prevent significant complications, the patient’s vital signs were closely monitored during the operation. After the treatment, the wound was cauterized to stop bleeding when the needle was withdrawn. It was protected with a band-aid, and hemostatic drugs and antibiotics were routinely applied for 3 days after the operation.
The patient’s electronic medical records and follow-up information were collected. Clinical information regarding age, sex, TNM stage, tumor differentiation degree, capsule integrity, adjacent to large blood vessel tumor, number of lesions, Child-Pugh liver function grade, hepatitis B surface antigen, combined portal vein collateral circulation, portal vein tumor thrombosis, vascular invasion, liver cirrhosis, antiviral therapy, smoking, drinking, maximum tumor diameter, AFP, platelets, prothrombin time, total bilirubin, Karnofsky score, albumin, α-L-fucosidase (AFU), γ-glutamyl transpeptidase (GGT), prognostic nutritional index (PNI), lymphocyte ratio, C-reactive protein, aspartate aminotransferase, and aminotransferase were collected.
Karnofsky score[6]: One week after the operation, the patient’s physical condition was assessed based on their current state, performance of normal activities, and level of self-care using the Karnofsky score method. The maximum score was 100, and a high score indicated good health. PNI = Serum albumin value (g/L) + 5 × total number of peripheral blood lymphocytes (× 109/L)[7]. Serum albumin was measured using a Mindray BS-280 automatic biochemical analyzer (Shenzhen Mindray Bio-medical Electronics Co. LTD). The total number of lymphocytes in the peripheral blood was determined using a DxH800 blood cell analyzer (Beckman Coulter).
The patients were followed up by telephone or outpatient follow-up after the operation. The follow-up started 1 week after the operation and ended when there was tumor recurrence and metastasis. During the follow-up, the number of tumor recurrences or metastases was counted. Patients who had tumor recurrence or metastasis were included in the recurrence and metastasis group, and the remaining patients were included in the non-recurrence or metastasis group. Tumor recurrence or metastasis: The level of AFP increases after surgery, and MRI or CT examination indicates that the original tumor has a blood supply or a new lesion in a distant location[8]. Tumor-free survival: There is no significant fluctuation of AFP after operation, and MRI or CT examination indicates that the original tumor has a blood supply or a new lesion in a distant location. The tumor lesions have no blood supply, no new lesions are at a distant location, and the tumor lesions are completely necrotic with no metastasis. The cut-off date for follow-up in this study was July 30, 2023.
The original data was analyzed using SPSS 23.0; mean ± SD was used to depict continuous variables that followed a normal distribution, and a t-test was used for intergroup comparisons. Categorical variables were represented as n (%), and the χ2 or rank-sum test was used for intergroup comparisons. Patient prognostic factors were examined using both univariate and multivariate logistic regression models. A nomogram prediction model was created in accordance with the prognostic model developed using logistic regression analysis of patients with HCC treated with RFA and TACE. The area under the curve (AUC) analysis was used to assess the model’s discriminative capability, and internal validation with 500 bootstrap iterations was used to assess the calibration effect via unreliability tests and calibration curves. The value of the model in terms of clinical applications was assessed using the decision curve analysis (DCA). The R software (version 4.1.2) was used for statistical analyses. Statistical significance was defined as P < 0.05.
Of the 150 patients with HCC in this study, 92 (61.33%) had recurrence or metastasis within 6 months of postoperative follow-up, constituting the recurrence and metastasis group, and 58 (38.67%) patients without recurrence or metastasis were included in the non-recurrence and metastasis group. Statistically significant differences were observed between the portal vein collateral circulation, portal vein tumor thrombosis, vascular invasion, liver cirrhosis, AFP, AFU, GGT, and PNI of the recurrence/metastasis groups and the non-recurrence/metastasis groups (P < 0.05), as presented in Table 1.
| Index | Recurrence and metastasis group (n = 92) | Non-recurrence and metastasis group (n = 58) | t/χ2/Z | P value |
| Gender | 1.039 | 0.308 | ||
| Male | 63 (68.48) | 35 (60.34) | ||
| Female | 29 (31.52) | 23 (39.66) | ||
| TNM stage | -0.051 | 0.960 | ||
| T2 | 24 (26.09) | 10 (17.24) | ||
| T3 | 19 (20.65) | 20 (34.48) | ||
| T4 | 49 (53.26) | 28 (48.28) | ||
| Degree of tumor differentiation | 2.592 | 0.107 | ||
| I/II | 60 (65.22) | 45 (77.59) | ||
| III/IV | 32 (34.78) | 13 (22.41) | ||
| Coated complete | 1.039 | 0.308 | ||
| Yes | 63 (68.48) | 35 (60.34) | ||
| No | 29 (31.52) | 23 (39.66) | ||
| The tumor is adjacent to large blood vessels | 0.575 | 0.448 | ||
| Yes | 16 (17.39) | 13 (22.41) | ||
| No | 76 (82.61) | 45 (77.59) | ||
| Number of lesions | 1.529 | 0.216 | ||
| ≤ 3 | 70 (76.09) | 49 (84.48) | ||
| > 3 | 22 (23.91) | 9 (15.52) | ||
| Child-Pugh | 1.558 | 0.212 | ||
| Grade A | 54 (58.70) | 28 (48.28) | ||
| Grade B | 38 (41.30) | 30 (51.72) | ||
| HBsAg | 0.214 | 0.643 | ||
| Positive | 65 (70.65) | 43 (74.14) | ||
| Negative | 27 (29.35) | 15 (25.86) | ||
| Combined portal collateral circulation | 8.272 | 0.004 | ||
| Yes | 42 (45.65) | 13 (22.41) | ||
| No | 50 (54.35) | 45 (77.59) | ||
| Portal vein tumor thrombosis | 19.244 | < 0.001 | ||
| Yes | 72 (78.26) | 25 (43.10) | ||
| No | 20 (21.74) | 33 (56.90) | ||
| Vascular invasion | 8.191 | 0.004 | ||
| Yes | 28 (30.43) | 6 (10.34) | ||
| No | 64 (69.57) | 52 (89.66) | ||
| Combined liver cirrhosis | 24.017 | < 0.001 | ||
| Yes | 60 (65.22) | 14 (24.14) | ||
| No | 32 (34.78) | 44 (75.86) | ||
| Antiviral therapy | 1.468 | 0.226 | ||
| Yes | 43 (46.74) | 33 (56.90) | ||
| No | 49 (53.26) | 25 (43.10) | ||
| Smoking | 0.702 | 0.402 | ||
| Yes | 54 (58.70) | 30 (51.72) | ||
| No | 38 (41.30) | 28 (48.28) | ||
| Alcohol drinking | 0.865 | 0.352 | ||
| Yes | 50 (54.35) | 27 (46.55) | ||
| No | 42 (45.65) | 31 (53.45) | ||
| Age (year) | 58.26 ± 12.95 | 60.11 ± 9.24 | 0.946 | 0.346 |
| Greatest tumor diameter (cm) | 3.87 ± 0.92 | 4.02 ± 0.58 | 1.110 | 0.269 |
| AFP (μg/L) | 457.26 ± 70.47 | 419.28 ± 67.73 | 3.263 | 0.001 |
| Blood platelet (× 109/L) | 102.45 ± 30.52 | 99.57 ± 30.26 | 0.565 | 0.573 |
| Prothrombin time (seconds) | 14.77 ± 2.21 | 14.81 ± 2.55 | 0.102 | 0.919 |
| Total bilirubin (μmol/L) | 19.66 ± 5.72 | 20.08 ± 4.39 | 0.477 | 0.634 |
| Cartesian score (U/L) score | 83.47 ± 20.22 | 79.87 ± 20.15 | 1.063 | 0.289 |
| Albumin (g/L) | 31.04 ± 3.22 | 30.89 ± 3.35 | 0.274 | 0.785 |
| AFU (U/L) | 42.36 ± 6.70 | 38.22 ± 5.53 | 3.935 | < 0.001 |
| GGT (U/L) | 45.37 ± 7.74 | 48.25 ± 8.45 | 2.142 | 0.034 |
| PNI | 42.39 ± 9.79 | 48.36 ± 8.23 | 3.862 | < 0.001 |
| NLR | 2.46 ± 0.72 | 2.57 ± 0.82 | 0.863 | 0.389 |
| CRP (mg/L) | 2.83 ± 0.82 | 2.91 ± 0.93 | 0.552 | 0.582 |
| AST (U/L) | 145.26 ± 38.57 | 150.11 ± 30.15 | 0.813 | 0.417 |
| ALT (U/L) | 85.39 ± 20.58 | 80.22 ± 25.21 | 1.372 | 0.172 |
When comparing clinical data from the two groups, indicators with statistically significant differences were included as independent variables, and the prognosis of patients with HCC treated with RFA and TACE was considered as dependent variable (0 = non-recurrence and metastasis, 1 = recurrence and metastasis). Multivariate logistic regression analysis was carried out. Portal vein tumor thrombosis, vascular invasion, liver cirrhosis, AFP, AFU, and PNI were independent prognostic factors for patients with HCC treated with RFA and TACE (P < 0.05) (Table 2).
| Variable | Assignment | β | SE | Wald | P value | OR | 95%CI | |
| Lower limit | Upper limit | |||||||
| Combined portal collateral circulation | 0 = No, 1 = Yes | 0.652 | 0.489 | 1.776 | 0.183 | 1.919 | 0.736 | 5.002 |
| Portal vein tumor thrombosis | 0 = No, 1 = Yes | 1.570 | 0.491 | 10.212 | 0.001 | 4.806 | 1.835 | 12.589 |
| Vascular invasion | 0 = No, 1 = Yes | 1.229 | 0.606 | 4.107 | 0.043 | 3.418 | 1.041 | 11.218 |
| Cirrhosis | 0 = No, 1 = Yes | 1.330 | 0.474 | 7.874 | 0.005 | 3.783 | 1.494 | 9.580 |
| AFP | 0.010 | 0.003 | 8.196 | 0.004 | 1.010 | 1.003 | 1.016 | |
| AFU | 0.127 | 0.041 | 9.385 | 0.002 | 1.135 | 1.047 | 1.231 | |
| GGT | -0.046 | 0.030 | 2.385 | 0.123 | 0.955 | 0.900 | 1.013 | |
| PNI | -0.083 | 0.028 | 9.174 | 0.002 | 0.920 | 0.872 | 0.971 | |
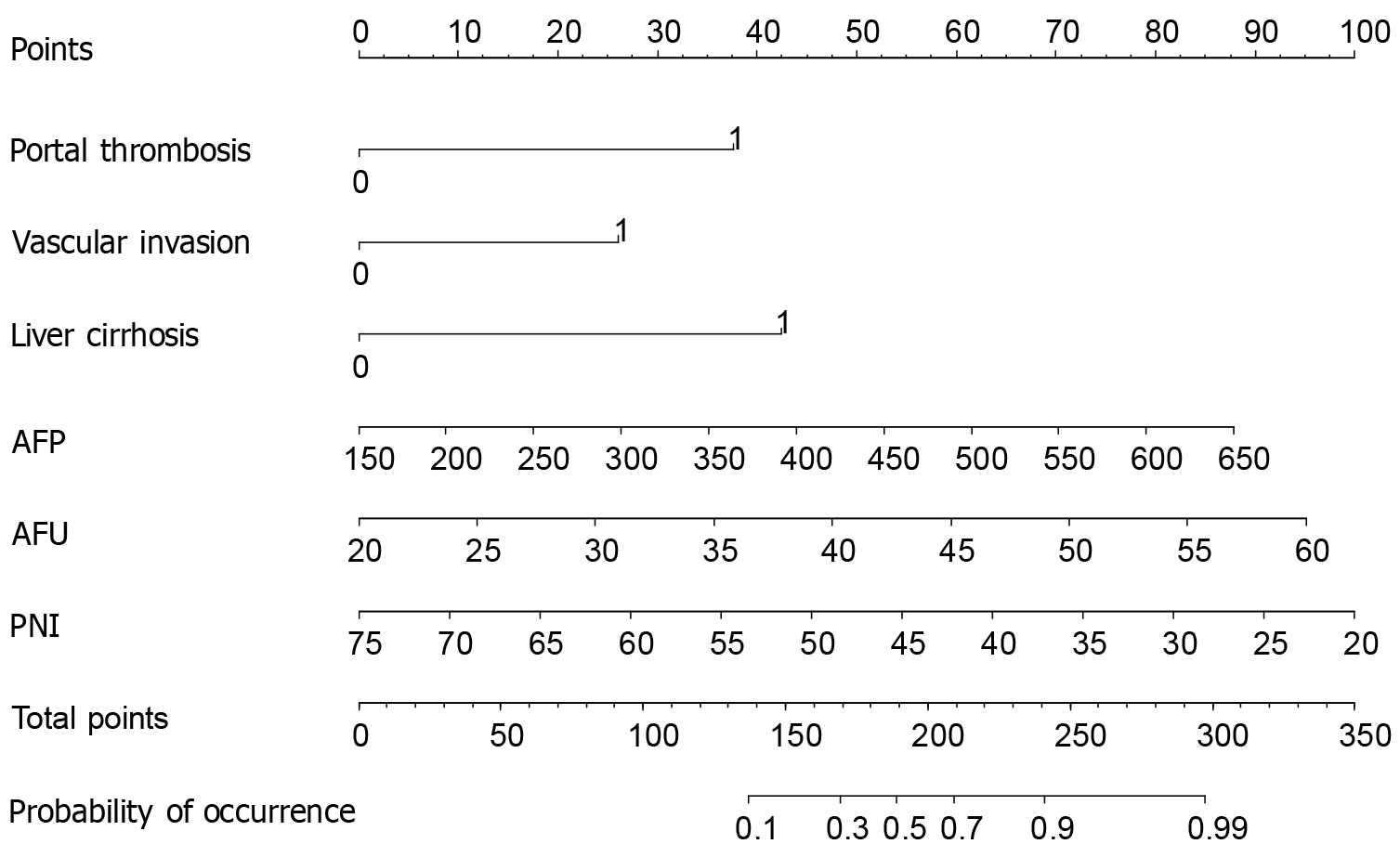
Portal vein tumor thrombosis, vascular invasion, liver cirrhosis, AFP, AFU, and PNI were selected as the predictive indices of the model. The coefficient of each predictor entering the model was as follows: Portal vein tumor thrombosis, 1.566; vascular invasion, 1.330; liver cirrhosis, 1.479; AFP, 0.009; AFU, 0.119; and PNI, -0.085. We established a nomogram-based model for predicting the prognosis of patients with HCC treated with RFA and TACE (Figure 1). The formula, based on the model, was presented as follows: -6.349 + 1.566 × portal vein tumor thrombosis + 1.330 × vascular invasion + 1.479 × liver cirrhosis + 0.009 × AFP + 0.119 × AFU -0.085 × PNI.
Prediction method: If a patient has portal vein tumor thrombosis, vascular invasion, and liver cirrhosis and the detected AFP, AFU, and PNI values are 400 μg/L, 35 U/L, and 45, respectively, the patient’s score is 32.5 + 28.0 + 31.0 + 45.0 + 37.5 + 54.0 = 228.0 points and the corresponding risk value is about 0.8, indicating that the probability of poor postoperative prognosis of this patient is 80%.
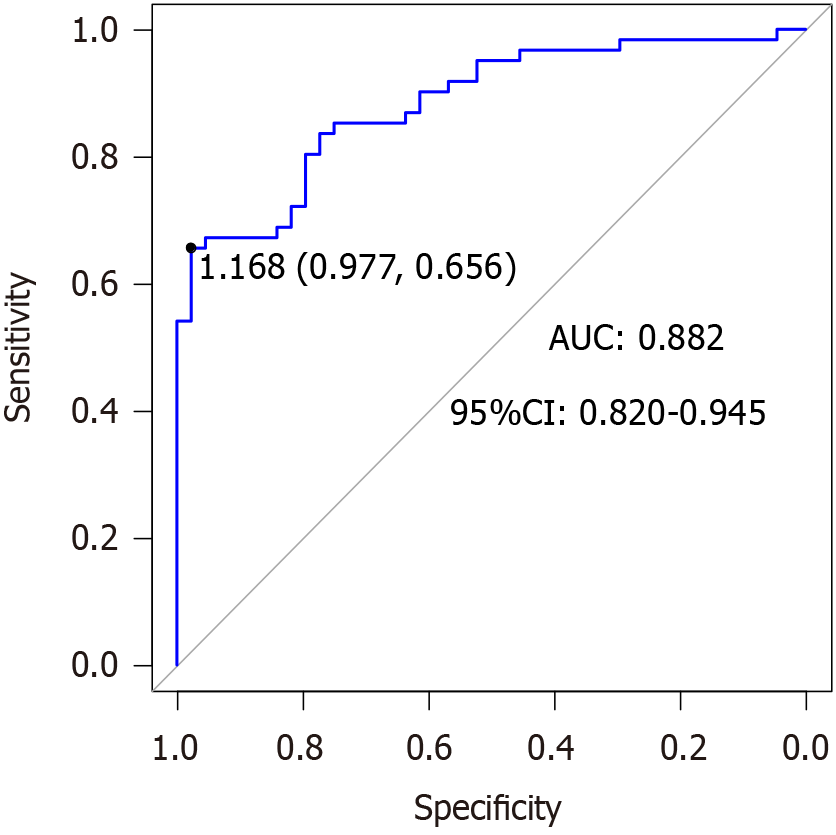
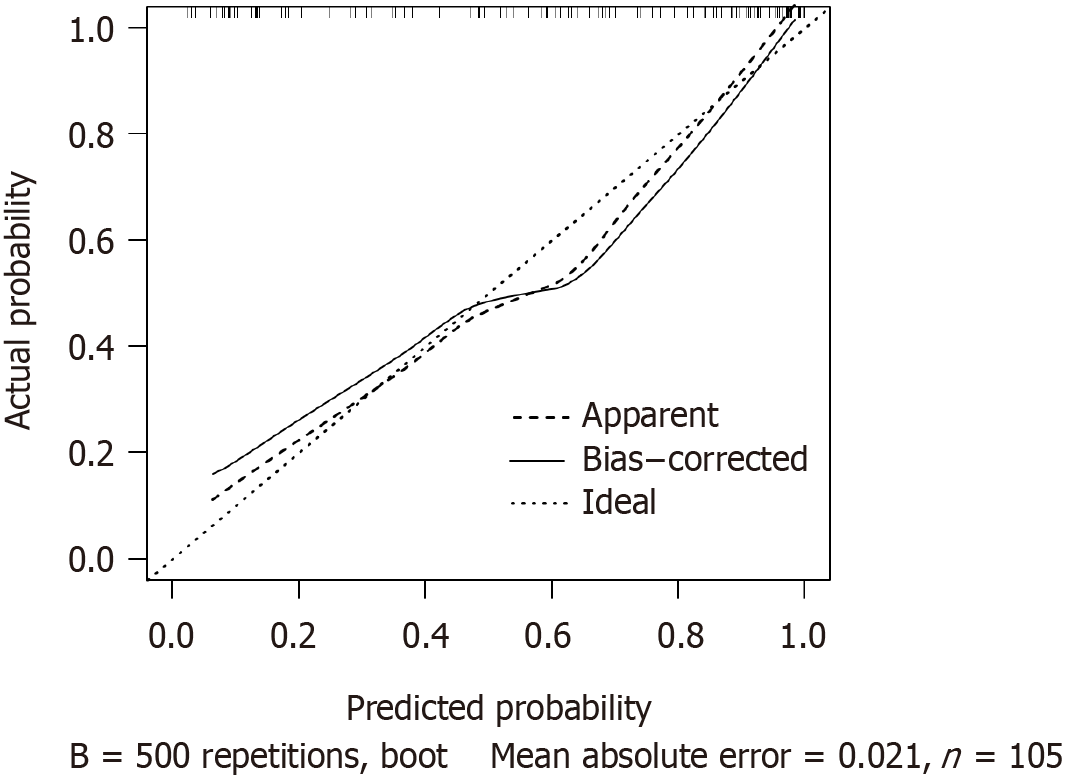
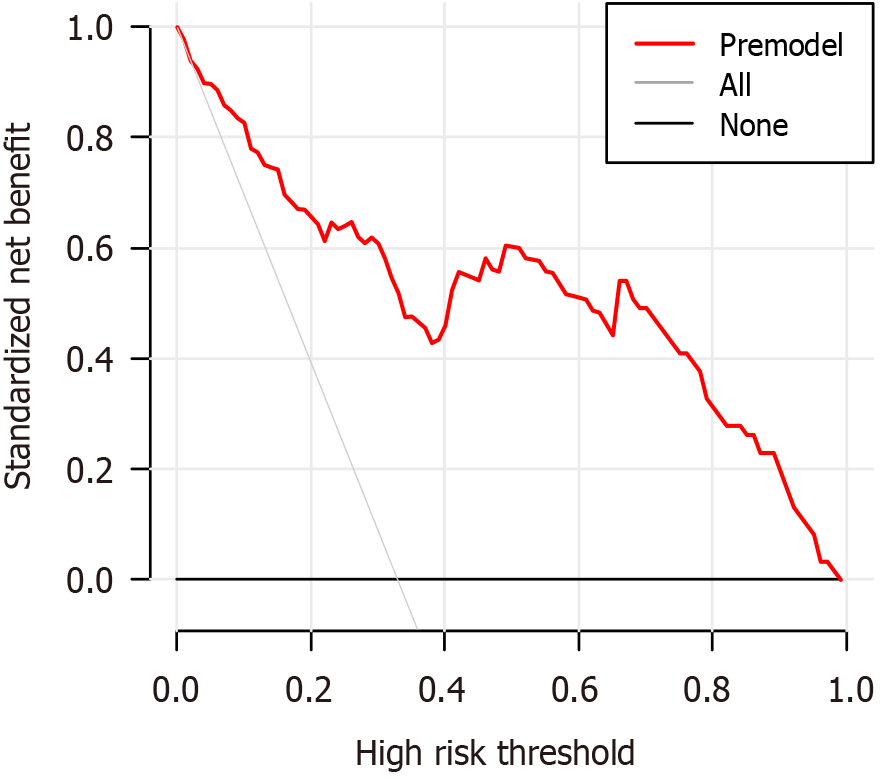
The AUC of the nomogram prediction model for HCC prognosis in patients receiving RFA and TACE was 0.882 (95%CI: 0.820-0.945), specificity was 0.977, and sensitivity was 0.656. After the Hosmer-Lemeshow test, χ2 = 3.552 and P = 0.895 (Figure 2). The results of the model’s internal validation showed a concordance index (CI) of 0.882. The trend in the calibration curve of the nomogram prediction model for the prognosis of patients with HCC treated with RFA and TACE was always near the diagonal line (Figure 3). The P value for the Hosmer-Lemeshow test was 0.973, with an Emax value of 0.014 and an Eavg value of 0.006, suggesting that this model fits the data perfectly. The DCA of the model is shown in Figure 4. With a threshold probability of < 85%, this model provides an additional value relative to either the treat-all or treat-none schemes.
RFA and TACE therapy can effectively improve the tumor necrosis rate, inhibit local tumor recurrence, and increase the patient’s survival rate. Moreover, the tumor response and short-term survival rates of those who received RFA and TACE therapy were better than those who received monotherapy. However, in clinical practice, not all patients with HCC who successfully received RFA and TACE achieved a good prognosis. In our study, the clinical records of 150 patients with primary liver cancer who underwent RFA and TACE were retrospectively examined, and the recurrence rate of tumor metastasis revealed within 3 years was 61.33%. Therefore, exploring a visual and efficient prediction method is of great significance for guiding clinicians in assessing the prognosis of patients with HCC who are receiving RFA and TACE early and taking intervening measures.
The results of this study revealed that portal vein tumor thrombosis, vascular invasion, liver cirrhosis, AFP, AFU, and PNI were independent predictors of prognosis in patients with HCC treated with RFA and TACE. A possible reason for this is that portal vein tumor thrombosis is a tumor thrombus formed by the sclerosis of the portal vein wall caused by blood stasis and backflow due to metastasis and compression of tumor cells through the portal vein circulation. Portal vein tumor thrombosis not only affects cardiac output and blood volume and hinders normal blood transport in the whole body, but it also spreads to the main portal vein with blood operation. This causes complex clinical symptoms and signs, reduces the patient’s quality of life, shortens their survival, and even endangers their lives[9,10]. Therefore, in the perioperative period, prophylactic infusion of cytokines can induce killer cells to delay the formation of tumor thrombi and improve surgical prognosis. Vascular invasion refers to a malignant tumor in the vascular system, mainly manifested as the formation of hepatic and portal vein tumor thrombus[11]. The patient liver blood vessels are surrounded by tumors, which increases the difficulty of surgery and the risk of residual tumor cells after surgery. After the vascular invasion, the tumor can spread and metastasize to the blood circulation, resulting in liver cancer recurrence or metastasis. Previous studies demonstrated that vascular invasion is an independent risk factor for HCC prognosis[12], which is consistent with the results of our study. Another study also pointed out that microvascular invasion is an independent risk factor for postoperative recurrence and metastasis of liver cancer[13]. Hence, a careful surgical plan should be formulated for patients with vascular invasion, and postoperative adjuvant therapy should be administered if necessary[14]. Liver cirrhosis is one of the pathological foundations of liver cancer; therefore, patients who have liver cirrhosis are more likely to experience liver cancer recurrence. Additionally, the survival rate of patients is reduced by the dual harm caused by liver cirrhosis and liver cancer. The enhancement of liver function increases the survival time of patients with HCC and cirrhosis[15]. Therefore, while treating patients with liver cancer, effective supportive treatment should be provided to enhance liver function reserve, slow the progression of liver cirrhosis, and increase patient survival time. Serum AFP is a clinical marker for assessing and identifying the recurrence and metastasis of liver cancer, and an elevated AFP level indicates that patients are in a state of larger tumor burden[16]. In addition, AFP can inhibit immune function and promote DNA synthesis in tumor cells, thereby mediating tumor proliferation and metastasis. According to our study’s findings, patients with a poor prognosis had higher serum AFP levels than did patients with a favorable prognosis, which could be caused by elevated AFP levels in patients with tumor recurrence or metastasis. According to a study on the relationship between AFP levels and the prognosis of patients with HCC, those with high AFP levels had a poor liver background and larger tumor burden, indicating that high preoperative AFP expression may be an important factor leading to postoperative liver cancer recurrence[17]. Therefore, patients with high AFP levels should be monitored in the clinic and followed up appropriately, and close attention should be paid to their surgical prognosis and timely interventions. AFU is a lysosomal acid hydrolase that promotes the metabolism of oligosaccharides, glycolipids, and glycoproteins. It is highly expressed in HCC and is used as a marker. The mechanism by which AFU affects the prognosis of liver cancer remains unclear, and the analysis may be related to the effect of tumor damage on the liver tissue to mediate the synthesis pathway of AFU and hinder its elimination process of AFU. Relevant research has shown that AFU may be related to the metastatic ability of tumors, and an increase in its level helps tumor cells escape immune recognition by the body[18]. It is speculated that AFU might be a major factor in the development and poor prognosis of HCC. Serum albumin and peripheral blood lymphocytes, which may indicate a patient’s nutritional state and immune system activity, are associated with the PNI. Relevant research has revealed a strong correlation between preoperative PNI and postoperative liver cancer recurrence[19]. HCC is a chronic wasting disease, which can easily lead to malnu
Currently, nomogram models predict the risk of disease prognosis in colon cancer, ovarian cancer, severe acute pancreatitis, and esophageal cancer and have achieved good results[21-24]. In this study, independent prognostic factors (portal vein tumor thrombosis, vascular invasion, liver cirrhosis, AFP, AFU, and PNI) in patients with HCC receiving RFA and TACE were used as predictors to establish a nomogram prediction model for prognosis. The trend of the calibration curve of the nomogram prediction model always fell near the diagonal line, indicating a good nomogram model calibration and prediction consistency. The AUC, specificity, and sensitivity were higher than 0.8, indicating that the nomogram model performed well when used to predict the prognosis of patients with HCC receiving RFA and TACE. The P value obtained in the Hosmer-Lemeshow test was higher than 0.05, the anticipated and actual risk values of the nomogram model did not differ statistically in any way, and the goodness of fit of the model was significant. The results of the internal verification revealed that the constructed nomogram prediction model was reliable, effective, and applicable to clinical prognosis prediction of HCC treated with RFA combined with TACE. Information on the predicted variables in the nomogram prediction model is reflected in the results of routine laboratory examinations, tumor marker detection, and imaging examinations of patients. The method of using a nomogram to assess risk is relatively simple and easy to implement. Moreover, the risk value can be quickly obtained, enabling clinicians to achieve high-efficiency predictions.
The prognosis of patients with HCC receiving RFA and TACE is affected by portal vein tumor thrombosis, vascular invasion, liver cirrhosis, AFP, AFU, and PNI. The goodness-of-fit and prediction performance of the prediction model is of clinical importance. The monogram prediction model helps clinicians identify patients with a high risk of recurrence and metastasis, which is important for early intervention and improved prognosis. The limitations of this study are that it was based on data from a single center and that the analysis was performed retrospectively, resulting in a small sample size and the possibility of overlooking potential confounders in the data, leading to selective bias in the outcomes. Therefore, further confirmation by prospective randomized controlled studies with larger and more reliable sample sizes is needed. The established nomogram prediction model was only verified internally, and lacks external verification based on data from other centers. Therefore, the reliability of the model still requires to be confirmed by additional evidence before it can be readily applied in clinical settings.
| 1. | Pinter M, Scheiner B, Peck-Radosavljevic M. Immunotherapy for advanced hepatocellular carcinoma: a focus on special subgroups. Gut. 2021;70:204-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 170] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 2. | Khemlina G, Ikeda S, Kurzrock R. The biology of Hepatocellular carcinoma: implications for genomic and immune therapies. Mol Cancer. 2017;16:149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 321] [Article Influence: 40.1] [Reference Citation Analysis (1)] |
| 3. | Jiang HY, Chen J, Xia CC, Cao LK, Duan T, Song B. Noninvasive imaging of hepatocellular carcinoma: From diagnosis to prognosis. World J Gastroenterol. 2018;24:2348-2362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 121] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (1)] |
| 4. | Dong YM, Sun J, Li YX, Chen Q, Liu QQ, Sun Z, Pang R, Chen F, Xu BY, Manyande A, Clark TG, Li JP, Orhan IE, Tian YK, Wang T, Wu W, Ye DW. Development and Validation of a Nomogram for Assessing Survival in Patients With COVID-19 Pneumonia. Clin Infect Dis. 2021;72:652-660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 5. | Qin S; Primary Liver Cancer Diagnosis and Treatment Expert Panel of the Chinese Ministry of Health. Guidelines on the diagnosis and treatment of primary liver cancer (2011 edition). Chin Clin Oncol. 2012;1:10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 6. | Ahluwalia M, Ali MA, Joshi RS, Park ES, Taha B, McCutcheon I, Chiang V, Hong A, Sinclair G, Bartek J Jr, Chen CC. An integrated disease-specific graded prognostic assessment scale for melanoma: contributions of KPS, CITV, number of metastases, and BRAF mutation status. Neurooncol Adv. 2021;3:vdaa152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Cadwell JB, Afonso AM, Shahrokni A. Prognostic nutritional index (PNI), independent of frailty is associated with six-month postoperative mortality. J Geriatr Oncol. 2020;11:880-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Colombo N, Sessa C, du Bois A, Ledermann J, McCluggage WG, McNeish I, Morice P, Pignata S, Ray-Coquard I, Vergote I, Baert T, Belaroussi I, Dashora A, Olbrecht S, Planchamp F, Querleu D; ESMO-ESGO Ovarian Cancer Consensus Conference Working Group. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease†. Ann Oncol. 2019;30:672-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 749] [Article Influence: 124.8] [Reference Citation Analysis (0)] |
| 9. | Lin X, Huang Y, Sun Y, Tan X, Ouyang J, Zhao B, Wang Y, Xing X, Liu J. 4E-BP1(Thr46) Phosphorylation Association with Poor Prognosis in Quantitative Phosphoproteomics of Portal Vein Tumor Thrombus Revealed that 4E-BP1Thr46 Phosphorylation is Associated with Poor Prognosis in HCC. Cancer Manag Res. 2020;12:103-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Zheng K, Zhu X, Fu S, Cao G, Li WQ, Xu L, Chen H, Wu D, Yang R, Wang K, Liu W, Wang H, Bao Q, Liu M, Hao C, Shen L, Xing B, Wang X. Sorafenib Plus Hepatic Arterial Infusion Chemotherapy versus Sorafenib for Hepatocellular Carcinoma with Major Portal Vein Tumor Thrombosis: A Randomized Trial. Radiology. 2022;303:455-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 98] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 11. | Zhang R, Ye J, Huang H, Du X. Mining featured biomarkers associated with vascular invasion in HCC by bioinformatics analysis with TCGA RNA sequencing data. Biomed Pharmacother. 2019;118:109274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Mokdad AA, Singal AG, Marrero JA, Zhu H, Yopp AC. Vascular Invasion and Metastasis is Predictive of Outcome in Barcelona Clinic Liver Cancer Stage C Hepatocellular Carcinoma. J Natl Compr Canc Netw. 2017;15:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Mehta N, Heimbach J, Harnois DM, Sapisochin G, Dodge JL, Lee D, Burns JM, Sanchez W, Greig PD, Grant DR, Roberts JP, Yao FY. Validation of a Risk Estimation of Tumor Recurrence After Transplant (RETREAT) Score for Hepatocellular Carcinoma Recurrence After Liver Transplant. JAMA Oncol. 2017;3:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 291] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 14. | Zhang YF, Shang H, Zeng XL, Ji H, Li YM, Lu HW. Postoperative adjuvant chemo (embolization) therapy for hepatocellular carcinoma with portal vein tumor thrombosis. Onco Targets Ther. 2018;11:5407-5417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Li L, Mo F, Hui EP, Chan SL, Koh J, Tang NLS, Yu SCH, Yeo W. The association of liver function and quality of life of patients with liver cancer. BMC Gastroenterol. 2019;19:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Qiu ZC, Li C, Zhang Y, Xie F, Yu Y, Leng SS, Chen TH, Wen TF. Tumor burden score-AFP-albumin-bilirubin grade score predicts the survival of patients with hepatocellular carcinoma after liver resection. Langenbecks Arch Surg. 2023;408:250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Chen T, Dai X, Dai J, Ding C, Zhang Z, Lin Z, Hu J, Lu M, Wang Z, Qi Y, Zhang L, Pan R, Zhao Z, Lu L, Liao W, Lu X. AFP promotes HCC progression by suppressing the HuR-mediated Fas/FADD apoptotic pathway. Cell Death Dis. 2020;11:822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 18. | Yu X, Zhang R, Yang T, Zhang M, Xi K, Lin Y, Wen Y, Wang G, Huang Z, Zhang X, Zhang L. Alpha-l-fucosidase: a novel serum biomarker to predict prognosis in early stage esophageal squamous cell carcinoma. J Thorac Dis. 2019;11:3980-3990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Yang J, Bao Y, Chen W, Duan Y, Sun D. Nomogram Based on Systemic Immune Inflammation Index and Prognostic Nutrition Index Predicts Recurrence of Hepatocellular Carcinoma After Surgery. Front Oncol. 2020;10:551668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Qu Z, Lu YJ, Feng JW, Chen YX, Shi LQ, Chen J, Rambaran N, Duan YF, He XZ. Preoperative Prognostic Nutritional Index and Neutrophil-to-Lymphocyte Ratio Predict Survival Outcomes of Patients With Hepatocellular Carcinoma After Curative Resection. Front Oncol. 2021;11:823054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 21. | Liu C, Hu C, Huang J, Xiang K, Li Z, Qu J, Chen Y, Yang B, Qu X, Liu Y, Zhang G, Wen T. A Prognostic Nomogram of Colon Cancer With Liver Metastasis: A Study of the US SEER Database and a Chinese Cohort. Front Oncol. 2021;11:591009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Sun H, Yan L, Chen H, Zheng T, Zhang Y, Wang H. Development of a nomogram to predict prognosis in ovarian cancer: a SEER-based study. Transl Cancer Res. 2020;9:5829-5842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Han D, Xu F, Li C, Zhang L, Yang R, Zheng S, Wang Z, Lyu J. A Novel Nomogram for Predicting Survival in Patients with Severe Acute Pancreatitis: An Analysis Based on the Large MIMIC-III Clinical Database. Emerg Med Int. 2021;2021:9190908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Semenkovich TR, Yan Y, Subramanian M, Meyers BF, Kozower BD, Nava R, Patterson GA, Kreisel D, Puri V. A Clinical Nomogram for Predicting Node-positive Disease in Esophageal Cancer. Ann Surg. 2021;273:e214-e221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |