Published online Aug 27, 2024. doi: 10.4240/wjgs.v16.i8.2602
Revised: June 8, 2024
Accepted: June 27, 2024
Published online: August 27, 2024
Processing time: 95 Days and 6.5 Hours
This study investigated the construction and clinical validation of a predictive model for neuroaggression in patients with gastric cancer. Gastric cancer is one of the most common malignant tumors in the world, and neuroinvasion is the key factor affecting the prognosis of patients. However, there is a lack of systematic analysis on the construction and clinical application of its prediction model. This study adopted a single-center retrospective study method, collected a large amo
To investigate the value of a model based on clinical data, spectral computed to
A retrospective analysis was performed on 80 gastric cancer patients who under
There were statistically significant differences in sex, carbohydrate antigen 199 expression, tumor thickness, Lauren classification and Borrmann classification between the two groups (all P < 0.05). Among the energy spectrum parameters, there were statistically significant differences in the single energy values (CT60-CT110 keV) at the arterial stage between the two groups (all P < 0.05) and statistically significant differences in CT values, iodide group values, standardized iodide group values and single energy values except CT80 keV at the portal vein stage between the two groups (all P < 0.05). The support vector machine model with the largest area under the curve was selected by image omics analysis, and its area under the curve, sensitivity, specificity, accuracy, P value and pa
The combined model based on clinical features, spectral CT parameters and imaging data has good value for the preoperative prediction of gastric cancer neuroinvasion.
Core Tip: By collecting clinical data of patients with single-center gastric cancer, a predictive model of neuroaggression was constructed and analyzed for clinical validation. The research included screening for relevant factors affecting gastric cancer neuroaggression, building predictive models using statistical and machine learning methods, and evaluating the accuracy and usefulness of the models through cross-validation and external validation. Finally, the performance of the model in clinical practical application is analyzed to provide clinicians with a reliable predictive tool aimed at optimizing the diagnosis and treatment strategies of gastric cancer and improving the prognosis and survival rate of patients.
- Citation: Lan YY, Han J, Liu YY, Lan L. Construction of a predictive model for gastric cancer neuroaggression and clinical validation analysis: A single-center retrospective study. World J Gastrointest Surg 2024; 16(8): 2602-2611
- URL: https://www.wjgnet.com/1948-9366/full/v16/i8/2602.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i8.2602
Stomach cancer is the fifth most common cancer and the third most common cause of cancer-related death worldwide[1]. PNI refers to the invasion of the nerve tract membrane or neuromuscular tract of the adjacent nerve by tumor cells, and this invasion is a key pathway of tumor invasion and is associated with a high risk of tumor recurrence[2-4]. At present, accurate assessment of the PNI relies only on postoperative pathology, and reliable preoperative prediction is highly important for the treatment and prognosis of gastric cancer patients[5]. Energy spectrum computed tomography (CT) involves multiparameter imaging on the basis of conventional CT single-parameter imaging and can optimize image contrast through single-energy imaging and material analysis technology for quantitative measurement of lesions, which has great clinical application value for qualitative, typing and staging diagnosis of gastric cancer[6-8]. By obtaining a large amount of data from conventional imaging images, imaging omics can extract quantitative data sets that are indirectly related to pathophysiological features[9]. At present, the number of imaging omics studies related to gastric cancer based on CT images is gradually increasing, but there are few reports[10-12] on the analysis of primary gastric cancer lesions based on clinical characteristics, energy spectrum CT parameters and imaging omics to establish a predictive PNI model.
As one of the most common malignant tumors in the world, gastric cancer has high morbidity and mortality rates and is a serious threat to human health[13]. The prognosis of gastric cancer patients is related to many factors, including the clinicopathological features of the tumor, the choice of treatment, and individual differences. Among these factors, neuroinvasion, as one of the important pathological features of gastric cancer, is highly important for prognosis asse
This study provides a scientific basis and provides technical support for prognosis assessment and treatment decisions for patients with gastric cancer. The establishment of an effective prediction model for neuroaggression can help clinicians identify high-risk patients in a timely manner and make individualized treatment plans in advance to maximize treatment efficacy and quality of life.
Eighty patients with gastric cancer in our hospital between January 2022 and August 2023 were retrospectively enrolled.
The inclusion criteria were as follows: (1) Patients who underwent radical surgery for gastric cancer + D2 lymph node dissection; (2) Patients who had no history of radiotherapy before surgery; (3) Patients whose postoperative TN was classified as advanced gastric cancer (T2-4aN0-3), and the clinicopathological data were complete; and (4) Patients whose energy spectrum data were available 1 week before surgery.
The exclusion criteria were as follows: (1) Had other tumors or a previous history of malignant tumors; (2) Had poor image quality or insufficient stomach filling and could not clearly delineate the contour of the lesion; or (3) Lacked clinicopathological data. This study was approved by the Ethics Committee of Peking University First Hospital, and all patients provided informed consent.
Age, sex, the tumor markers A-fetoprotein, carcinoembryonic antigen, carbohydrate antigen 125 (CA125), and carbohydrate antigen 199 (CA199) were collected. CA199, tumor thickness, degree of differentiation, Lauren classification, Borrmann classification, presence or absence of PNI.
GE 256-slice revolution CT in gemstone spectral imaging (GSI) mode was used to perform enhanced scanning of the abdominal artery stage and portal vein stage. The patient was placed in a supine position with hands raised on both sides of the head, and the scan was conducted from the top of the diaphragm to the lower poles of both kidneys. The tube voltage was quickly switched between 140 kVp and 80 kVp, the tube current was 375 mAs, the pitch was 1.375:1, the detector was 64 mm, the collimator was 0.625 mm × 40 mm, the matrix was 512 × 512, the display field was 40 cm, the layer thickness was 5 mm, the layer spacing was 5 mm, and the reconstruction thickness was 1.25 mm. Ioferol (320 mg/mL) was injected at 80-100 mL at 3.5 mL/second through the anterior elbow vein, and 30 mL of sodium chloride aqueous solvent was immediately injected at the same rate. Enhanced images of the arterial phase and portal vein phase were obtained 30 seconds and 70 seconds after injection, respectively.
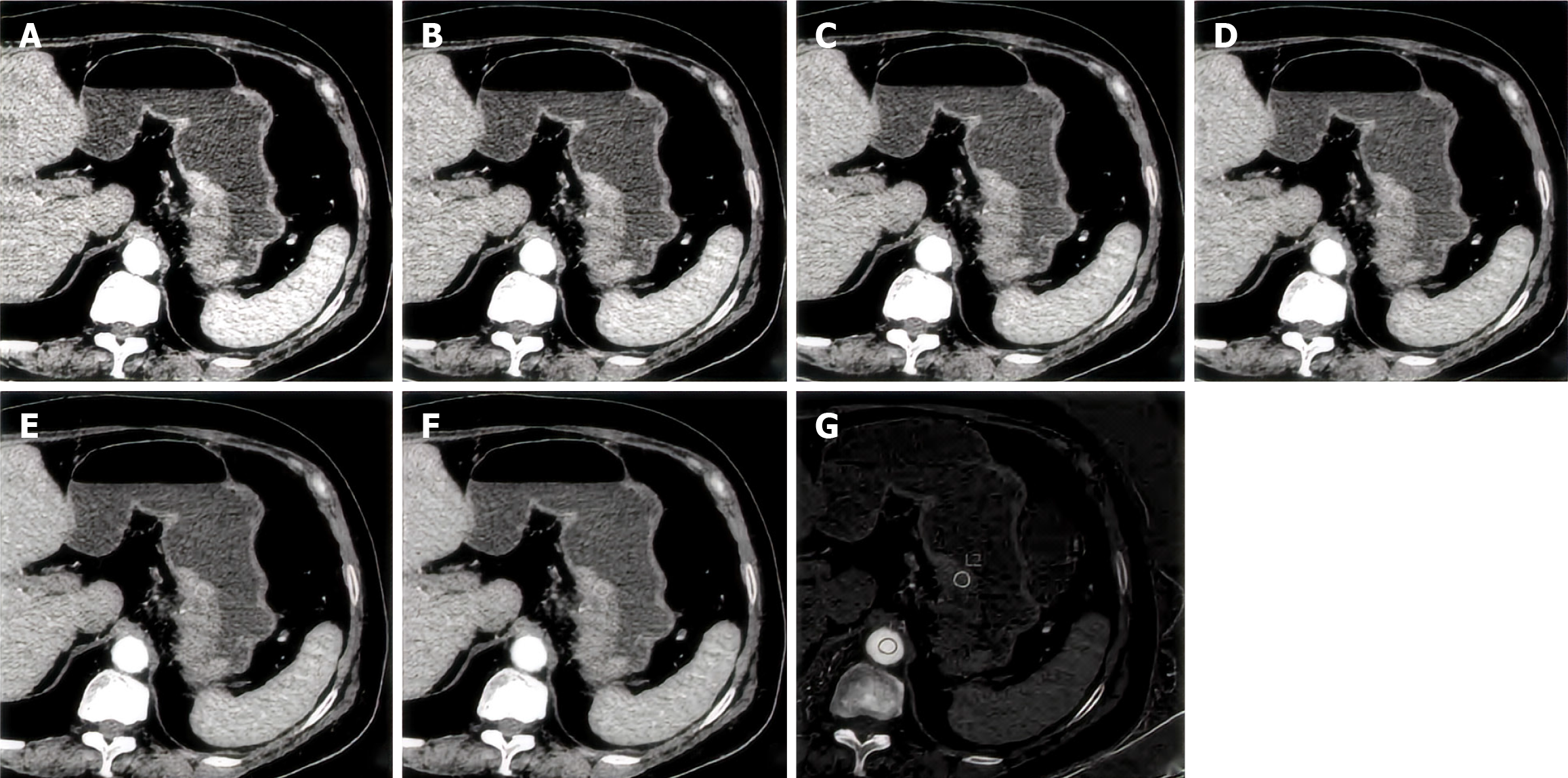
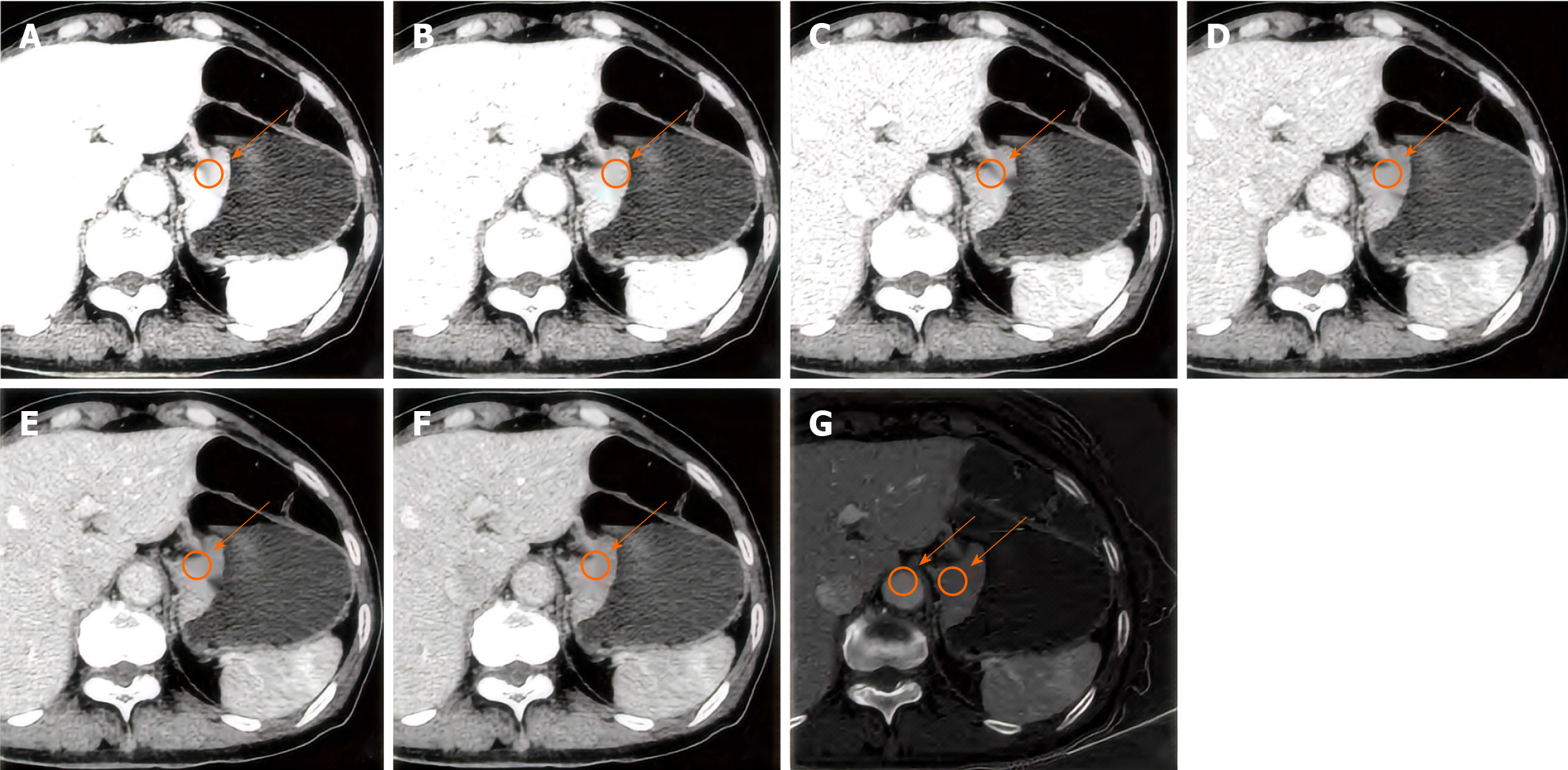
The images were independently analyzed by a radiologist using GSI Viewer analysis software and verified by a senior abdominal diagnostician. To determine the site of the primary lesion of gastric cancer, the tumor thickness (maximum short diameter) at the transverse position and the largest tumor level were measured. In addition, the values of arteriovenous two-phase CT, single energy (CT60-CT110 keV), iodine concentration, IC), normalized IC in the same abdominal aorta (ICao), and normalized IC (NIC, IC/ICao) are shown in Figures 1 and 2.
Image segmentation: The enhanced images of the arterial stage and portal vein stage were imported into 3D Slicer 4.11 software (www.slicer.org) in DICOM format, and a region of interest was manually outlined from the largest tumor layer and its upper and lower layers (a total of 3 Layers).
Feature extraction: The image was resampled to 1 mm × 1 mm × 1 mm, and 214 image omics features, including shape features, first-order features, gray co-occurrence matrix features, gray dependence matrix features, gray run matrix features, grayscale size region matrix features, and adjacent gray difference matrix features, which were all from the original image, were extracted by the PyRadiomics plug-in.
Feature selection: All features with statistically significant differences (P < 0.05) were retained by the independent sample t test or rank sum test, and the omics features with feature correlation > 0.8 were deleted according to the Pearson correlation coefficient and then included in the support vector machine (SVM) model. After 5 cross-validations, the final image omics features were selected according to the optimal area under the curve (AUC) of the model.
Model construction: The clinical data with statistically significant differences in univariate analysis, spectral CT parameters and image omics features selected according to statistical analysis and SVM were included in the logistic regression algorithm. The maximum likelihood ratio stepwise forward method was used to establish a clinical model, energy spectrum CT model and image omics model, and then the clinical + energy spectrum model, clinical + image omics model, energy spectrum + image omics model and clinical + energy spectrum + image omics model were esta
MATLAB and SPSS 25.0 software were used. Normally distributed data were expressed as mean ± SD and compared using the independent sample t test. Nonnormally distributed data are represented by media (Q1, Q3) and were compared by the Mann-Whitney U test. The statistical data are expressed as cases or percentages and were compared using the χ2 test. The Pearson correlation coefficient was used to further retain the omics features, the logistic regression algorithm was applied to establish the model, and a receiver operating characteristic curve was drawn to compare the diagnostic efficiency of the different models. The AUC, 95%CI, sensitivity and specificity were calculated, and the optimal threshold was determined according to the Youden index. Finally, the Hosmer-Lemeshow test was used to test the goodness of fit of the prediction model, and P > 0.05 indicated that the model was well fitted.
Among the 80 patients, 56 were males and 24 were females, with an average age of 65.57 ± 7.67 years. There were 54 PNI-positive patients and 26 PNI-negative patients. There were statistically significant differences in sex, CA199, tumor thickness, Lauren classification, and Borrmann classification between the two groups (all P < 0.05), while there were no statistically significant differences in age, alpha-fetoprotein, carcinoembryonic antigen, or CA125 (all P > 0.05), as shown in Table 1.
| Project | PNI positive group (n = 54) | PNI negative group (n = 26) | T/χ2/Z | P value |
| Age (years, mean ± SD) | 65.00 ± 8.75 | 66.76 ± 4.82 | -0.823 | 0.416 |
| Sex | 4.786 | 0.029 | ||
| Male | 42 (78) | 14 (54) | ||
| Female | 12 (22) | 12 (46) | ||
| Alpha fetoprotein, ng/mL | 2.980 (2.160, 3.650) | 2.590 (1.700, 3.780) | 0.578 | 0.568 |
| Carcinoembryonic antigen (ng/mL) | 56.735 ± 268.309 | 2.109 ± 1.085 | 0.729 | 0.470 |
| CA125 (U/mL, mean ± SD) | 124.600 ± 540.554 | 9.191 ± 4.435 | 0.765 | 0.449 |
| CA199 [U/mL, M (Q1, Q3)] | 16.485 (11.890, 36.635) | 6.77 (3.260, 16.335) | 3.133 | 0.001 |
| Tumor thickness (mm, mean ± SD) | 20.149 ± 6.410 | 14.746 ± 5.065 | 2.664 | 0.011 |
| Lauren typing | 15.949 | 0.000 | ||
| Intestinal type | 16 (30) | 20 (77) | ||
| Diffuse type | 16 (30) | 2 (8) | ||
| Mixed type | 22 (40) | 4 (15) | ||
| Borrmann classification | 13.219 | 0.004 | ||
| I | 2 (3) | 2 (7) | ||
| Ⅱ | 8 (15) | 12 (47) | ||
| Ⅲ | 34 (63) | 6 (23) | ||
| IV | 10 (19) | 6 (23) | ||
| Differentiation degree | 2.825 | 0.244 | ||
| High | 10 (19) | 2 (8) | ||
| Middle | 18 (33) | 8 (31) | ||
| Low | 26 (48) | 16 (61) |
There were no significant differences in CT, IC, the ICao or the NIC in the arterial phase between the two groups (all P > 0.05), while there were significant differences in the single energy values of CT60 keV to CT110 keV between the two groups (all P < 0.05). There were statistically significant differences in the CT, IC, NIC and other single energy values except for the CT80 keV at the portal vein stage (all P < 0.05), but there were no statistically significant differences in the other parameters (Table 2).
| Project | PNI positive group (n = 54) | PNI negative (n = 26) | T/Z value | P value |
| Arterial phase | ||||
| CT value [Hu, M (Q1, Q3)] | 67.240 (56.800, 80.290) | 59.130 (52.585, 71.875) | 1.141 | 0.264 |
| CT60 kev [Hu, M (Q1, Q3)] | 88.040 (72.260, 107.690) | 62.370 (60.235, 75.330) | 3.018 | 0.002 |
| CT70 kev [Hu, M (Q1, Q3)] | 68.690 (59.260, 86.120) | 56.750 (50.760, 67.735) | 2.584 | 0.009 |
| CT80 kev (Hu, mean ± SD) | 61.944 ± 14.476 | 50.405 ± 9.996 | 2.584 | 0.014 |
| CT90 kev (Hu, mean ± SD) | 54.610 ± 11.875 | 45.160 ± 8.884 | 2.540 | 0.015 |
| CT100 kev (Hu, mean ± SD) | 49.912 ± 10.568 | 41.404 ± 8.025 | 2.562 | 0.014 |
| CT110 kev (Hu, mean ± SD) | 46.061 ± 9.261 | 38.783 ± 7.537 | 2.463 | 0.018 |
| IC [μg/cm³, M (Q1, Q3)] | 14.400 (11.320, 18.720) | 11.310 (10.375, 13.815) | 1.863 | 0.064 |
| ICao (μg/cm³, mean ± SD) | 105.161 ± 21.732 | 114.863 ± 18.730 | -1.380 | 0.176 |
| NIC [M (Q1, Q3)] | 0.135 (0.097, 0.205) | 0.097 (0.090, 0.125) | 1.891 | 0.060 |
| Portal venous phase | ||||
| CT value (Hu, mean ± SD) | 97.302 ± 21.606 | 78.460 ± 15.938 | 2.792 | 0.008 |
| CT60 kev[Hu, M (Q1, Q3)] | 120.317 ± 28.755 | 97.011 ± 23.240 | 2.544 | 0.015 |
| CT70 kev[Hu, M (Q1, Q3)] | 94.877 ± 21.566 | 80.289 ± 19.614 | 2.061 | 0.046 |
| CT80 kev (Hu, mean ± SD) | 78.673 ± 17.013 | 65.790 ± 14.930 | 2.329 | 0.125 |
| CT90 kev (Hu, mean ± SD) | 67.918 ± 14.126 | 57.403 ± 12.745 | 2.273 | 0.029 |
| CT100 kev (Hu, mean ± SD) | 60.452 ± 12.191 | 51.541 ± 11.360 | 2.212 | 0.033 |
| CT110 kev (Hu, mean ± SD) | 55.154 ± 10.891 | 47.410 ± 10.376 | 2.138 | 0.039 |
| IC [μg/cm³, M (Q1, Q3)] | 55.154 ± 10.891 | 18.270 (13.440, 18.960) | 2.902 | 0.003 |
| ICao [ug/cm³, M (Q1, Q3)] | 48.350 (40.200, 52.150) | 44.180 (36.690, 51.945) | 0.462 | 0.648 |
| NIC (mean ± SD) | 0.528 ± 0.184 | 0.402 ± 0.098 | 2.799 | 0.008 |
In this study, 47 features were screened through independent sample t tests and rank sum tests, and the Pearson correlation coefficient was selected to delete the feature pairs with correlations > 0.8. The remaining 16 omics features were included in the SVM to establish multiple models. According to the order of the model AUCs from largest to smallest, the model with the largest AUC and its features were selected (Firstorder_Media/firstorder_90Percentile/firstorder_RootMeanSquared/Glsimplanted largeareaemphasis). The AUC, sensitivity, specificity, accuracy, P value and parameters of the PNI SVM model were 0.843, 0.923, 0.714, 0.925, < 0.001, and c:g 2.64:10.56, respectively.
According to the logistic regression maximum likelihood ratio stepwise forward method, clinical data, sex, tumor thickness, Borrmann classification, the energy spectrum CT parameter CT60 keV at the arterial stage, the NIC at the portal vein stage, and the first-order (median) imaging characteristics were found to be independent predictors of the PNI in patients with gastric cancer (Table 3). The clinical model, energy spectrum CT model, imaging model, clinical + energy spectrum model, clinical + imaging model, energy spectrum + imaging model, clinical + energy spectrum + imaging model, and clinical + energy spectrum + imaging model were established, among which the combined clinical + energy spectrum + imaging model had the best diagnostic efficiency. The AUC, optimal threshold, Jorden index, sensitivity and specificity were 0.927 (95%CI: 0.850-1.000), 0.879, 0.778, 0.778, and 1.000, respectively (Table 4). The Hosmer-Lemeshow test values of the 7 models were 0.973, 0.761, 0.858, 0.761, 0.737, 0.529, and 0.944, respectively, with P > 0.05, indicating that all the models had good fitting effects.
| Factor | β | Corrected OR (95%CI) | P value |
| Clinical features | |||
| Sex | -5.259 | 0.005 (0-0.318) | 0.012 |
| Tumor thickness | 0.351 | 1.421 (1.051-1.920) | 0.022 |
| Borrmann typing | 2.167 | 8.732 (1.486-51.324) | 0.016 |
| Spectral CT parameters | |||
| Arterial phase CT60 kev | 0.065 | 1.067 (1.012-1.124) | 0.016 |
| Portal phase NIC | 0.065 | 1.067 (1.012-1.126) | 0.016 |
| Imaging omics features | |||
| First-order (median) | 0.078 | 1.081 (1.015-1.151) | 0.016 |
| Model name | AUC (95%CI) | Optimal threshold | Yoden index | Sensitivity | Specificity |
| Clinical model | 0.858 (0.692-1.000) | 0.486 | 0.772 | 0.926 | 0.845 |
| Spectral CT model | 0.832 (0.680-0.983) | 0.531 | 0.695 | 0.925 | 0.769 |
| Imaging omics model | 0.897 (0.778-1.000) | 0.718 | 0.809 | 0.963 | 0.845 |
| Clinical + spectral model | 0.772 (0.608-0.936) | 0.643 | 0.735 | 0.889 | 0.845 |
| Clinical and imaging omics models | 0.842 (0.717-0.967) | 0.67 | 0.584 | 0.815 | 0.769 |
| Energy spectrum + imaging omics model | 0.846 (0.720-0.972) | 0.809 | 0.553 | 0.63 | 0.923 |
| Clinical + energy spectrum + imaging omics model | 0.927 (0.850-1.000) | 0.879 | 0.778 | 0.778 | 1.00 |
Nerves, blood vessels, lymph nodes, etc., constitute the microscopic environment of tumors and play a crucial role in the process of cancer progression[19]. The preoperative prediction of the PNI is highly important for the individualized treatment of gastric cancer patients[20]. In this study, a model was established based on clinical data, energy spectrum CT parameters, and image omics characteristics for the noninvasive prediction of the PNI in gastric cancer patients to identify high-risk recurrent gastric cancer patients and optimize their preoperative decision-making[21-23]. The combined clinical + energy spectrum + imaging model had the best predictive power, with an AUC of 0.927 (95%CI: 0.850-1.000).
Men are more likely to have PNI-positive gastric cancer, which may be related to poor lifestyle habits such as smoking and drinking. Previous studies[24-26] have shown that the thickness and Borrmann classification of gastric cancer are related to PNI, possibly due to the strong invasiveness of Borrmann types III-IV, and the larger the tumor is, the wider the invasion range, which easily leads to PNI-positive results, which is consistent with the results of this study. The composition analysis and single-energy imaging techniques of energy spectrum CT can be used to evaluate angiogenesis and blood supply in gastric cancer, which are closely related to the PNI status[27]. In this study, IC and CT60-CT110 keV in the primary lesions of gastric cancer patients were measured, and the results showed that arterial CT60 keV and portal NIC were found to be independent predictors of PNI in gastric cancer patients, which was consistent with the results of related studies[28-30], and this inconsistency may be due to differences in the selected energy spectrum parameters.
The imaging features can reflect the heterogeneity and biological characteristics of tumor lesions[31]. At present, researchers have used imaging omics to predict the PNI status of gastric cancer patients[32]. In relevant studies[33-35], 11 omics characteristics and 2 clinical factors were incorporated into SVM and logistic regression models to predict the preoperative PNI of patients with gastric cancer, and it was found that the AUC of the comprehensive parameter model of SVM in the test set was the highest at 0.82 (0.69-0.94). Another study[36] screened 5 PNI texture features from 271 texture features and used 8 machine learning algorithms to predict the PNI, with an average AUC of 0.482-0.754 and an accuracy of 54%-68.2%, among which the naive Bayes algorithm had the best performance in predicting the PNI[37-40]. In contrast to the above studies, this study not only relies on imaging characteristics to establish a prediction model but also establishes a clinical model based on clinical data, an energy spectrum model based on energy spectrum CT parameters, a combined clinical + energy spectrum model, a clinical + imaging model, an energy spectrum + imaging model, and a clinical + energy spectrum + imaging model. The results showed that the combined clinical + energy spectrum + imaging model had the best predictive efficiency and greatly improved the ability to predict the PNI before surgery.
This was a single-center retrospective study without a validation set, which may have led to selection bias. The sample size is small, which may cause sample error. Omics features were extracted from only 3 tumor types, and whole-tumor studies should be carried out in the future. The inclusion of energy spectrum parameters was not comprehensive enough, and the effective atomic number was not explored in this study.
The combined clinical + energy spectrum + imaging model has good value for the preoperative prediction of the PNI and is helpful for accurately and effectively predicting the PNI in patients with gastric cancer before surgery.
| 1. | Xu Q, Sun Z, Li X, Ye C, Zhou C, Zhang L, Lu G. Advanced gastric cancer: CT radiomics prediction and early detection of downstaging with neoadjuvant chemotherapy. Eur Radiol. 2021;31:8765-8774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 2. | Shen Y, Kang HK, Jeong YY, Heo SH, Han SM, Chen K, Liu Y. Evaluation of early gastric cancer at multidetector CT with multiplanar reformation and virtual endoscopy. Radiographics. 2011;31:189-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | de Vries L, Harding AT. Mechanisms of Neuroinvasion and Neuropathogenesis by Pathologic Flaviviruses. Viruses. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 4. | Staniuk T, Małkowski B, Śrutek E, Szlęzak P, Zegarski W. Comparison of FLT-PET/CT and CECT in gastric cancer diagnosis. Abdom Radiol (NY). 2016;41:1349-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Gertsen EC, Borggreve AS, Brenkman HJF, Verhoeven RHA, Vegt E, van Hillegersberg R, Siersema PD, Ruurda JP; Dutch Upper Gastrointestinal Cancer Audit (DUCA) Group. Evaluation of the Implementation of FDG-PET/CT and Staging Laparoscopy for Gastric Cancer in The Netherlands. Ann Surg Oncol. 2021;28:2384-2393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Foley KG, Coomer W, Coles B, Bradley KM. The impact of baseline (18)F-FDG PET-CT on the management and outcome of patients with gastric cancer: a systematic review. Br J Radiol. 2022;95:20220437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 7. | Liu J, Qiu J, Wang K, Liu J, Sun X, Zhang J, Wang X, Wei J, Wu B, Wang X, Qin N. An investigation on gastric cancer staging using CT structured report. Eur J Radiol. 2021;136:109550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Fairweather M, Jajoo K, Sainani N, Bertagnolli MM, Wang J. Accuracy of EUS and CT imaging in preoperative gastric cancer staging. J Surg Oncol. 2015;111:1016-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Zhao S, Bi Y, Wang Z, Zhang F, Zhang Y, Xu Y. Accuracy evaluation of combining gastroscopy, multi-slice spiral CT, Her-2, and tumor markers in gastric cancer staging diagnosis. World J Surg Oncol. 2022;20:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Wu L, Zheng Y, Liu J, Luo R, Wu D, Xu P, Wu D, Li X. Comprehensive evaluation of the efficacy and safety of LPV/r drugs in the treatment of SARS and MERS to provide potential treatment options for COVID-19. Aging (Albany NY). 2021;13:10833-10852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 11. | Chen PT, Shih TTF. Editorial for "Comparison of MRI and CT-Based Radiomics and Their Combination for Early Identification of Pathological Response to Neoadjuvant Chemotherapy in Locally Advanced Gastric Cancer". J Magn Reson Imaging. 2023;58:924-925. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Li J, Zhang C, Guo H, Li S, You Y, Zheng P, Zhang H, Wang H, Bai J. Non-invasive measurement of tumor immune microenvironment and prediction of survival and chemotherapeutic benefits from (18)F fluorodeoxyglucose PET/CT images in gastric cancer. Front Immunol. 2022;13:1019386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Wu L, Zhong Y, Wu D, Xu P, Ruan X, Yan J, Liu J, Li X. Immunomodulatory Factor TIM3 of Cytolytic Active Genes Affected the Survival and Prognosis of Lung Adenocarcinoma Patients by Multi-Omics Analysis. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 62] [Reference Citation Analysis (0)] |
| 14. | Mazzei MA, Bagnacci G, Gentili F, Capitoni I, Mura G, Marrelli D, Petrioli R, Brunese L, Cappabianca S, Catarci M, Degiuli M, De Manzoni G, De Prizio M, Donini A, Romario UF, Funicelli L, Laghi A, Minetti G, Morgagni P, Petrella E, Pittiani F, Rausei S, Romanini L, Rosati R, Ianora AAS, Tiberio GAM, Volterrani L, Roviello F, Grassi R. Structured and shared CT radiological report of gastric cancer: a consensus proposal by the Italian Research Group for Gastric Cancer (GIRCG) and the Italian Society of Medical and Interventional Radiology (SIRM). Eur Radiol. 2022;32:938-949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Wu L, Liu Q, Ruan X, Luan X, Zhong Y, Liu J, Yan J, Li X. Multiple Omics Analysis of the Role of RBM10 Gene Instability in Immune Regulation and Drug Sensitivity in Patients with Lung Adenocarcinoma (LUAD). Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 55] [Reference Citation Analysis (0)] |
| 16. | Wu L, Li X, Yan J. Commentary: Machine learning developed an intratumor heterogeneity signature for predicting prognosis and immunotherapy benefits in cholangiocarcinoma. Transl Oncol. 2024;45:101995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 17. | Sun Z, Jin L, Zhang S, Duan S, Xing W, Hu S. Preoperative prediction for lauren type of gastric cancer: A radiomics nomogram analysis based on CT images and clinical features. J Xray Sci Technol. 2021;29:675-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Wu L, Zheng Y, Ruan X, Wu D, Xu P, Liu J, Wu D, Li X. Long-chain noncoding ribonucleic acids affect the survival and prognosis of patients with esophageal adenocarcinoma through the autophagy pathway: construction of a prognostic model. Anticancer Drugs. 2022;33:e590-e603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 70] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 19. | Jiang Y, Jin C, Yu H, Wu J, Chen C, Yuan Q, Huang W, Hu Y, Xu Y, Zhou Z, Fisher GA Jr, Li G, Li R. Development and Validation of a Deep Learning CT Signature to Predict Survival and Chemotherapy Benefit in Gastric Cancer: A Multicenter, Retrospective Study. Ann Surg. 2021;274:e1153-e1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 20. | Nie T, Liu D, Ai S, He Y, Yang M, Chen J, Yuan Z, Liu Y. A radiomics nomogram analysis based on CT images and clinical features for preoperative Lauren classification in gastric cancer. Jpn J Radiol. 2023;41:401-408. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Wu L, Zhong Y, Yu X, Wu D, Xu P, Lv L, Ruan X, Liu Q, Feng Y, Liu J, Li X. Selective poly adenylation predicts the efficacy of immunotherapy in patients with lung adenocarcinoma by multiple omics research. Anticancer Drugs. 2022;33:943-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 22. | Burbidge S, Mahady K, Naik K. The role of CT and staging laparoscopy in the staging of gastric cancer. Clin Radiol. 2013;68:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Guo Q, Sun Q, Bian X, Wang M, Dong H, Yin H, Dai X, Fan G, Chen G. Development and validation of a multiphase CT radiomics nomogram for the preoperative prediction of lymphovascular invasion in patients with gastric cancer. Clin Radiol. 2023;78:e552-e559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 24. | Wu L, Li H, Liu Y, Fan Z, Xu J, Li N, Qian X, Lin Z, Li X, Yan J. Research progress of 3D-bioprinted functional pancreas and in vitro tumor models. IJB. 2024;10:1256. [DOI] [Full Text] |
| 25. | Kim AY, Kim HJ, Ha HK. Gastric cancer by multidetector row CT: preoperative staging. Abdom Imaging. 2005;30:465-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Liu S, Shi H, Ji C, Zheng H, Pan X, Guan W, Chen L, Sun Y, Tang L, Guan Y, Li W, Ge Y, He J, Liu S, Zhou Z. Preoperative CT texture analysis of gastric cancer: correlations with postoperative TNM staging. Clin Radiol. 2018;73:756.e1-756.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Wang K, Guo D, Yan H, Wu Z, Xue L. Enhancing Diagnostic Accuracy for Gastric Cancer: Integration of Multi-Slice Spiral CT and Gastrointestinal Angiography. Altern Ther Health Med. 2024;30:118-123. [PubMed] |
| 28. | Wu L, Li X, Qian X, Wang S, Liu J, Yan J. Lipid Nanoparticle (LNP) Delivery Carrier-Assisted Targeted Controlled Release mRNA Vaccines in Tumor Immunity. Vaccines (Basel). 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 29. | Nakajo M, Kajiya Y, Tani A, Jinguji M, Nakajo M, Yoshiura T. FLT-PET/CT diagnosis of primary and metastatic nodal lesions of gastric cancer: comparison with FDG-PET/CT. Abdom Radiol (NY). 2016;41:1891-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Jiang Y, Yuan Q, Lv W, Xi S, Huang W, Sun Z, Chen H, Zhao L, Liu W, Hu Y, Lu L, Ma J, Li T, Yu J, Wang Q, Li G. Radiomic signature of (18)F fluorodeoxyglucose PET/CT for prediction of gastric cancer survival and chemotherapeutic benefits. Theranostics. 2018;8:5915-5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 31. | Botet JF, Lightdale CJ, Zauber AG, Gerdes H, Winawer SJ, Urmacher C, Brennan MF. Preoperative staging of gastric cancer: comparison of endoscopic US and dynamic CT. Radiology. 1991;181:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 222] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 32. | Gertsen EC, de Jongh C, Brenkman HJF, Mertens AC, Broeders IAMJ, Los M, Boerma D, Ten Bokkel Huinink D, van Leeuwen L, Wessels FJ, van Hillegersberg R, Ruurda JP. The additive value of restaging-CT during neoadjuvant chemotherapy for gastric cancer. Eur J Surg Oncol. 2020;46:1247-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Banba Y, Kanazawa T, Seto H. CT evaluation of gastric cancer: depth of tumor invasion and pancreas invasion. Radiat Med. 1998;16:161-166. [PubMed] |
| 34. | Masci GM, Ciccarelli F, Mattei FI, Grasso D, Accarpio F, Catalano C, Laghi A, Sammartino P, Iafrate F. Role of CT texture analysis for predicting peritoneal metastases in patients with gastric cancer. Radiol Med. 2022;127:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 35. | Takao M, Fukuda T, Iwanaga S, Hayashi K, Kusano H, Okudaira S. Gastric cancer: evaluation of triphasic spiral CT and radiologic-pathologic correlation. J Comput Assist Tomogr. 1998;22:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Wu L, Chen X, Zeng Q, Lai Z, Fan Z, Ruan X, Li X, Yan J. NR5A2 gene affects the overall survival of LUAD patients by regulating the activity of CSCs through SNP pathway by OCLR algorithm and immune score. Heliyon. 2024;10:e28282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 37. | Li J, Xu S, Wang Y, Fang M, Ma F, Xu C, Li H. Spectral CT-based nomogram for preoperative prediction of perineural invasion in locally advanced gastric cancer: a prospective study. Eur Radiol. 2023;33:5172-5183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 38. | Kadowaki K, Murakami T, Yoshioka H, Kim T, Takahashi S, Tomoda K, Narumi Y, Nakamura H. Helical CT imaging of gastric cancer: normal wall appearance and the potential for staging. Radiat Med. 2000;18:47-54. [PubMed] |
| 39. | Choi MH, Kim KA, Hwang SS, Byun JY. CT-quantified muscle and fat change in patients after surgery or endoscopic resection for early gastric cancer and its impact on long-term outcomes. Medicine (Baltimore). 2018;97:e13878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | de Jongh C, van der Meulen MP, Gertsen EC, Brenkman HJF, van Sandick JW, van Berge Henegouwen MI, Gisbertz SS, Luyer MDP, Nieuwenhuijzen GAP, van Lanschot JJB, Lagarde SM, Wijnhoven BPL, de Steur WO, Hartgrink HH, Stoot JHMB, Hulsewe KWE, Spillenaar Bilgen EJ, van Det MJ, Kouwenhoven EA, Daams F, van der Peet DL, van Grieken NCT, Heisterkamp J, van Etten B, van den Berg JW, Pierie JP, Eker HH, Thijssen AY, Belt EJT, van Duijvendijk P, Wassenaar E, Wevers KP, Hol L, Wessels FJ, Haj Mohammad N, Frederix GWJ, van Hillegersberg R, Siersema PD, Vegt E, Ruurda JP; PLASTIC Study Group. Impact of (18F)FDG-PET/CT and Laparoscopy in Staging of Locally Advanced Gastric Cancer: A Cost Analysis in the Prospective Multicenter PLASTIC-Study. Ann Surg Oncol. 2024;31:4005-4017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |